Abstract
Estrogen receptor (ER) α has been studied extensively in familial breast cancers but there are limited data on ERβ and its isoforms. This is an important issue since many BRCA1-associated tumours are “triple negative” and are resistant to conventional and targeted therapies. We performed an immunohistochemical study of pan-ERβ, ERβ1 and ERβ2 in a cohort of 123 familial breast carcinomas (35 BRCA1, 33 BRCA2 and 55 BRCAX) using a cut-off for positivity at 20% (Shaaban et al. in Clin Cancer Res 14:5228–5235, 2008). BRCA1 cancers were more likely to be nuclear ERα negative and nuclear pan-ERβ positive (21/32, 66%) when compared with BRCA2 (2/29, 7%) and BRCAX cancers (11/49, 22%) (both P < 0.001). For survival analysis, expression was also stratified using cut-offs defined by Bates et al. (Breast Cancer Res Treat 111:453–459, 2008) (score out of 7). Cytoplasmic ERβ2 expression correlated with shorter overall survival at 15 years regardless of cut-off used (both P < 0.046) At a cut-off score of 6 out of 7, cytoplasmic ERβ2 expression correlated with a poorer response to chemotherapy in both univariate (P = 0.011) and multivariate analyses including grade, lymph node status and chemotherapy as an interaction variable (P = 0.045, Hazard ratio 1.22, 95% CI 1.004–9.87). A similar trend was seen in a univariate analysis with a cut-off of 20% although this did not reach statistical significance (P = 0.057). Expression of nuclear ERβ1 was associated with a favourable response to endocrine therapy at 15 years regardless of cut-offs employed (both P < 0.025). However, this did not reach statistical significance in a multivariate analysis (P > 0.05). Since a significant proportion of ERα negative familial breast carcinomas are positive for nuclear ERβ1 and cytoplasmic ERβ2, the different ERβ isoforms and their intracellular location may need to be assessed, to identify patients that may benefit from hormonal and chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that 5–10% of all breast cancers are attributable to inherited mutations in susceptibility genes, of which the two most important are BRCA1 and BRCA2 [1]. BRCA1 tumours show a so called triple negative phenotype being oestrogen receptor (ER), progesterone receptor (PgR) and HER2 negative [1]. They also harbour p53 mutations [2] and express basal and myoepithelial markers [3–5]. No similarly defined phenotype has been described for BRCA2 tumours which usually show a ductal, no special type morphology and ER positivity [6].
Strategies using selective oestrogen receptor modulators such as tamoxifen-targeting tumours that express ERα have resulted in improvements in relapse-free and overall survival [7]. The advent of trastuzumab and lapatinib has similarly revolutionized treatment for HER2-amplified breast cancer [8, 9]. However, the resistance of BRCA1 basal-like breast cancers to conventional agents have made these tumours difficult to treat [10]. Nevertheless, there are data to suggest that tamoxifen may prevent development of contralateral breast cancer in women with a strong family history and increases survival in BRCA1 breast-cancer-affected patients [11, 12].
Estrogens mediate their role in the progression of breast cancer and through two transcription factors, ERα and ERβ. It is becoming apparent that ERβ plays an important role in breast cancer progression [13]. In contrast to ERα, ERβ expression is progressively lost during transition from normal breast to invasive carcinoma [14, 15]. Recent studies on sporadic breast cancers have shown that the expression of ERβ isoforms 1, 2 and 5 may have important prognostic implications [16–18].
To our knowledge, there are two reports documenting the expression of ERβ in familial breast cancers. The first study in 44 familial cancer patients (16 BRCA1, 12 BRCA2 and 16 BRCAX) [19] and the second in 48 patients with BRCA1 founder mutation positive women [20]. However, there are no data regarding the range of expression for its different isoforms and the cellular pattern of expression for either BRCA1- or BRCA2-associated tumours. Thus, we have performed a comprehensive immunohistochemical analysis of pan-ERβ, ERβ1 and ERβ2 in a large cohort of BRCA1, BRCA2 and BRCAX tumours with survival data. Our aims are to document the range of expression of ERβ in familial breast cancer, and to determine the relationship between ERβ and conventional clinicopathological parameters and survival. We have also evaluated the relationship between ERβ and intrinsic breast cancer subtypes including basal-like, luminal, HER2 and null types.
Materials and methods
Patients
147 cases of familial breast carcinomas from female patients were collected from the kConFab biorepository between 1980 and 2005. Classification of BRCA1 and BRCA2 mutations and sequence variants was according to designations listed for research purposes on the kConFab website (www.kconfab.org). The BRCAX breast cancers are defined by breast cancer in families without a known BRCA1 and BRCA2 pathogenic mutation, who met kConFab category 1 and 1B eligibility criteria. Of the 147 cases, 18 cases were excluded due to the lack of tissue available and a further 6 cases were excluded due to the absence of tumour on the array. The final cohort was composed of 123 cases including 35 BRCA1, 33 BRCA2 and 55 BRCAX cases (Table 1). All the patients had operable breast carcinomas and were not diagnosed with metastatic disease at the time of presentation. Patients were followed up for a median period of 64.0 (range 0.4–298.8) months. During this time, 38 patients relapsed and 31 died from breast cancer (deaths unrelated to breast cancer were censored). Breast-cancer-specific survival was defined as time from primary surgical excision to breast-cancer-related death.
Using stratification of intrinsic phenotypes based on Nielsen et al. [21] tumours were placed into luminal (ERα positive, HER2 negative, cytokeratin (CK) 5/6 negative or positive), basal (HER2 and ERα negative; CK5/6 positive), HER2 (HER2 positive, ERα and CK5/6 negative or positive) and null/negative (HER2, ERα and CK5/6 negative).
Immunohistochemistry
Tumour tissue microarrays (1-mm cores), with a fourfold redundancy, were prepared from formalin-fixed, paraffin-embedded tissue blocks of 129 tumours. Sections were cut, dewaxed, placed through graded alcohol and water. Antigen retrieval was performed in PT Link using low pH EnVision FLEX Target Retrieval Solution (Dako, Denmark) for 20 min at 100°C. Endogenous peroxidase was blocked with EnVision FLEX Peroxidase-Blocking Reagent (Dako, Denmark) before incubating the sections with mouse monoclonal antibodies pan-ERβ (Clone 14C8, Abcam, 1/200), ERβ1 (Clone PPG5/10, GeneTex, 1/15) [17] and ERβ2 (Clone 57/3, Serotec, 1/10) [18] for overnight at 4°C. Antigen–antibody complex was detected using Envision FLEX system (EnVision FLEX/HRP and EnVision FLEX DAB+ Chromogen). The specificity of both nuclear and cytoplasmic staining for these antibody clones have been previously established via peptide absorption studies [18, 22].
HER2 chromogenic in situ hybridisation (CISH) and immunoperoxidase staining for ERα, PgR, HER2, CK5/6 and EGFR were performed for all tumours. HER2 CISH was performed using the Invitrogen Spotlight system (Invitrogen, California, USA). Tumour cells were regarded as positive for amplification if there were more than six signals per nucleus [23]. Tumours in the equivocal group (4–6 signals) were further probed with chromosome 17 and considered amplified with a ratio of >2.2 [24].
Immunohistochemical scoring and cut-off levels
ERβ was scored for nuclear and cytoplasmic staining using a cut-off of 20%, as defined by Shaaban et al. [18]. For survival analysis, the data were also analysed using cut-offs defined by Bates et al. [25]: the intensity of staining was scored as negative = 0, weak = 1, moderate = 2 or strong = 3 (Figure S1). The percentage of tumour cells was scored as: 0 = 0; 1–10 = 1, 11–50 = 2, 51–80 = 3, 81–100 = 4. The intensity and the percentage of positive tumour cells were added together to give a maximum score of 7. Previously defined median cut-offs of 7 for nuclear expression and 6 for cytoplasmic expression were used to groups into positive and negative tumours [25]. The highest score from the 4 cores of the tissue array was used where any discordance between cores was noted. For HER2, EGFR and CK5/6, the same cut-offs were derived from Neilsen et al. An Allred score of >2/8 was considered as positive for ERα [26].
Statistical analysis
Correlations were evaluated using, the Kruskal–Wallis or Chi-square tests where appropriate. Kaplan–Meier survival curves were calculated for breast-cancer-specific death, and were compared at 5 and 15 years using a log rank test. Binary logistic regression was used for multivariate analyses and the Cox proportional hazard regression model was used to identify independent prognostic factors for breast-cancer-specific survival. Analyses were performed with SPSS 16.0 (SPSS Inc., IL, USA). A 2-tailed P value test was used in all analyses and a P value of less than 0.05 was considered as statistically significant.
Results
ERβ expression in familial breast cancers and their relationship with clinicopathological parameters
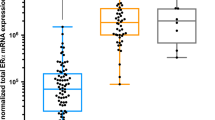
Pan-ERβ was expressed both in the nuclear and in the cytoplasmic compartments. This ranged from focal weak positivity to widespread strong positivity. Using a cut-off of 20% staining the most common nuclear ER phenotype in BRCA1 cancers was n(nuclear)ERα-n(pan)ERβ+ (65.6%) (Table 2). BRCA1 cancers were significantly more likely have nERα–nERβ− and nERα–nERβ+ phenotype when compared with BRCA2 and BRCAX cancers (both P < 0.001). In contrast, BRCA2 and BRCAX cancers were significantly (P < 0.001) more likely to be nuclear ERα positive (the most common phenotype being nERα+ nERβ+) (75.9 and 61.2%, respectively) when compared with BRCA1 (12.5%) (Table 2).
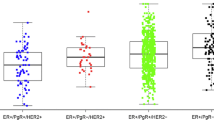
The distributions of pan-ERβ, ERβ1 and ERβ2 expressions stratified by BRCA status and intrinsic phenotypes are shown in Tables S1a–S1f. Correlation with ERβ expression was performed using a cut-off of 20% [18]. No differences in pan-ERβ, ERβ1 or ERβ2 expression when tumours were stratified by BRCA status (all P > 0.05). Basal-type familial breast cancers were significantly less likely to be nuclear pan-ERβ positive (26/36, 72.2%) when compared with luminal familial cancers (44/49, 89.8%) (P = 0.036). There were no significant differences in ERβ1 and ERβ2 expressions between familial luminal and basal cancers (P > 0.005).
ERα expression correlated with positive nuclear pan-ERβ expression (P = 0.014) and negative cytoplasmic ERβ2 expression (P = 0.024). Patients with positive cytoplasmic ERβ2 were more likely to receive chemotherapy (P = 0.042) (Table 3). There was no significant association between pan-ERβ, ERβ1 or ERβ2 expression and tumour size, grade, lymph node status, PR, Her-2 and treatment with endocrine therapy (all P > 0.05).
ERβ and survival analysis in familial breast cancers
Analyses for survival were performed at 5 and 15 years using two different cut-offs as defined by Shaaban et al. (20% staining) and Bates et al. (7 out of 7 nuclear staining and 6 out of 7 cytoplasmic staining). Expression of cytoplasmic ERβ2 in familial breast cancers correlated with shorter breast-cancer-specific survival at 15 years irrespective of cut-offs employed (P = 0.045 at 20% and P = 0.002 at score of 6) (Table 4; Fig. 1a, b). A similar trend was seen at 5 years although this was not statistically significant (P = 0.226 at 20% and P = 0.061 at score of 6). There was a trend for nuclear ERβ1 expression to be associated with survival at 15 years although this was not statistically significant (P = 0.229 at 20% and P = 0.064 at score of 7). There was no correlation between survival and pan-ERβ, cytoplasmic ERβ1 or nuclear ERβ2 expression (P > 0.05).
Kaplan Meier curves of 15 year breast-cancer-specific survival stratified by cytoplasmic ERβ2, cut-off for positivity at a score of 6 out of 7 (b, d, f, h), and at 20% (a, c, e, g). All familial cancers (a, b), basal-type cancers (c, d), BRCA1 cancers (e, f) and cancers treated with chemotherapy (g, h)
The tumours were then analysed based on their intrinsic subtypes. For basal-type familial breast cancers, cytoplasmic ERβ2 expression was associated with shorter overall survival at 5 and 15 years at a cut-off of 6 out of 7 (P = 0.039 and P = 0.011, respectively) (Table 4; Fig. 1d). At a cut-off of 20% a similar trend was seen although it did not reach statistical significance (P = 0.182 at 5 years and P = 0.159 at 15 years) (Table 4; Fig. 1c). No differences in survival were observed for luminal, HER2 and null subtypes when stratified by nuclear or cytoplasmic ERβ expression (all P > 0.05).
When the tumours were analysed according to their BRCA status, there was a non-significant trend for BRCA1 tumours with positive cytoplasmic ERβ2 to have a poorer 15-year survival (P = 0.306 at 20% and P = 0.068 at 6 out of 7) (Table 4; Fig. 1e, f). There was no correlation between ERβ expression and survival for BRCA2 and BRCAX cancers.
Survival analysis by treatment with endocrine therapy
Analysis of the familial cancers by treatment group was then performed. In patients treated with tamoxifen, positive nuclear ERβ1 expression correlated with longer 15-year survival (P = 0.024 at 20% and P = 0.021 at score of 7) (Fig. 2a, b). This did not reach statistical significance in a multivariate analysis using the Cox regression model, with grade, lymph node status, plus endocrine treatment and nuclear ERβ1 as interaction variables (P = 0.203). No correlation was seen between pan-ERβ, ERβ1 or ERβ2 expression and survival in patients not treated with tamoxifen (P > 0.05).
Survival analysis by treatment with chemotherapy
For patients treated with chemotherapy, cytoplasmic ERβ2 expression at a cut-off score of 6, correlated with poorer survival at 15 years (P = 0.003) (Table 4; Fig. 1h). At a cut-off 20%, a trend was present, but this did not reach statistical significance (P = 0.057) (Fig. 1g). The survival curves at 5 years showed a similar trend although this did not reach statistical significance (P = 0.159 at a cut-off score of 6). No such differences were seen in patients not treated with chemotherapy (P > 0.05 irrespective of cut-offs used). The significance of cytoplasmic ERβ2 on response to chemotherapy at 15 years was confirmed by multivariate analysis including chemotherapy and non-threshold cytoplasmic ERβ2 (score out of 7) as interaction variables (P = 0.045, hazard ratio 1.22, 95% CI 1.004–9.87) (Table 5). This however did not reach statistical significance at a cut-off of 20% (P = 0.056, hazard ratio 1.21, 95% CI 1.00–1.48) (Table 5).
Expression of nuclear ERβ1 (cut-off at score of 7) correlated with better 15-year survival in patients treated with chemotherapy (P = 0.029), however, this did not reach statistical significance on a multivariate analysis, including grade, lymph node status plus non-threshold ERβ1 score out of 7 and chemotherapy as interaction variables (P = 0.979, hazard ratio 1.00, 95% CI 0.83–1.20), or at a cut-off of 20% (P = 0.339). There was no correlation between pan-ERβ expression and chemotherapy response (P > 0.05).
Discussion
In this series of familial breast cancers nuclear pan-ERβ was expressed in 81% of cases, with no significant difference between the three BRCA1 (77%), BRCA2 (84%) and BRCAX (84%) groups. This is similar to 84% (94% BRCA1, 75% BRCA2 and 81% BRCAX) obtained by Daidone et al. [19] and higher than 42% reported in a series of 48 patients with founder BRCA1 mutation [20]. Although potentially due to differing patient cohorts, the discrepancy with the latter study may be due to their use of a different polyclonal antibody. We have used a monoclonal pan-ERβ antibody that has been validated by others and is likely to reflect true positivity [22]. Indeed, there was no significant variation across the four molecular subtypes in familial breast is in keeping with previous studies using this validated antibody [17].
Recent studies on sporadic cancers using the same antibody clone have yielded discordant results regarding the impact of nuclear ERβ1 on prognosis including: better survival particularly in triple negative cancers and postmenopausal women [16], better survival in node negative luminal A tumours [17], worse survival in node positive luminal B tumours [17] and no impact on prognosis [18]. In this study of familial cancers, while there was a trend for nuclear ERβ1 to be associated with a better prognosis, this did not reach statistical significance. Again this may be due to different cohorts, cut-offs employed and number of the familial cancers available for our study. Nuclear ERβ1, however, was predictive of response to endocrine therapy at both cut-offs, in concordance with 10 out of 13 previous ERβ studies as reviewed by Fox et al. [27]. This is supported by cell line studies where the induction of ERβ expression enhanced the anti-proliferative effects of tamoxifen [28]. This may have important treatment implications, particularly for BRCA1 cancers, since a significant proportion of these cases was negative for ERα but positive for nuclear ERβ1 (77%, 20/26).
In addition to nuclear expression, in our other studies [25] and in accordance with other investigators [14, 18, 22, 29], we also noted cytoplasmic expression. The cytoplasmic staining present for the ERβ antibody clones used in our study is likely to be specific as it has been terminated by peptide absorption in previous studies [18, 22]. This is in keeping ERα and ERβ having a non-genomic signalling function, and the role of ERα and ERβ in the transcription of mtDNA in the mitochondrion [30, 31]. Indeed, immunoblotting of subcellular fractions have confirmed the presence of ERβ within the nucleus, cytoplasm and the caveolae of plasma membranes [32].
Although there was no significant correlation between either nuclear or cytoplasmic ERβ2 and classical prognostic factors including size, grade and lymph node status it is interesting to note that cytoplasmic positivity was associated with poorer survival at 15 years regardless of cut-offs employed. For basal-type cancers, this was significant at 5 and 15 years, when a cut-off score of 6 was used. A similar trend was seen at a cut-off of 20%, however, this did not reach statistical significance. The absence of a statistical association at this cut-off may be due to the limited number of cases with <20% staining, but increasing the cohort size in familial breast cancer is difficult. Similarly, there was a trend for shorter overall survival in BRCA1 tumours. Overall, these findings are consistent with a previous study by Shaaban et al. [18], where cytoplasmic ERβ2 was associated with a poorer prognosis.
The effect of ERβ on basal-type and BRCA1 cancers noted in our study are supported by cell line studies where the introduction of ERβ into a ERα negative cell line MDA-MB-435 resulted in increased proliferation, invasiveness and metastasis [33]. Whereas in ERα positive cells, the introduction of ERβ led to the inhibition of genes associated with proliferation [27]. Extranuclear ERβ may have rapid non-genomic effects including stimulation of cell proliferation via G protein, ERK and c-Jun kinase activation [32, 34, 35]. In addition, induction of ERβ-dependent transcription of mtDNA (COXI, COXII and ND1 subunit complex 1) in the mitochondrion may result in alterations in energy metabolism, abnormal growth and inhibition of apoptosis [31, 36–38].
Furthermore, the expression of cytoplasmic ERβ2 in our study was associated with shorter survival at 15 years in patients receiving chemotherapy. This was significant at a cut-off score of 6, and was also significant in a multivariate analysis with non-threshold data (score out of 7) and chemotherapy as interaction variables. Multivariate analysis at a 20% cut-off did not reach statistical significance (P = 0.056), however, this may be due to the limited number of tumours with ≤20% expression treated with chemotherapy. Assessment of ERβ2 expression may be of clinical relevance, as a proportion of familial triple negative cancers, which are resistant to targeted therapies, express cytoplasmic ERβ2 in >20% of cells (32/40, 80%).
Chemotherapeutic agents such as cyclophosphamide, doxorubicin and paclitaxel initiate apoptosis by increasing permeability of the mitochondrial membrane, either through induction of p53/bcl-2 expression (secondary to DNA damage), or by the generation of reactive oxygen species [31, 39, 40]. This leads to Ca2+ overload of the mitochondrial matrix and dissipation of the electrochemical gradient which drives ATP generation, resulting in swelling and rupture of the mitochondrion, followed by the release of pro-apoptotic proteins [31, 41]. Mitochondrial ERβ may block apoptosis by promoting transcription of respiratory chain protein mt-DNA (such as subunits of ATP synthase, complex III and IV), leading to increased ATP production and the neutralisation of reactive oxygen species [31]. Inhibition of apoptosis may also occur via direct inhibition of the Ca2+ uniporter by mitochondrial estrogen receptors [42]. The role of ERβ in the inhibition of apoptosis is further supported by the lowering of resting mitochondrial membrane potential following mitochondrial ERβ knockdown [43]. Chemotherapy resistance may be further enhanced by the rapid non-genomic effects of extranuclear ERβ on cell proliferation [31].
The correlation between cytoplasmic ERβ2, but not ERβ1 with survival provides further evidence to support the different transactivating properties of the different ERβ isoforms [44]. While ERβ1 is the only fully functional isoform and may form ERβ1 homodimers, ERβ2 to 5 cannot form homodimers, but may form heterodimers with ERβ1 only. Under the stimulation of estrogens, ERβ1 preferentially forms heterodimers with ERβ2-5 with enhanced transactivating properties when compared to ERβ1 homodimers [44].
A correlation between loss of ERα protein and BRCA1 mutation has been reported [5, 45, 46]. There are limited data on the relationship between ERβ and BRCA1, however, it is known under the influence of the phytoestrogen genistein, BRCA1 inhibits ERβ but not ERα reporter activity [47]. The presence of BRCA1 mutations may therefore enhance ERβ activity in ERα negative tumours.
In summary, this is the first study to comprehensively analyse the subcellular expression of ERβ and its different isoforms in familial breast cancer. Our study highlights the impact cytoplasmic ERβ2 on prognosis and response to treatment. Since it has been reported that 5–10% of patients with ERα negative breast cancer respond to tamoxifen [7, 48] and that this effect is may be predicted by ERβ expression, the clinical diagnostic measurement of nuclear ERβ1 may identify patients with ERα negative tumours that may benefit from endocrine therapy.
References
Palacios J, Honrado E, Osorio A, Cazorla A, Sarrio D, Barroso A, Rodriguez S, Cigudosa JC, Diez O, Alonso C et al. (2003) Immunohistochemical characteristics defined by tissue microarray of hereditary breast cancer not attributable to BRCA1 or BRCA2 mutations: differences from breast carcinomas arising in BRCA1 and BRCA2 mutation carriers. Clin Cancer Res 9(10 Pt 1):3606–3614
Sensi E, Tancredi M, Aretini P, Cipollini G, Naccarato AG, Viacava P, Bevilacqua G, Caligo MA (2003) p53 inactivation is a rare event in familial breast tumors negative for BRCA1 and BRCA2 mutations. Breast Cancer Res Treat 82(1):1–9
Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, Bishop T, Benitez J, Rivas C, Bignon YJ et al (2005) Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res 11(14):5175–5180
Laakso M, Loman N, Borg A, Isola J (2005) Cytokeratin 5/14-positive breast cancer: true basal phenotype confined to BRCA1 tumors. Mod Pathol 18(10):1321–1328
Jacquemier J, Padovani L, Rabayrol L, Lakhani SR, Penault-Llorca F, Denoux Y, Fiche M, Figueiro P, Maisongrosse V, Ledoussal V et al (2005) Typical medullary breast carcinomas have a basal/myoepithelial phenotype. J Pathol 207(3):260–268
Armes JE, Egan AJ, Southey MC, Dite GS, McCredie MR, Giles GG, Hopper JL, Venter DJ (1998) The histologic phenotypes of breast carcinoma occurring before age 40 years in women with and without BRCA1 or BRCA2 germline mutations: a population-based study. Cancer 83(11):2335–2345
Group EBCTC (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 351(9114):1451–1467
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355(26):2733–2743
Hudis CA (2007) Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med 357(1):39–51
Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, Sledge GW, Carey LA (2008) Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res 14(24):8010–8018
Gronwald J, Tung N, Foulkes WD, Offit K, Gershoni R, Daly M, Kim-Sing C, Olsson H, Ainsworth P, Eisen A et al (2006) Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer 118(9):2281–2284
Narod SA, Brunet JS, Ghadirian P, Robson M, Heimdal K, Neuhausen SL, Stoppa-Lyonnet D, Lerman C, Pasini B, de los Pasini P et al (2000) Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet 356(9245):1876–1881
Speirs V (2008) The evolving role of oestrogen receptor beta in clinical breast cancer. Breast Cancer Res 10(5):111
Shaaban AM, O’Neill PA, Davies MP, Sibson R, West CR, Smith PH, Foster CS (2003) Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol 27(12):1502–1512
Speirs V, Skliris GP, Burdall SE, Carder PJ (2002) Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J Clin Pathol 55(5):371–374
Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G (2008) Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol 26(22):3727–3734
Novelli F, Milella M, Melucci E, Di Benedetto A, Sperduti I, Perrone-Donnorso R, Perracchio L, Venturo I, Nistico C, Fabi A et al (2008) A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res 10(5):R74
Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT et al (2008) Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res 14(16):5228–5235
Daidone MG, Veneroni S, Cappelletti V, Radice P, Pierotti MA, Younes M (2002) Estrogen receptor-beta expression in hereditary breast cancer. J Clin Oncol 20(17):3752–3753 (author reply 3753)
Litwiniuk MM, Roznowski K, Filas V, Godlewski DD, Stawicka M, Kaleta R, Breborowicz J (2008) Expression of estrogen receptor beta in the breast carcinoma of BRCA1 mutation carriers. BMC Cancer 8:100
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L et al (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10(16):5367–5374
Skliris GP, Parkes AT, Limer JL, Burdall SE, Carder PJ, Speirs V (2002) Evaluation of seven oestrogen receptor beta antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol 197(2):155–162
Cayre A, Mishellany F, Lagarde N, Penault-Llorca F (2007) Comparison of different commercial kits for HER2 testing in breast cancer: looking for the accurate cutoff for amplification. Breast Cancer Res 9(5):R64
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145
Bates GJ, Fox SB, Han C, Launchbury R, Leek RD, Harris AL, Banham AH (2008) Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptorbeta in primary invasive breast carcinomas. Breast Cancer Res Treat 111(3):453–459
Leake R, Barnes D, Pinder S, Ellis I, Anderson L, Anderson T, Adamson R, Rhodes T, Miller K, Walker R (2000) Immunohistochemical detection of steroid receptors in breast cancer: a working protocol. UK Receptor Group, UK NEQAS, The Scottish Breast Cancer Pathology Group, and The Receptor and Biomarker Study Group of the EORTC. J Clin Pathol 53(8):634–635
Fox EM, Davis RJ, Shupnik MA (2008) ERbeta in breast cancer—onlooker, passive player, or active protector? Steroids 73(11):1039–1051
Hodges-Gallagher L, Valentine CD, El Bader S, Kushner PJ (2008) Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res Treat 109(2):241–250
Jensen EV, Cheng G, Palmieri C, Saji S, Makela S, Van Noorden S, Wahlstrom T, Warner M, Coombes RC, Gustafsson JA (2001) Estrogen receptors and proliferation markers in primary and recurrent breast cancer. Proc Natl Acad Sci USA 98(26):15197–15202
Speirs V, Walker RA (2007) New perspectives into the biological and clinical relevance of oestrogen receptors in the human breast. J Pathol 211(5):499–506
Chen JQ, Cammarata PR, Baines CP, Yager JD (2009) Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta 1793(10):1540–1570
Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW (2002) ERbeta has nongenomic action in caveolae. Mol Endocrinol 16(5):938–946
Hou YF, Yuan ST, Li HC, Wu J, Lu JS, Liu G, Lu LJ, Shen ZZ, Ding J, Shao ZM (2004) ERbeta exerts multiple stimulative effects on human breast carcinoma cells. Oncogene 23(34):5799–5806
Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS et al (2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104(5):719–730
Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER (2004) Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol 18(12):2854–2865
Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, Becker K, Yates JR 3rd, Felding-Habermann B (2007) Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res 67(4):1472–1486
Pedram A, Razandi M, Wallace DC, Levin ER (2006) Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell 17(5):2125–2137
Simpkins JW, Yang SH, Sarkar SN, Pearce V (2008) Estrogen actions on mitochondria–physiological and pathological implications. Mol Cell Endocrinol 290(1–2):51–59
Fulda S, Debatin KM (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25(34):4798–4811
Moll UM, Wolff S, Speidel D, Deppert W (2005) Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol 17(6):631–636
Leung AW, Halestrap AP (2008) Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta 1777(7–8):946–952
Lobaton CD, Vay L, Hernandez-Sanmiguel E, Santodomingo J, Moreno A, Montero M, Alvarez J (2005) Modulation of mitochondrial Ca(2+) uptake by estrogen receptor agonists and antagonists. Br J Pharmacol 145(7):862–871
Yang SH, Sarkar SN, Liu R, Perez EJ, Wang X, Wen Y, Yan LJ, Simpkins JW (2009) Estrogen receptor beta as a mitochondrial vulnerability factor. J Biol Chem 284(14):9540–9548
Leung YK, Mak P, Hassan S, Ho SM (2006) Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci USA 103(35):13162–13167
Foulkes WD, Metcalfe K, Sun P, Hanna WM, Lynch HT, Ghadirian P, Tung N, Olopade OI, Weber BL, McLennan J et al (2004) Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin Cancer Res 10(6):2029–2034
Hosey AM, Gorski JJ, Murray MM, Quinn JE, Chung WY, Stewart GE, James CR, Farragher SM, Mulligan JM, Scott AN et al (2007) Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst 99(22):1683–1694
Cabanes A, Wang M, Gustafsson J-A, Hilakivi-Clarke L: BRCA1 effects on estrogen receptor (ER){alpha} and ER{beta} activity are ligand dependent. AACR Meeting Abstracts 2004, 2004(1):660-b-
Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Eden P, Peterson C, Malmstrom P, Isola J, Borg A et al (2007) Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res 13(7):1987–1994
Acknowledgements
We wish to thank Heather Thorne, Eveline Niedermayr, the kConFab research nurses and staff, the staff and of the Family Cancer Clinics, the Clinical Follow Up Study (funded by NHMRC Grants 145684, 288704 and 454508). kConFab is supported by grants from the National Breast Cancer Foundation, the National Health and Medical Research Council (NHMRC) and by the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia. This study was partly funded by the Victorian Breast Cancer Research Consortium, the NHMRC, the Royal College of Pathologists of Australasia and the Victorian Cancer Biobank.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
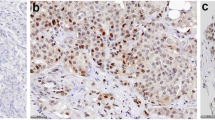
(A) Invasive carcinoma with weak nuclear and cytoplasmic pan-ERβ staining, (B) Invasive carcinoma with strong nuclear and cytoplasmic pan-ERβ staining. (C) Invasive carcinoma with weak nuclear and cytoplasmic ERβ1 staining, (D) Invasive carcinoma with strong nuclear and cytoplasmic ERβ1 staining, (E) Invasive carcinoma with weak nuclear and cytoplasmic ERβ2 staining, (F) Invasive carcinoma with strong nuclear and cytoplasmic ERβ2 staining. Supplementary material 1 (DOC 4436 kb)
Rights and permissions
About this article
Cite this article
Yan, M., Rayoo, M., Takano, E.A. et al. Nuclear and cytoplasmic expressions of ERβ1 and ERβ2 are predictive of response to therapy and alters prognosis in familial breast cancers. Breast Cancer Res Treat 126, 395–405 (2011). https://doi.org/10.1007/s10549-010-0941-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0941-9