Abstract
Objectives
This study evaluated the incidence of late cardiotoxicity after dose-dense and -intense adjuvant sequential doxorubicin (A), paclitaxel (T), and cyclophosphamide (C) for breast cancer (BC) with ≥ 4 involved ipsilateral axillary lymph nodes.
Methods
Patients were enrolled from 1994 to 2001 after definitive BC surgery if ≥4 axillary nodes were involved. Planned treatment was A 90 mg/m2 q 14 days × 3, T 250 mg/m2 q 14 days × 3, C 3 g/m2 q 14 days × 3 with filgrastim (G) support. Left ventricular ejection fraction (LVEF) was monitored using equilibrium radionuclide angiography (ERNA) before the initiation of chemotherapy, and after three cycles of each chemotherapeutic agent. At a median follow-up of 7 years, we obtained ERNA scans on 32 patients to evaluate the long-term cardiotoxicity of this regimen.
Results
Eighty-five eligible patients enrolled on the treatment protocol. Clinical heart failure developed in one patient. Seven (8%) patients had LVEF < 50% at the end of therapy. No cardiac-related deaths occurred. Thirty-two (46%) of 69 surviving patients have consented to late cardiac imaging. At a median follow-up of 7 years, the median absolute change in LVEF from baseline was -5.5%; [range (−8%) to (+36%)], and from the end of chemotherapy was −2.0%; [range (−25%) to (+16%)]. Four patients (12%) had a LVEF < 50%; two of these four patients had an LVEF of < 50% at the end of chemotherapy.
Conclusions
Late development of asymptomatic decline in cardiac function may occur after dose-dense and -intense adjuvant therapy, but is uncommon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthracycline cardiomyopathy has been a recognized side effect of this class of drugs since its introduction in the 1960s. In 1973, Lefrak et al. reported a 30% incidence of congestive heart failure (CHF) in patients who received >550 mg/m2 of doxorubicin [1]. Von Hoff et al. later demonstrated a continuum of increasing risk as the cumulative amount of administered drug increased [2]. They reported an overall incidence of drug-induced CHF of 2.2% in patients who received a median total dose of 390 mg/m2 of doxorubicin. Similarly, Swain et al. reported an estimated incidence of CHF of 5% for patients receiving 400 mg/m2 of doxorubicin, with an increasing risk of CHF with increasing doxorubicin dose [3].
Delayed cardiotoxicity is subclassified as early subacute cardiotoxicity occurring <1 year after the completion of treatment, or late cardiotoxicity occurring >1 year after the cessation of chemotherapy [4]. Delayed cardiotoxicity has been reported in approximately 5% of patients treated with doxorubicin [5, 6]. The majority of patients who develop late cardiotoxicity are those patients who had previously developed early subacute cardiotoxicity [4]. Late cardiotoxicity may not be apparent until years to decades after the administration of anthracyclines [7–10, 30, 35]. Risk factors for late-onset cardiotoxicity include age ≤18 or ≥65 at time of treatment, increasing cumulative dose or dose intensity of anthracyclines, mediastinal radiation therapy (RT), and female gender [3, 11, 12, 29, 34]. Typically patients with late-onset cardiotoxicity have reduced left ventricular (LV) mass, mass index, and compliance, increasing susceptibility to cardiac stressors [12–14, 42]. Numerous studies have reported the long-term effect of anthracyclines on cardiac function with childhood exposure, but long-term cardiotoxicity of anthracyclines in adults has not been extensively studied [8, 9, 40, 42].
Paclitaxel only rarely causes cardiomyopathy when administered as a single agent [15]. However, paclitaxel may enhance the cardiotoxicity of doxorubicin when given in combination [16]. Cyclophosphamide is not cardiotoxic at conventional doses, but myocardial damage has been reported at myeloablative doses as given in transplantation [17–22]. Rose and coworkers treated 52 metastatic breast cancer (BC) patients with sequential high dose alkylating agents and stem cell rescue. Use of paclitaxel for stem cell mobilization was associated with a mean absolute decrease in left ventricular ejection fraction (LVEF) of 3.4% (P = 0.032), which was relative to no significant change in LVEF (−1.3%, P = 0.23) in patients who only received high dose cyclophosphamide for mobilization, but mobilization with sequential paclitaxel and cyclophosphamide resulted in a mean absolute drop of 4.9% in LVEF (P = 0.008) [23].
Due to the quantitative nature and high reproducibility, equilibrium radionuclide angiography (ERNA) is an excellent noninvasive method for monitoring LVEF during the course of doxorubicin chemotherapy. ERNA reproducibly detects relatively small changes, and ERNA monitoring cost effectively permits doxorubicin administration with a low risk of clinical heart failure [24–26, 28]. A comparison between radionucleotide and echocardiographic LVEF showed better intra- and inter-observer reproducibility with the radionucleotide LVEF [27]. Repeat ERNA assessments of LVEF vary between 2 and 4%, while repeat echocardiogram assessments of LVEF vary between 13 and 17% range [27]. Therefore, when a precisely reproducible measurement is required for clinical trial monitoring or patient management decisions, the authors recommended ERNA as the method of choice. Schwartz et al. have proposed guidelines for LVEF monitoring with serial ERNA scans during treatment with doxorubicin [28]. For patients with normal cardiac function, they recommend an ERNA at baseline, follow-up ERNA scans after cumulative doses of 240–300 mg/m [2] and 400–450 mg/m [2], and then before each subsequent dose of doxorubicin. More frequent studies are recommended for patients with impaired cardiac function, known heart disease, radiation exposure or simultaneous exposure to other cardiotoxic agents.
Eighty-five BC patients with ≥4 involved axillary nodes enrolled in a clinical trial of adjuvant dose-dense and -intense sequential doxorubicin (A), paclitaxel (T), and cyclophosphamide (C) [29]. Because of the dose-dense and -intense anthracycline therapy investigated in the trial, rigorous cardiac testing with ERNA scans was implemented to monitor patients’ LVEF during this trial. Patients underwent ERNA before the initiation of chemotherapy and after three cycles of each chemotherapeutic agent. The low rate of clinical or subclinical acute cardiotoxicity has been previously published. In the current study, the long-term cardiotoxicity of chemotherapy as manifested by clinical and/or radiographic impairment of cardiac ejection fraction (EF) was evaluated. We report this long-term evaluation of cardiac function at a median of 7 years follow-up.
Patients and methods
Eighty-five women with primary BC, histologic involvement of at least four axillary lymph nodes and no evidence of distant metastases were enrolled in a clinical trial of dose-dense and -intense adjuvant chemotherapy between March 1994 and April 2001. Eligibility criteria and pre-study evaluation have been previously published [29]. Patients were ineligible if the LVEF was <50%.
Treatment plan
Patients received doxorubicin 90 mg/m2 intravenously on days 1, 15, and 29. ERNA was repeated after three doses of doxorubicin. Patients who had an absolute decrease in LVEF of ≥25% or a LVEF <50%, were removed from study. Paclitaxel 250 mg/m2 were administered by continuous infusion over 24 h on days 43, 57, and 71. ERNA was repeated after three doses of paclitaxel. The same cardiac function guidelines were applied. Cyclophosphamide 3 g/m2 were administered intravenously on days 85, 99, and 113, with 24 h of hyperhydration and ERNA was repeated. Supportive and adjunctive measures have been previously published [29]. After completing chemotherapy, patients received RT at the discretion of their medical and radiation oncologists. Separate internal mammary lymph node fields were not used in an attempt to minimize exposure of the heart to RT.
Follow-up ERNA scans
The current study which investigates whether patients who participated in the clinical trial of dose-dense and -intense adjuvant therapy had suffered late cardiac dysfunction was reviewed and approved by the Yale University Human Investigation Committee. Beginning in February 2004, the 69 surviving patients were contacted by mail and their participation in the current study was requested. Thirty-two patients wished to enroll and gave written informed consent. A physician or physician extender performed a history and physical examination. ERNA was performed at Yale New-Haven Hospital (YNHH) in the Department of Nuclear Cardiology. Patients who had a recent ERNA scan or an echocardiogram ordered by their local oncologist, cardiologist, or primary care physician as part of their standard medical care, gave consent to collection of the results of these studies and related medical records, and did not undergo repeat scanning for this study.
Results
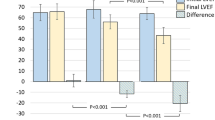
Thirty-two of 69 surviving patients enrolled in this follow-up study. Twenty-eight of the 32 patients had an ERNA performed at YNHH, and data were collected on an additional 4 patients who had recent cardiac evaluation as part of their standard medical care. Two of these four patients had echocardiography rather than ERNA. The median age of patients enrolling for this study was 56.7 years, with a range of 41.1–70.9 years. The median age of all 69 surviving patients is 55.0 years (range, 40.9–77.2 years). The median absolute change in cardiac EF between a pre-chemotherapy baseline and the current assessment of EF was −5.5% (range −8% to +36%) (Fig. 1). The median absolute change between baseline and the post-chemotherapy cardiac EF was −5.0% (range −28% to +15%) (Fig. 1). The mean absolute change between post-chemotherapy and the current cardiac EF was −2.0% (range −25% to +16%) (Fig. 1). Ten of the 32 patients had an absolute drop of >10% in EF at the end of chemotherapy, while 7 of the 32 patients had an absolute drop of >10% in EF at a median of 8.3 years follow-up when compared with the post-chemotherapy EF. For the majority of patients, the largest drop in LVEF occurred between the baseline and immediately post-ATC chemotherapy cardiac EF. However, a comparison of the changes in EF between pre- and post-chemotherapy with changes in EF between post-chemotherapy and current time was not statistically significant (P = 0.257, two-tailed distribution).
Equilibrium radionuclide angiography data for pre- and post-chemotherapy were available on all 85 patients who had enrolled in the ATC study. The median absolute change in EF between pre- and post-chemotherapy for the remaining 53 of original 89 patients who did not enroll in this ERNA follow-up study was −2.0%. A comparison of the changes between pre- and post-chemotherapy EF for patients who enrolled in the follow-up ERNA study and patients who did not enroll in the follow-up ERNA study did not reveal a significant difference in change in EF (P = 0.165, two-tailed distribution). The changes in the EF of all patients enrolled in this follow-up ERNA study are illustrated below in Figs. 2, 3.
Of the 32 patients that followed up in this study, 14 patients received left-sided post-chemotherapy RT, 16 received right-sided post-chemotherapy RT, and 2 patients received no RT. Of the 37 patients who were alive and did not participate in this study, 11 patients received left-sided, 21 received right-sided, and 5 patients received no post-chemotherapy RT. There was no significant difference in the absolute change in EF from pre-chemotherapy to current time in patients who received left- (median −5.5%) versus right-sided (median −7.0%) RT (P = 0.175). Similarly, patients who received left- (median −3.5%) versus right-sided (median −2.0%) RT had no significant difference in change in EF from post-chemotherapy to the current time (P = 0.152).
Four patients had a measured EF of ≤50% in this current study. These patients are described in Table 1. All four of these patients had Class I symptoms according to the Functional Classification of New York Heart Association (NYHA). The drop in EF to <50% in patients 3 and 4 is possibly related to chemotherapy. Patient 3 had an EF of 43%, and was evaluated by a cardiologist (F.L.) who found the patient without signs or symptoms of CHF. An echocardiogram showed an EF of 65% and no valvular lesions or left ventricular hypertophy (LVH). She was treated for previously undiagnosed hypertension and received an ACE inhibitor and a diuretic, and remained asymptomatic. Patient 4 had a drop in EF from a pre-chemotherapy baseline of 65% to a post-chemotherapy value of 37%. Following her last dose of cyclophosphamide, she had symptoms of dyspnea on exertion. She was seen by a cardiologist and managed with digoxin, a diuretic, and an ACE inhibitor. She was then asymptomatic for 2 years, but then developed digoxin toxicity. Digoxin was discontinued and the patient remained asymptomatic on an ACE inhibitor and diuretic. On her study visit in December 2005, she remained asymptomatic with a normal physical examination. However, EF by ERNA was 45%. She was referred to a cardiologist (F.L.); echocardiogram showed an EF of 55% and no valvular lesions or LVH. No further treatment was recommended.
Discussion
Anthracycline cardiomyopathy has been reported to occur five or more years after completion of therapy [7–10, 10–10, 32–10, 34, 35, 39]. Myocyte damage or death leading to decreased ventricular contractility is thought to be responsible for early anthracycline cardiotoxicity, while late anthracycline cardiotoxicity is thought to be due to chronically increased ventricular wall stress resulting from depressed ventricular contractility and an inappropriately thin ventricular wall [12, 30].
The relationship between peak chemotherapy dose and immediate cardiotoxicity has been examined by Hudis et al. who published results of short-term change in LVEF in 42 women who were treated with ATC and followed by a similar ERNA surveillance during the study [31]. Of these 42 patients, 2 evaluable patients had >10% decrease in LVEF, and both of these patients recovered to a <10% decrease from baseline prior to receiving paclitaxel. After completing the sequence of doxorubicin and paclitaxel, there were three evaluable patients with >10% decrease in LVEF, and one of these three patients had recovery of LVEF prior to cyclophosphamide. Finally, after completing high dose cyclophosphamide, four evaluable patients had >10% decrease in LVEF [31]. Long-term cardiotoxicity from this study has not been reported.
The relationship between cumulative anthracycline dose and long-term effects on cardiac toxicity has been explored in various studies. In a study where two cohorts of adult patients received 225 or 450 mg/m2 of doxorubicin along with 500 mg/m2 of cyclophosphamide for BC, the estimated risk per 100 patient years of a cardiac event was 3.4 times higher than the low dose cohort [32]. Bonneterre et al. followed 150 BC patients treated with six cycles of adjuvant FEC. At a median follow-up of 102 months, of the 85 patients treated with epirubicin 100 mg/m2; 2 patients experienced symptomatic CHF, and 18 patients experienced grade 1 and 2 clinically asymptomatic left ventricular dysfunction (LVD). Of the 65 patients treated with epirubicin 50 mg/m2, there was one case of grade 1 LVD [33]. In a larger French retrospective review of eight trials in which 2,553 patients were treated with adjuvant epirubicin, a 0.7% incidence of LVD was found at a median of 84 months of follow-up [34]. Other studies with adjuvant epirubicin have found an incidence of CHF of 0–2% at median follow-up ranging from 39 to 74 months [35–39].
Long-term doxorubicin cardiotoxicity has been more extensively studied in children than adults [8, 9, 27, 30, 32]. The most significant predictors of abnormal cardiac function were the cumulative dose of doxorubicin, receiving cardiac RT or mediastinal RT and length of follow-up [40–43].
Two patients in this study had long-term cardiotoxicity without evidence of a mechanism other than prior chemotherapy. Both patients were treated with standard CHF therapy and improved by LV function and clinical assessment to essentially an asymptomatic status. Thus, the mechanism of their LVD was reversible and quite possibly unrelated to prior anthracycline therapy. Both of these patients had ERNA scans that documented an EF <50%, but both had a follow-up echocardiogram that showed an EF >50%. ERNA scan remains the principal diagnostic modality for diagnosing CHF and provides a more accurate and reproducible measurement of LVEF than echocardiogram [27]. Echocardiograms were performed on these patients to add additional clinical information that would bear on treatment. Echocardiogram provides a visual estimate of LVEF, and also provides important information on cardiac chamber sizes, integrity of cardiac valvular function, and the presence or absence of hypertrophy.
There is concern that both paclitaxel and cyclophosphamide may augment the cardiotoxicity of doxorubicin. There is pharmacokinetic evidence that the administration of paclitaxel shortly after doxorubicin may potentiate the cardiotoxic potential of doxorubicin [44]. The elimination of doxorubicin is slowed by paclitaxel, resulting in prolonged exposure to doxorubicin. In a study of neoadjuvant doxorubicin followed directly by a 3-h infusion of paclitaxel, 4 of 31 patients had a decrease in LVEF below 40% and 3 of 31 patients had a decrease in LVEF >20% from their baseline values [45]. In a larger study that reported the incidence of cardiac toxicity in 657 patients with metastatic BC treated by a combination of concomitant doxorubicin (50–60 mg/m2) and paclitaxel (175–220 mg/m2), the cardiac toxicity rate was 25% when the total doxorubicin dose was >440 mg/m2, whereas it was <5% when the amount was <380 mg/m2 [46]. Because the chemotherapeutic agents in our study were given sequentially and every 2 weeks, a pharmacokinetic interaction between the paclitaxel and doxorubicin is an unlikely cause of the reported short- or long-term cardiotoxicity. Several studies have demonstrated that the sequential administration of paclitaxel after anthracyclines does not significantly affect the EF of patients who have been pre-treated with anthracyclines with a subsequent anthracycline-related drop in EF.
Preclinical and clinical data have demonstrated that high dose cyclophosphamide may be cardiotoxic. The pathophysiology of high dose cyclophosphamide-associated cardiac toxicity is thought to depend upon toxic endothelial damage followed by extravasation of toxic metabolites with subsequent myocyte damage and interstitial hemorrhage and edema. These changes usually occur during or within a few weeks after administration [47]. The long-term cardiac effects of high dose cyclophosphamide have not been well studied. High dose cyclophosphamide-associated cardiac toxicity is thought to be related to the dose and schedule of drug and it is not related to the cumulative drug dose. No pharmacokinetic parameter has been consistently associated with cardiotoxicity [47]. Several studies have shown that there is no consistent relationship between previous anthracycline exposure and development of cardiac toxicity following high dose alkylating therapy [48–55]. We previously reported that 76 of the 85 enrolled patients had evaluable post-cyclophosphamide ERNA scans, and the median drop in LVEF immediately after cyclophosphamide was −2% (range, −18 to +15%), with 6 patients having a drop in EF of ≥10%. In the current follow-up study, 3 of the 32 patients had a history of a >10% drop in LVEF immediately after cyclophosphamide. One out of these three patients (patient 4 as described in the Results section) had a drop in LVEF that was sustained in long-term follow-up, while the other two patients had recovery of LVEF in this follow-up study (patients 10 and 16 in Figs. 2, 3). Hudis et al. reported that after completing high dose cyclophosphamide, 4 (11%) out of 37 evaluable patients had ≥10% decrease in LVEF [31]. Although the cyclophosphamide dose in bone marrow transplant recipients may be higher than those we used, or cyclophosphamide may be given together with potentiating agents in these patients, data on repeated use of high dose cyclophosphamide that is not myeloablative are scarce [56, 57]. The incidence of symptomatic cardiac failure from high dose cyclophosphamide therapy has ranged from 0 to 28% [58–61].
In our study an exploratory analysis did not reveal significant differences in EF among patients with left- or right-sided post-chemotherapy RT, but real differences may be obscured by the small sample size. However, it is notable that three of four patients with cardiac problems were radiated for left-sided lesions. Review of the radiation ports indicated a small amount of left ventricle in the radiation field. Although numerous studies have indicated a minimal effect of radiation on LV function with modern radiation techniques, it is possible that subsets of patients such as those treated in this study with more aggressive forms of systemic therapy, are at higher risk and even small volumes of heart, which may be in the tangential radiation field, may further compromise cardiac function. Ongoing investigations using intensity-modulated radiotherapy, respiratory gating, and other techniques, are currently being evaluated in an effort to further minimize cardiac radiation [62–66].
Radiation therapy may augment anthracycline toxicity by contributing to the loss of myocytes and damaging coronary vessels and myocardial microvasculature [67]. In patients with Hodgkin’s disease treated with mediastinal RT and in post-masectomy BC patients treated with RT, there is an increase in long-term cardiovascular mortality [68–71]. A retrospective analysis of 825 women treated with CMF alone or with CMF and doxorubicin, reported acute radiation-related cardiotoxicity. In this review, 61% of women had received doxorubicin, and 44% of patients had received breast RT, with 22% of women in the case series receiving left-sided breast RT. Patients were followed for a median of 80 months. In the patients treated with doxorubicin, no cases of CHF were reported in patients who had underwent right breast RT, however, 3 of the 114 who had received doxorubicin and left breast RT had CHF within 1 month after completing adjuvant treatment [72]. Late cardiac toxicity with anthracyclines and RT may also manifest after several years. Shapiro et al. reported that patients treated with ten cycles of cyclophosphamide 500 mg/m2 and bolus doxorubicin 45 mg/m2 and who received left-sided RT with tangential fields or anterior RT for internal mammary nodes had a higher risk of CHF or myocardial infarction (MI) at a median follow-up of 6.0 years [32]. Pein et al. found that after 15 years of follow-up, pediatric patients treated with anthracyclines and RT had an increased risk of CHF when compared with patients who received anthracyclines without RT [43]. However, newer techniques of RT delivery may lessen the potential of cardiotoxicity. Poutanen et al. and Sorensen et al. did not find any difference in echocardiographic parameters between patients receiving anthracyclines alone versus anthracyclines in combination with RT [41, 73]. Bonneterre et al. did not find a significant difference in left- versus right-sided RT BC patients who had received adjuvant epirubicin based regimens [33].
In conclusion, follow-up ERNA scans on 32 out of 69 surviving BC patients previously treated with dose-dense and -intense ATC adjuvant chemotherapy revealed evidence of an EF below 50% in 4 (12%) of 32 patients, of which two events were likely related to chemotherapy administration. All four of these patients had NYHA Functional Classification Class I symptoms. Long-term cardiotoxicity of the ATC regimen does not appear to significantly contribute to the morbidity or mortality of the regimen. The potential for delayed cardiotoxicity should continue to be explored in other BC adjuvant and neoadjuvant trials, particularly in light of the recent advances with dose-dense therapy as well as with adjuvant trastuzumab.
References
Lefrak EA, Pitha J, Rosenheim S et al (1973) A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 32:302–314
Von Hoff DD, Maxwell WL, Basa P et al (1979) Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91:710–717
Swain SM, Whaley FS, Ewer MS (2003) Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97(11):2869–2879
Grenier MA, Lipshultz SE (1998) Epidemiology of anthracycline cardiotoxicity in children and adults. Semin Oncol 25(Suppl. 10):72–85
Botti C, Vici P, Lopez M, Scinto AF, Cognetti F, Cavaliere R (1995) Prognostic value of lymph node metastasis after neoadjuvant chemotherapy for large sized operable cancer of the breast. J Am Coll Surg 181:202–208
Bonadonna G, Valagussa P (1984) Contribution of prognostic factors of adjuvant chemotherapy in breast cancer. Recent Results Cancer Res 96:34–45
Haq MM, Legha SS, Choksi J (1985) Doxorubicin-induced congestive heart failure in adults. Cancer 56(6):1361–1365
Leandro J, Dyck J, Poppe D (1994) Cardiac dysfunction late after cardiotoxic therapy for childhood cancer. Am J Cardiol 74:1152–1156
Jakacki R, Silber J, Larsen R et al (1991) Cardiac dysfunction following a “low risk” cardiotoxic treatment for childhood malignancy [Abstract]. Pediatr Res 29:143A
Lamonte CS, Yeh SD, Straus DJ (1986) Long term follow up of cardiac function in patients with Hodgkin’s disease treated with mediastinal irradiation and combination chemotherapy including doxorubicin. Cancer Treat Rep 70:439–444
Green DM, Grigoriev YA, Nan B et al (2001) Congestive heart failure after treatment for Wilms’ tumor: a report from the National Wilms’. J Clin Oncol 19(7):1926–1934
Lipshultz SE, Lipsitz SR, Mone SM et al (1995) Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 332:1738–1743
Silber JH, Jakacki RI, Larsen RL et al (1993) Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol 21:477–479
BuLock FA, Marin RP, Mott MG (1995) Increased risk of cardiac dysfunction after anthracyclines in girls [letter]. Med Pediatr Oncol 24:143–144
Shek TW, Luk IS, Ma L et al (1996) Paclitaxel-induced cardiotoxicity. An ultrastructural study. Arch Pathol Lab Med 120(1):89–91
Sparano JA (1998) Use of dexrazoxane and other strategies to prevent cardiomyopathy associated with doxorubicin–taxane combinations. Semin Oncol 25(4 Suppl. 10):66–71
Fraiser LH, Kanekal S, Kehrer JP (1991) Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs 42(5):781–795
Dunphy FR, Spitzer G, Buzdar AU et al (1990) Treatment of estrogen receptor-negative or hormonally refractory breast cancer with double high-dose chemotherapy intensification and bone marrow support. J Clin Oncol 8:1207–1216
Jones RB, Shpall EJ, Shogan J et al (1990) The Duke AFM program. Intense induction chemotherapy for metastatic breast cancer. Cancer 66:431–436
Peters WP, Ross M, Vredengurgh JJ et al (1993) High-dose chemotherapy and autologous bone marrow support as consolidation after standard-dose adjuvant therapy for high risk primary breast cancer. J Clin Oncol 11:1132–1143
Antman K, Ayash L, Elias A et al (1992) A phase II study of high dose cyclophosphamide, thiotepa, and carboplatin with autologous marrow support in women with measurable advanced breast cancer responding to standard dose therapy. J Clin Oncol 10:102–110
Budman DR, Berry DA, Cirrincione CT et al (1998) Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst 90:1205–1211
Bou-Khalil J, Rose M, Psyrri A (2003) Sequential high-dose alkylating therapy and stem cell support for high-risk stage III breast cancer. Breast J 9(6):472–477
Alexander J, Dainiak N, Berger HJ et al (1979) Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med 300:278–283
Piver MS, Marchetti DL, Parthasarathy KL et al (1985) Doxorubicin hydrochloride (Adriamycin) cardiotoxicity evaluated by sequential radionuclide angiocardiography. Cancer 56:76–80
Ritchie JL, Singer JW, Thorning D et al (1980) Anthracycline cardiotoxicity: clinical and pathologic outcomes assessed by radionuclide ejection fraction. Cancer 46:1109–1116
Van Royen N, Jaffe CC, Krumholz HM et al (1996) Comparison and reproducibility of visual echocardiographic and quantitative radionucleotide left ventricular ejection fractions. Am J Cardiol 77:845–850
Schwartz RG, McKenzie WB, Alexander J et al (1987) Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med 82:1109–1118
Abu-Khalaf MM, Windsor S, Ebisu K et al (2005) Five-year update of an expanded phase II study of dose-dense and -intense doxorubicin, paclitaxel and cyclophosphamide (ATC) in high-risk breast cancer. Oncology 69(5):372–383
Lipshultz SE, Colan SD (1993) The use of echocardiography and Holter monitoring in the assessment of anthracycline-treated patients. In: Bricker JT, Green DM, DiAngeo G (eds) Long term complications of treatment of children and adolescents for cancer. Wiley-Liss, Philadelphia, pp. 45–62
Hudis C, Riccio L, Seidman A et al (1998) Lack of increased cardiac toxicity with sequential doxorubicin and paclitaxel. Cancer Invest 16(2):67–71
Shapiro CL, Hardenbergh PH, Gelman R (1998) Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol 16(11):3493–3501
Bonneterre J, Roche H, Kerbrat P (2004) Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French Adjuvant Study Group. J Clin Oncol 22(15):3070–3079
Fumoleau P, Roche H, Kerbrat P et al (2006) Long-term cardiac toxicity after adjuvant epirubicin-based chemotherapy in early breast cancer: French Adjuvant Study Group results. Ann Oncol 17(1):85–92
Chacon R, Galvez C, Romero Acuna L et al (1992) A clinical analysis of cardiac toxicity and other life-threatening toxicities in patients receiving anthracyclines as adjuvant treatment in breast cancer: Pronacam Cooperative Group. Proc Am Soc Clin Oncol 11 (abstract 111)
Basser RL, Abraham R, Bik To L et al (1999) Cardiac effects of high-dose epirubicin and cyclophosphamide in women with poor prognosis breast cancer. Ann Oncol 10:53–58
Levine MN, Pritchard KI, Bramwell VHC et al (2002) A randomized trial comparing CEF to CMF in premenopausal women with node-positive breast cancer: update of NCIC CTG MA 5. Breast Cancer Res Treat 76(abstract 17, Suppl. 1):533
Piccart MJ, Di Leo A, Beauduin M et al (2001) Phase III trial comparing two dose levels of epirubicin combined with cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer. J Clin Oncol 19:3103–3110
Wils JA, Bliss JM, Coombes MG et al (1999) Epirubicin plus tamoxifen versus tamoxifen alone in node-positive postmenopausal patients with breast cancer: a randomized trial of the International Collaborative Cancer Group. J Clin Oncol 17:1988–1998
Lipshultz SE, Colan SD, Gelber RD et al (1991) Late cardiac effects of doxorubicin in childhood lymphoblastic leukemia. N Engl J Med 324:808–815
Poutanen T, Tikanoja T, Riiknen P et al (2003) Long term prospective follow up study of cardiac function after cardiotoxic therapy for malignancy in children. J Clin Oncol 2(12):2349–2356
Steinherz L, Steinherz P, Tan C et al (1991) Cardiac toxicity 4 to 20 years after completing anthracycline therapy. J Am Med Assoc 266(12):1672–1677
Pein F, Sakiroglu O, Dahan M et al (2004) Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumour at the Institut Gustave Roussy. Br J Cancer 91(1):37–44
Saad SY, Najjar TA, Alashari M (2004) Cardiotoxicity of doxorubicin/paclitaxel combination in rats: effect of sequence and timing of administration. J Biochem Mol Toxicol 18(2):78–86
Magne N, Largillier R, Marcy PY et al (2005) Cardiac toxicity assessment in locally advanced breast cancer treated neoadjuvantly with doxorubicin/paclitaxel regimen. Support Care Cancer 13(10):819–825
Gianni L, Dombernowsky P, Sledge G et al (2001) Cardiac function following combination therapy with paclitaxel and doxorubicin: an analysis of 657 women with advanced breast cancer. Ann Oncol 12(8):1067–1073
Morandi1 P, Ruffini PA, Benvenuto GM (2005) Cardiac toxicity of high-dose chemotherapy. Bone Marrow Transplant 35:323–334
Morandi P, Ruffini PA, Benvenuto GM et al (2001) Serum cardiac troponin I levels and ECG/Echo monitoring in breast cancer patients undergoing high-dose (7 g/m(2)) cyclophosphamide. Bone Marrow Transplant 28:277–282
Ayash LJ, Wright JE, Tretyakov O et al (1992) Cyclophosphamide pharmacokinetics: correlation with cardiac toxicity and tumor response. J Clin Oncol 10:995–1000
Braverman AC, Antin JH, Plappert MT et al (1991) Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol 9:1215–1223
Petros WP, Broadwater G, Berry D et al (2002) Association of highdose cyclophosphamide, cisplatin, and carmustine pharmacokinetics with survival, toxicity, and dosing weight in patients with primary breast cancer. Clin Cancer Res 8:698–705
Bearman SI, Petersen FB, Schor RA et al (1990) Radionuclide ejection fractions in the evaluation of patients being considered for bone marrow transplantation: risk for cardiac toxicity. Bone Marrow Transplant 5:173–177
Fujimaki K, Maruta A, Yoshida M et al (2001) Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant 27:307–310
Fraiser LH, Kanekal S, Kehrer JP (1991) Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs 42:781–795
Lehmann S, Isberg B, Ljungman P, Paul C (2000) Cardiac systolic function before and after hematopoietic stem cell transplantation. Bone Marrow Transplant 26:187–192
Goldberg MA, Antin JH, Guinan EC et al (1986) Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood 68(5):1114–1118
Gottdiener JS, Appelbaum FR, Ferrans VJ et al (1981) Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med 141(6):758–763
Tiersten A, Wob J, Jacobson C et al (2004) Cardiac toxicity observed in association with high-dose cyclophosphamide-based chemotherapy for metastatic breast cancer. Breast 13:341–346
Cazin B, Gorin N, Laporte J et al (1986) Cardiac complications after bone marrow transplantation. Cancer 10:2061–2069
Ayash L, Wright J, Tretyakov O et al (1992) Cyclophosphamide pharmacokinetics: correlation with cardiac toxicity and tumor response. J Clin Oncol 10:995–1000
Bearman S, Petersen F, Schor R et al (1990) Radionuclide ejection fractions in the evaluation of patients being considered for bone marrow transplantation: risk for cardiac toxicity. Bone Marrow Transplant 5:173–177
Marks LB, Yu X, Prosnitz RG et al (2005) The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 63:214–233
Das SK, Bell M, Marks LB et al (2004) A preliminary study of the role of modulated electron beams in intensity modulated radiotherapy, using automated beam orientation and modality selection. Int J Radiat Oncol Biol Phys 59:602–617
Deigert F, Gunn W, Lindemann F et al (1995) A blended beam technique to decrease toxic effects of post mastectomy irradiation by combining and sequentially mixing electrons and photons. Med Dosim 20:183–190
Landau D, Adams EJ, Webb S et al (2000) Cardiac avoidance in breast radiotherapy: a comparison of simple shielding techniques with intensity-modulated radiotherapy. Radiother Oncol 60:247–255
Remouchamps VM, Vicini FA, Sharpe MB et al (2003) Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys 55:392–406
Pihkala J, Saarinen UM, Lundstrom U et al (1996) Myocardial function in children and adolescents after therapy with anthracyclines and chest irradiation. Eur J Cancer 32A:97–103
Hancock SL, Tucker MA, Hopp RT et al (1993) Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. J Am Med Assoc 270:1949–1955
Haybittle JL, Brinkley D, Houghton D et al (1989) Postoperative radiotherapy and late mortality: evidence from the Cancer Research Campaign trial for early breast cancer. Br Med J 298:1611–1614
Host H, Brenhovd IO, Loeb M (1986) Postoperative radiotherapy in breast cancer—long term results from the Oslo study. Int J Radiat Oncol Biol Phys 12:727–732
Jones JM, Ribeiro GG (1989) Mortality patterns over 34 years of breast cancer patients in a clinical trial of post-operative radiotherapy. Clin Radiol 40:204–208
Valagussa P, Zambetti M, Biasi S et al (1994) Cardiac effects following adjuvant chemotherapy and breast irradiation in operable breast cancer. Ann Oncol 5:209–216
Sorensen K, Levitt GA, Bull C et al (2003) Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer 97(8):1991–1998
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu-Khalaf, M.M., Juneja, V., Chung, G.G. et al. Long-term assessment of cardiac function after dose-dense and -intense sequential doxorubicin (A), paclitaxel (T), and cyclophosphamide (C) as adjuvant therapy for high risk breast cancer. Breast Cancer Res Treat 104, 341–349 (2007). https://doi.org/10.1007/s10549-006-9413-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9413-7