Abstract
Purpose
Aromatase inhibitors (AI), which decrease circulating estradiol concentrations in post-menopausal women, are associated with toxicities that limit adherence. Approximately one-third of patients will tolerate a different AI after not tolerating the first. We report the effect of crossover from exemestane to letrozole or vice versa on patient-reported outcomes (PROs) and whether the success of crossover is due to lack of estrogen suppression.
Methods
Post-menopausal women enrolled on a prospective trial initiating AI therapy for early-stage breast cancer were randomized to exemestane or letrozole. Those that discontinued for intolerance were offered protocol-directed crossover to the other AI after a washout period. Changes in PROs, including pain [Visual Analog Scale (VAS)] and functional status [Health Assessment Questionnaire (HAQ)], were compared after 3 months on the first versus the second AI. Estradiol and drug concentrations were measured.
Results
Eighty-three patients participated in the crossover protocol, of whom 91.3% reported improvement in symptoms prior to starting the second AI. Functional status worsened less after 3 months with the second AI (HAQ mean change AI #1: 0.2 [SD 0.41] vs. AI #2: −0.05 [SD 0.36]; p = 0.001); change in pain scores was similar between the first and second AI (VAS mean change AI #1: 0.8 [SD 2.7] vs. AI #2: −0.2 [SD 2.8]; p = 0.19). No statistical differences in estradiol or drug concentrations were found between those that continued or discontinued AI after crossover.
Conclusions
Although all AIs act via the same mechanism, a subset of patients intolerant to one AI report improved PROs with a different one. The mechanism of this tolerance remains unknown, but does not appear to be due to non-adherence to, or insufficient estrogen suppression by, the second AI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selective aromatase inhibitors (AIs) decrease circulating estrogen concentration in post-menopausal women by preventing conversion of adrenal-derived precursors to estradiol and estrone in peripheral tissue [1]. AIs have been shown to be more effective than the selective receptor modulator tamoxifen in the adjuvant and metastatic settings [2, 3]. However, tolerance of AI therapy can be poor due to treatment-emergent toxicities, primarily AI-induced musculoskeletal symptoms (AIMSS), which can lead to early discontinuation [4, 5].
It is desirable for patients to continue treatment with adjuvant endocrine therapy for the optimal duration in order to maximize breast cancer outcomes [6]. In the adjuvant setting, we and others have reported that some patients intolerant of an initially prescribed AI persist with a second AI treatment [4, 7, 8]. In the Exemestane and Letrozole Pharmacogenetics (ELPh) trial, conducted by the Consortium on Breast Cancer Pharmacogenomics (COBRA), women with ER positive early-stage breast cancer were randomly assigned to either adjuvant letrozole or exemestane. In a substudy, we observed that approximately one-third of women intolerant of the first AI were able to tolerate the second after a brief washout period [4]. Since all AIs act via the same mechanism and have similar toxicity profiles, it is unclear why patients intolerant of one AI would be able to tolerate a different one. In addition, it is unknown which patients are more likely to tolerate a second AI medication. In this report, we further characterize the patient-reported outcomes (PROs) and serum estradiol and drug concentrations during treatment with the first versus second AI medications in the ELPh trial to gain further insights into the mechanisms of this tolerance and of the patient experience following the switch from one AI to another.
Materials and methods
Study participants
Post-menopausal women were eligible for enrollment on the ELPh trial if they had stage 0-III hormone receptor-positive breast cancer and were initiating treatment with an AI. Details of the trial have been previously published (clinicaltrials.gov NCT00228956) [4, 9]. Prior to enrollment, all indicated surgery, chemotherapy, and/or radiation therapy were completed. Prior tamoxifen therapy was permitted. No prior AI therapy for any reason was allowed. Institutional Review Board approval was obtained at all three participating sites (Johns Hopkins University, Indiana University, University of Michigan). Before undergoing protocol-directed procedures, patients were required to provide written informed consent.
Study procedures
Patients were randomized 1:1 to treatment with letrozole (Femara; Novartis, Basel, Switzerland) 2.5 mg orally daily or exemestane (Aromasin; Pfizer, New York, NY) 25 mg orally daily. After 132 patients had been enrolled, an amendment to the protocol allowed patients with self-reported intolerance to the AI to which they were originally randomized to crossover to the other study-provided AI. Following crossover, patients discontinued the first AI medication and remained off therapy during a washout period of 2–8 weeks per protocol. Following the washout, patients started treatment on the second AI until discontinuation for any reason or completion of study follow-up.
Blood samples were collected at baseline and at 3 months on the first AI and after 1–3 months on the second AI for evaluation of serum estradiol and drug concentration. Serum samples were assayed for estradiol using an ultrasensitive gas chromatography tandem mass spectroscopy assay as previously described [10]; the lower limit of quantification (LLOQ) of this assay was 0.625 pg/mL. Letrozole and exemestane concentrations were measured by mass spectrometry as previously described [11,12,13].
PROs, described below, were obtained before and during treatment with the first AI, at the time of discontinuation of the first AI, and at baseline and 3 months following initiation of the second AI. The objective of the current PRO analysis was to compare the differences in early changes in symptom burden (3 months to baseline) during treatment with the first and second AI (Online Supplement 1). The medical record was queried to record the date of discontinuation of the second AI therapy if the patient stopped treatment after the end of the 6-month crossover period, or the date of last follow-up.
Validated tools to measure pain [Visual Analog Scale (VAS)], functional status [modified Health Assessment Questionnaire (HAQ)] [14], health-related quality of life (EuroQOL VAS) [15], depression [Center for Epidemiologic Studies–Depression (CESD)] [16], anxiety [Hospital Anxiety and Depression Scale (HADS-A)] [17], and symptom burden [18] were utilized. Clinically significant change in symptom burden was defined as a change of at least 2.0 points for the pain VAS and 0.22 points for the HAQ. [19, 20]. For symptom burden, as previously described [21], we derived six separate symptom clusters (musculoskeletal, mood, vasomotor, cognitive, weight/body image, and vulvovaginal) from a 47-item tool, composed largely of items from the Breast Cancer Prevention Trial Symptom Checklist [18]. Scores for each item ranged from 0 to 4, with a higher score indicating worse symptom burden. In addition to the above instruments, patients completed two questions, “Do you still have the symptoms that you had when you were taking the first study medication?” and “Do you currently have any bone, joint, or muscle pain?” at baseline (following washout), 1, 3, and 6 months following crossover to the second AI medication.
Statistical considerations
In this exploratory analysis of patients who crossed over from one AI medication to another because of intolerance, we examined the difference in pain, functional status, quality of life, depression, anxiety, and symptom burden during treatment on the two different AI medications. For all PROs except the EuroQOL and the two single-item crossover questions, mean change scores from baseline to 3 months were calculated such that a negative mean change in each PRO indicated the score decreased from baseline (improved) and a positive mean change in each PRO indicated the score increased from baseline (worsened). For the EuroQOL, a negative mean change from baseline to 3 months indicated QOL worsened and a positive mean change QOL improved. The PRO scores were compared within patients between their first and second AI using the Wilcoxon signed rank test. PRO evaluation was considered missing at any time point after the patient had discontinued either AI. The two crossover questionnaire responses were analyzed descriptively.
The differences in estradiol and drug concentrations at 1–3 months of the second AI therapy between those that continued and discontinued the second AI were assessed using Wilcoxon rank sum test.
Multiple comparisons were not controlled for due to the exploratory nature of the analysis. P < 0.05 was considered statistically significant.
Results
Patient characteristics
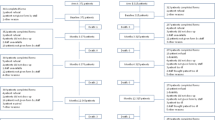
Of the 83 patients who discontinued the first AI medication and agreed to participate in the crossover study, 34 (41%) initially treated with letrozole switched to exemestane, and 49 (59%) initially treated with exemestane switched to letrozole; the proportions of those who started with each AI and switched were not statistically significantly different (p = 0.10) (Fig. 1). As previously reported [4], approximately one-third of patients who were unable to tolerate a first AI persisted in taking the second. Baseline characteristics for all the eligible patients in the current analysis are listed in Table 1.
Serum estradiol and drug concentrations following crossover
Estradiol and drug concentrations were analyzed following crossover to assess if there were pharmacodynamic and pharmacokinetic differences that would provide insight into patient tolerance of AI therapy following crossover. Of the 83 patients enrolled in the crossover study, 66 (80%) had estradiol concentrations assessed 1–3 months after initiation of treatment with the second AI (Fig. 1). Forty-nine of the 83 (59%) patients had estradiol concentrations assessed both 1–3 months after initiation of treatment with the second AI and 3 months after the first AI. Thirty-eight of 49 (77.6%) patients had estradiol concentrations below the LLOQ during treatment with both AI #1 and #2 (Fig. 2a). Six of 49 (12.2%) patients’ estradiol concentrations increased above the LLOQ during AI #2, four of 49 (8.2%) patients’ decreased below the LLOQ after switching therapy, and one (2%) remained above the LLOQ during treatment with both AI medications. There was no difference in estradiol concentrations between those that continued or discontinued after crossover to letrozole (p = 0.15) or exemestane (p = 0.27). Similarly, there was no difference in drug concentrations between those that continued or discontinued after crossover to letrozole (p = 0.39) or exemestane (p = 0.53) (Fig. 2b). Estradiol and drug concentrations were not available from the remaining 34 subjects because of technical errors or inability to obtain blood.
Serum estradiol (E2) and drug concentrations 1–3 months after initiation of the second AI. Exemestane (E) is represented by solid circles and letrozole (L) by open circles. Columns designate whether patients switched from E to L, or vice versa, and are divided by whether patients persisted on (continued) or discontinued the second AI medication by the 6-month time point. a Serum E2 concentrations. The lower limit of quantification (LLOQ) was 0.625 pg/mL and b Serum letrozole and exemestane concentrations
Change in symptom burden during treatment with the second AI
Immediately following the washout period (baseline) and serially during treatment with the second AI, patients were queried about (1) change in symptoms compared to the first AI medication (Fig. 3a) and (2) current bone, joint, or muscle pain (Fig. 3b). When queried about change in symptoms during the washout period, 9 of 46 responding patients (19.5%) reported no longer having the symptoms they had while on the first AI and 33 (71.7%) reported improved symptoms. Five of 48 responding patients (10.4%) reported no bone, joint, or muscle pain and 18 (37.5%) reported mild symptoms at the initiation of the second AI medication. After 6 months, 3 of the 35 patients (8.5%) who remained on the second AI medication continued to report no bone, joint, or muscle pain and 13 (37%) reported mild symptoms.
Differences in patient-reported outcomes during first versus second AI medications
As shown in Online Supplement 1, PROs were assessed prior to initiation of therapy (baseline) and after 3 months of treatment with the first AI medication and at the same time points before and during treatment with the second AI medication. Compared to the first AI, during the first 3 months of treatment with the second AI medication, patients reported statistically significantly less worsening of functional status (HAQ mean change AI #1: 0.20 [SD 0.41] vs. AI #2: −0.05 [SD 0.36]; p = 0.001) (Table 2). No statistically significant difference in the change in pain VAS or musculoskeletal cluster was observed when comparing symptoms during the initial 3 months of treatment with the first and second AI.
Depression worsened significantly less during the initial 3 months of treatment with the second AI medication compared to the same time period during treatment with the first AI (mean change CESD AI #1: 1.3 [SD 8.3] vs. AI #2: 0.2 [SD 6.8]; p = 0.03). Similarly, analysis of the mood symptom cluster showed that patients reported development of fewer adverse mood symptoms during the first 3 months on the second AI compared to the first AI (mean change AI #1: 0.2 [SD 0.5] vs. AI #2: 0 [SD 0.2]; p = 0.02). In addition, as compared to the first AI, patients reported statistically significantly less worsening of vasomotor symptoms during the first 3 months of treatment with the second AI (mean change AI #1: 0.2 [SD 0.7] vs. AI #2: −0.2 [SD 0.7]; p = 0.01). No other statistically significant differences were observed between changes in PROs during the first compared to the second AI.
Discussion
In the current analysis of the ELPh trial, using prospectively collected PROs in a protocol-directed crossover substudy, we observed that patients who had discontinued initial treatment with one AI medication because of toxicity were less likely to report negative impacts on functional status, depression, and vasomotor symptoms during treatment with an alternate AI medication. These findings were noted even though both AIs act via the same mechanism of action and have similar side effect profiles.
Several possible mechanisms might explain these curious findings. First, if AIMSS and other toxicities of the AIs are due to extremely low estradiol concentration, it is possible that if the second AI is less effective in lowering estradiol concentrations than the first, which could result in improved tolerance of the second AI medication. Our data do not support this theory. In the crossover cohort, there were no differences in circulating estradiol concentrations after 1-3 months of treatment between those who ultimately persisted on the second AI medication and those who discontinued treatment because of toxicity.
A second possible explanation for apparent tolerance of the second AI is non-compliance with treatment. However, examination of serum drug concentrations during treatment demonstrated similar concentrations in those that continued and those that discontinued the second AI. In addition, if a patient was not taking the medication as directed, then her estradiol concentration would be expected to be greater, which, as described above, was not what was observed. Therefore, almost all patients were likely taking the medication as reported.
A third possibility might involve inherited germline pharmacogenomics, resulting in differences in drug metabolism, estrogen signaling, or tolerance of associated pain. We previously reported an association between a specific single nucleotide polymorphism in the gene encoding estrogen receptor alpha and decreased persistence with exemestane but not letrozole, although this finding has not yet been independently verified [22]. Further, one might postulate that off-target effects account for the tolerance of one drug, but not the other. However, although slightly higher for exemestane vs. letrozole, a large proportion of patients in both initial groups was unable to tolerate one or the other, and we observed tolerance of the second AI regardless of the initially assigned AI medication.
The washout period itself may allow for improved compliance by some unclear physiologic change in symptom perception after a short break in toxicity, or alternatively, simply a psychological one. Indeed, in our study, 91% of patients reported improved or resolved symptoms following the washout period (Fig. 3b). Similarly, in the ATOLL trial, patients reported improvements in pain and functional status following a 1-month washout period [23]. However, our study was not powered to reliably explore any associations between improvement in pain symptoms following washout and duration of therapy on the second AI medication. If the improvement is due to a physiologic or a psychologic mechanism, it should not matter what AI was started as the “second” treatment—either an alternative (such as in the ELPh trial) or the same drug as initially assigned. To our knowledge, no study has formally tested if restarting the same AI after a brief period of discontinuation would similarly lead to improved tolerance of the AI medication.
On the other hand, greater patient willingness to proceed with next line therapy might influence subsequent tolerance. Multiple studies have shown that most patients are willing to accept adjuvant therapy even with minimal efficacy [24, 25]. However, limited data exist to support that patients remain similarly accepting if they discontinue prior adjuvant treatment due to toxicity. Unexpectedly, symptom severity on the first AI was higher in those that pursued crossover than those who declined (data not shown) suggesting that the degree of toxicity does not influence willingness to consider an alternate AI.
A key strength of the ELPh trial is the prospective collection of PROs during AI therapy both before and after the crossover event. In addition, and in contrast to previously reported experiences of crossover from one AI to another, the current analysis provides a comparison of switching from a steroidal to a non-steroidal AI, and vice versa, as well as assessment of circulating biomarkers. Limitations of this analysis include the small sample size, short duration of follow-up after crossover, lack of randomization to crossover versus not, and missing data. All three previously reported crossover studies similarly limited follow-up to 6 months [7, 8, 23]. Future research examining the impact of crossover on long-term tolerance of medication, or intermittent discontinuation of treatment, may reveal additional information about factors limiting tolerance of this class of medication.
In summary, we compared PROs in a prospective study of patients who were intolerant to initial AI therapy and who switched to a different AI and evaluated if any such variance might be related to differences in estradiol suppression. Despite finding no significant difference in circulating estradiol concentrations, patients reported modestly fewer symptoms on the second AI medication compared to the first. This study provides additional evidence that switching from one AI to another is an option for managing bothersome treatment-emergent symptoms and that the mechanism for this tolerance is unlikely to be related to a differential effect on estradiol suppression between AIs. A greater understanding of why an individual patient can tolerate one AI better than another may yield insights into initial treatment selection and optimize compliance with minimal impact on quality of life for breast cancer survivors.
References
Johnston SRD, Dowsett M (2003) Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer 3:821–831. doi:10.1038/nrc1211
Rugo HS, Rumble RB, Macrae E et al (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 34:3069–3103. doi:10.1200/JCO.2016.67.1487
Burstein HJ, Lacchetti C, Anderson H et al (2016) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression. J Clin Oncol 34:1689–1701. doi:10.1200/JCO.2015.65.9573
Henry NL, Azzouz F, Desta Z et al (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30:936–942. doi:10.1200/JCO.2011.38.0261
Crew KD, Greenlee H, Capodice J et al (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877–3883. doi:10.1200/JCO.2007.10.7573
Makubate B, Donnan PT, Dewar JA et al (2013) Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer 108:1515–1524. doi:10.1038/bjc.2013.116
Yardley D, Green NB, Papish S, et al (2009) Rheumatologic Evaluation of Adjuvant Letrozole in Post-Menopausal Breast Cancer Patients Discontinuing Anastrozole Due to Grade 2-3 Arthralgia—Myalgia. In: San Antonio Breast Cancer Symp. p Cancer Res 2009;69(24 Suppl):Abstract nr 805
Renshaw L, McHugh R, Williams L (2007) Comparison of joint problems reported by patients in a randomized adjuvant trial of anastrozole and letrozole. In: Breast Can Res Treat. p 106(Suppl 1):S108–S109. Abstract 2072
Henry NL, Chan H-P, Dantzer J et al (2013) Aromatase inhibitor-induced modulation of breast density: clinical and genetic effects. Br J Cancer 109:2331–2339. doi:10.1038/bjc.2013.587
Santen RJ, Demers L, Ohorodnik S et al (2007) Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 72:666–671. doi:10.1016/j.steroids.2007.05.003
Hertz DL, Kidwell KM, Seewald NJ et al (2016) Polymorphisms in drug-metabolizing enzymes and steady-state exemestane concentration in postmenopausal patients with breast cancer. Pharmacogenomics J. doi:10.1038/tpj.2016.60
Robarge JD, Desta Z, Nguyen AT et al (2017) Effects of exemestane and letrozole therapy on plasma concentrations of estrogens in a randomized trial of postmenopausal women with breast cancer. Breast Cancer Res Treat 161:453–461. doi:10.1007/s10549-016-4077-4
Desta Z, Kreutz Y, Nguyen AT et al (2011) Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin Pharmacol Ther 90:693–700. doi:10.1038/clpt.2011.174
Bruce B, Fries JF (2003) The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol 30:167–178
Pickard AS, Wilke CT, Lin H-W, Lloyd A (2007) Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics 25:365–384
Carpenter JS, Andrykowski MA, Wilson J, et al Psychometrics for two short forms of the center for epidemiologic studies-depression scale. Issues Ment Health Nurs 19:481–94
Stafford L, Judd F, Gibson P et al (2014) Comparison of the hospital anxiety and depression scale and the center for epidemiological studies depression scale for detecting depression in women with breast or gynecologic cancer. Gen Hosp Psychiatry 36:74–80. doi:10.1016/j.genhosppsych.2013.08.010
Ganz PA, Day R, Ware JE et al (1995) Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst 87:1372–1382
Farrar JT, Pritchett YL, Robinson M et al (2010) The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain 11:109–118. doi:10.1016/j.jpain.2009.06.007
Wolfe F, Michaud K, Strand V (2005) Expanding the definition of clinical differences: from minimally clinically important differences to really important differences. Analyses in 8931 patients with rheumatoid arthritis. J Rheumatol 32:583–589
Kadakia KC, Snyder CF, Kidwell KM et al (2016) Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncologist. doi:10.1634/theoncologist.2015-0349
Henry NL, Skaar TC, Dantzer J et al (2013) Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast Cancer Res Treat 138:807–816. doi:10.1007/s10549-013-2504-3
Briot K, Tubiana-Hulin M, Bastit L et al (2010) Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat 120:127–134. doi:10.1007/s10549-009-0692-7
Duric VM, Fallowfield LJ, Saunders C et al (2005) Patients’ preferences for adjuvant endocrine therapy in early breast cancer: what makes it worthwhile? Br J Cancer 93:1319–1323. doi:10.1038/sj.bjc.6602874
Ravdin PM, Siminoff IA, Harvey JA (1998) Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol 16:515–521
Acknowledgements
This study was supported in part by U01-GM61373 and 5T32-GM08425 (DAF), M01-RR00042 (UM), M01-RR00750 (IU), and M01-RR00052 (JHU). NJS was supported (in part) by Cancer Center Biostatistics Training Grant 5T32-CA083654 (to J. Taylor). NLH was a Damon Runyon-Lilly Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (Grant number CI-53-10) and by an American Cancer Society Research Scholar Grant. Additional support was provided by Fashion Footwear Association of New York/QVC Presents Shoes on Sale (DFH). In addition, the ELPh trial was supported by grants from Pfizer, Inc. and Novartis Pharma AG, who provided study medication.
Funding
Abbvie, Merck, Pfizer, MedImmune, Celgene, Puma Biotechnology, and Novartis (VS); Pfizer and Novartis (AMS); AstraZeneca, Janssen Research and Development, Puma Biotechnology, Pfizer, Eli Lilly Company (DFH); WellPoint and Genentech (CFS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Employment or Leadership Position: None, Consultant or Advisory Role: Walgreens and Pfizer (CFS); Pfizer (DFH), Stock Ownership: Immunomedics (CFS) Oncoimmune and InBiomotion (DFH), Honoraria: Pfizer (AMS); Lilly (DFH), All other authors had no conflicts of interest to disclose.
Additional information
David A. Flockhart was Deceased.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2017_4260_MOESM1_ESM.docx
Supplementary material 1 (DOCX 73 kb) Online Supplement 1—Diagram of the primary patient reported outcome analysis. PRO patient reported outcomes, AI aromatase inhibitor; Δ change
Rights and permissions
About this article
Cite this article
Kadakia, K.C., Kidwell, K.M., Seewald, N.J. et al. Prospective assessment of patient-reported outcomes and estradiol and drug concentrations in patients experiencing toxicity from adjuvant aromatase inhibitors. Breast Cancer Res Treat 164, 411–419 (2017). https://doi.org/10.1007/s10549-017-4260-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4260-2