Opinion statement
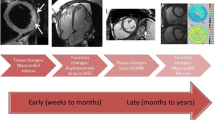
Recent advances in cancer treatment and research have greatly improved survival rates for patients with cancer. However, many of these cancer survivors are developing cardiac disease—most commonly heart failure as a result of this treatment. Certain chemotherapeutic agents, including anthracyclines and trastuzumab, have been linked to cardiotoxicity-induced cardiomyopathy in cancer patients. It has been reported as early as during infusion and as late as several years following treatment. Radiation therapy, particularly to the left breast, has also been linked to cardiac disease. The responsibility of cardiac monitoring has traditionally fallen on oncologists using assessment of LVEF through multigated acquisition (MUGA) scans or echocardiograms. The “formal” definition of cardiotoxicity, as a 5 to 10% decrease in LVEF from its baseline, even though not validated, is currently used by clinicians to alter treatment, but it has been recently challenged, as a possible irreversible late stage of a myocardial insult. Furthermore, it falls into the interobserver variability range of echocardiography. The growing field of medicine called cardio-oncology is based on emerging research that has shown that more advanced imaging modalities can help detect cardiotoxicity early, allowing the patient to receive treatment and avoid developing heart failure from cancer treatment. While traditional imaging still has its place in cardiac monitoring, cardiac magnetic resonance imaging is the most accurate and detailed imaging modality available to assess cardiotoxicity. Our own pilot cardiac MRI study suggests that a normal left ventricular remodeling to chemotherapy, when patients have not developed heart failure symptoms, could occur over time. Perhaps, knowing a baseline normal response could help us to define a more accurate definition of cardiotoxicity by CMR. Here, we discuss various imaging modalities and emerging techniques that can assist in detecting early signs of cardiotoxicity and thus reduce the incidence of cardiac disease in cancer survivors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While traditional imaging (“echocardiography and nuclear medicine”) still has its place in cardiac monitoring, cardiac magnetic resonance imaging is the most accurate and detailed imaging modality available to assess cardiotoxicity [1], and, therefore, can be used to confirm an abnormal LVEF by echocardiography (Fig.1). Cancer and cardiovascular diseases are among the leading causes of death in the US and worldwide [2, 3]. It is not surprising that both groups of diseases are connected, especially due to cardiotoxicity related to chemotherapy or radiotherapy and thromboembolic complication both from the cancer itself or its treatment. Breast cancer treatment is often related to cardiovascular complications due to anthracyclines use and radiotherapy. Otherwise, heart failure patients have a higher incidence of cancer [4].
Anthracyclines comprise a class of chemotherapy that has greatly improved cancer treatment outcomes. Unfortunately, this class of drugs is also associated with a high incidence of irreversible cardiac damage, which leads to heart failure. This type of irreversible cardiotoxicity is referred to as type I cardiotoxicity and may occur in acute, early, or late stages [5, 6]. While the exact pathophysiology is not fully understood, the oxidative stress hypothesis is the predominantly accepted view. This hypothesis suggests that cardiomyocytes are damaged through the production of reactive oxygen and peroxidation of the cell membrane [5]. Higher lifetime cumulative doses of anthracyclines are directly related to the incidence of heart failure. Doxorubicin, for example, is associated with a 5% incidence of heart failure for cumulative doses of 400 mg/m2 and a 48% incidence for cumulative doses of 700 mg/m2 [5].
While several chemo agents can potentially cause cardiotoxicity(Table 1), the most frequently ones associated with left ventricular dysfunction are antracycline-based agents [5]. Targeted therapies have also helped significantly improve rates of cancer survivorship. Trastuzumab, a monoclonal antibody, targets and inhibits human epidermal growth factor receptor 2 (HER2), which has greatly improved survivorship outcomes for patients with HER2 positive breast cancer. However, Trastuzumab is also associated with cardiotoxicity, which presents as heart failure. The incidence of trastuzumab-induced cardiotoxicity is higher among patients who have also been treated with anthracyclines and cyclophosphamide [7]. The mechanism that causes heart failure with trastuzumab is different than with anthracyclines, and is usually reversible, as it is not believed to cause myocyte death. However, it is important to monitor cardiac function during trastuzumab therapy in order to start pharmacologic intervention and reduce treatment interruptions [5].
Radiation therapy for cancer has also been associated with cardiotoxicity. This risk is greatest in patients who have received left breast radiation in addition to cardiotoxic chemotherapy. Radiation-induced cardiotoxicity is typically manifested as heart failure, valve disease, and coronary artery disease. These effects may not be detected until years after treatment, thus demonstrating a need for close surveillance in patients who have been treated with radiation [5].
Although left ventricular dysfunction (LVD) is the most noted manifestation of cardiotoxicity, it is important to note that other forms of cardiac disease also arise from cancer and its treatment. Valve disease has also been noted in patients with cancer, which may be attributed to radiation therapy, opportunistic infections, and rarely chemotherapy. In addition, chemotherapy-induced pancytopenia and indwelling central line catheters may also predispose patients to bacteremia and endocarditis [8]. The relation between the chemotherapeutic agent, cardiovascular toxicity,reversibilty, and imaging modality recommended is depicted in Table 1.
History of cardiotoxicity detection
The term “cardiotoxicity” refers to a variety of cardiac diseases, which can potentially arise from cancer treatment. However, LVD is the most commonly reported manifestation of cardiotoxicity from chemotherapy [9] and has been the standard by which cardiac function has been monitored throughout cancer treatment.
Myocardial biopsy was the diagnostic method of choice in the 1970s to identify left ventricular dysfunction. This procedure was, however, invasive and time-consuming, and its use has declined significantly with improved cardiac imaging modalities and reduced cumulative doses of chemotherapy [8]. Left ventricular ejection fraction (LVEF) has since become the measurement by which cardiotoxicity is defined. Traditional monitoring of LVEF includes baseline and post-treatment assessments [10]. However, earlier detection of subclinical myocardial dysfunction, before a decrease in LVEF is apparent, could lead to identifying, intervening, and possibly preventing late adverse cardiac outcomes. While a universal consensus for the definition of cardiotoxicity does not yet exist, the most commonly used definition is based on a reduction of LVEF from baseline. Cardiotoxicity is thus defined as a decline of LVEF of ≥5% from baseline in symptomatic patients or ≥10% in asymptomatic patients to less than 55% [10, 11]. Emerging data suggests, however, that left ventricular dysfunction may not be reversible after a certain point [8], and more advanced imaging techniques are needed to predict and detect cardiotoxicity earlier. The recommended timing of imaging is before, during, and after cancer treatment.
Some research has suggested that the biomarkers brain natriuretic peptide and troponin may play a role in the early detection of cardiotoxicity. Troponin is considered the most reliable biomarker for detecting myocardial damage, and an early increase in troponin after administration of anthracyclines has been associated with an increased probability of the development of cardiotoxicity and cardiac events [8, 12]. In addition, persistently high troponin levels after anthracycline exposure have been associated with an increase in the severity of cardiotoxicity and a higher incidence of cardiovascular events [8]. In addition to troponin, elevations in BNP may also offer some predictability when it comes to cardiotoxicity. While there is limited data about the relationship between BNP and cardiotoxicity, one study found that an increase in BNP to >200 pg/mL was associated with a significantly higher risk of developing cardiotoxicity [8]. While biomarkers alone are not sufficient to determine cardiotoxicity, they should be monitored and taken into consideration with the rest of the clinical picture. The traditional risk factors for cardiovascular disease (obesity, smoking, CAD, HTN, hyperlipidemia, family history) also place a patient at a higher risk to develop cardiotoxicity. (see Appendix).
Current imaging modalities and techniques for the detection of cardiotoxicity
Nuclear medicine. Multigated acquisition scan (MUGA)
MUGA has been the most widely used imaging modality in the past for evaluating left ventricular function due to its availability, accuracy, and reproducibility [5]. It has been less frequently used, however, with improved imaging techniques including echocardiography and cardiac MRI. The main disadvantage of MUGA is excess radiation exposure. In addition, MUGA does not provide information about right ventricular function or atrial sizes, and it is not able to detect valvular or pericardial disease [8]. Because a decline in LVEF may indicate permanent cardiac damage, it is important to be able to visualize and assess all of the cardiac structures in order to identify the earliest predictors of cardiotoxicity. Multigated radionuclide angiography (MUGA) uses 99mTc-erythrocytes to visualize the cardiac blood pool using a gated acquisition γ camera [13]. Planar images obtained during the cardiac cycle derive accurate and reproducible data of left ventricle function and volumes. Data of a small study showing high sensitivity and specificity was not confirmed by a poll of three trials comprising 630 patients of a randomized sample comparing doxorubicin to placebo [14, 15]. This larger retrospective analysis showed that 66% of patients experiencing doxorubicin-related chronic HF had no clinically relevant decline in LVEF value assessed by MUGA, suggesting that MUGA is not accurate on heart failure prediction of cancer patients treated with chemotherapy. Although MUGA is widely available and generally cost-effective, newer echocardiography technology and cardiac magnetic resonance imaging (CMR) have recently emerged as the preferred imaging modalities for the detection of cardiotoxicity.
SPECT (single photon emission computed tomography) acquires 3D scanned images with high reproducibility that correlates well with MUGA and echocardiography ejection fractions [16]. Different tracers with diverse characteristics and objectives area used and some hold some promise to have advantages in clinical use. In order to determine LV function, 99mTC gated blood-pool SPECT (99mTC GBPS) can obtain 3D images to derive left and right ventricular ejection fraction and regional wall motion abnormality. However, it tends to underestimate LVEF and does not have a good predictive value for development of heart failure [17]. Otherwise, there are also issues of radioactivity and tracer supply.
PET (positron emission tomography) due to its high spatial and temporal resolution remains a superb technique for both myocardial metabolism and perfusion. This leads PET to a high sensitivity and specificity for myocardial viability. PET is also useful for metastatic lesions, in addition to response to chemotherapy and cardiotoxicity [18]. Nevertheless, there is still doubt about PET’s ability to early detect myocardial dysfunction and predict heart failure in chemotherapy patients. Myocardial metabolism alterations in FDG-PET showed the possibility that increased glucose utilization is an evidence of cellular lesions previous to cardiotoxicity in patients using adriamycin [19].
Echocardiogram (echo)
New techniques in echocardiography have led to its becoming more widely used in the detection of cardiotoxicity. Echo is widely preferred due to its availability, cost-effectiveness, and lack of radiation exposure. It is also the preferred imaging method for evaluating valve disease in cancer patients [8]. Newer technology has made echocardiogram appropriate for serial evaluation of left ventricular function and structure [5]. 3D echo is currently the preferred method for determining LVEF, but when this is not a viable option, LVEF is best determined with 2D echo using the biplane Simpson method [5]. The reliability and reproducibility of the Simpson method is based on the drawing of endomyocardial LV borders, geometrical assumptions, and image quality. The interobserver variability to detect cardioxicity was calculated as 10% (IC 95%). The definition of cardiotoxicity is a 10% decrease in LVEF (or a 5% drop with HF symptoms), leading to a compromise in accuracy and detection [8, 11, 20,21,22,23,24].
The use of 3D echo to calculate LVEF in cancer patients may improve variability, if performed properly, but will not affect early detection of cardiotoxicity [25].
Strain image has normal values and variability well documented for global, longitudinal, and radial strain [26]. Peak GLS strain reduction of 10–15% can predict cardio toxicity early in the treatment [27]. Even though there is promising data on regional and 3D strains studies, its use is investigational at this point [28, 29].
The addition of contrast to 2D echocardiography is believed to have incremental value by decreasing interobserver variability, but advantages of using contrast in 3D exams are not that clear. It reduces variability over 2D in cancer-treated patients; however, it has not demonstrated a prognostic value benefit [30, 31••].
Echocardiography is also very useful in evaluation of RV function and diagnosis of valve and pericardial disease associated both with drugs and radiotherapy [31••]. However, CMR is the gold standard method to evaluate RV morphology and function, pericardial disease, and valve regurgitation.
With the growing realization that a decrease in LVEF is a late manifestation of cardiotoxicity, current advances seek to determine early predictors in order to initiate treatment earlier and prevent irreversible cardiac damage. Traditional 2D echocardiography lacks the ability to detect subtle changes in left ventricular function [8], which has led to the development of better techniques to detect early signs of cardiotoxicity. Several clinical studies have shown that using echocardiography to measure myocardial deformation can help identify subclinical LVD and has significant predictive value for the development of cardiotoxicity [11]. Myocardial strain imaging is measured with echocardiography using tissue doppler imaging (TDI) software and 2D and 3D speckle-tracking echocardiography [11]. Many studies have found that a decrease in myocardial deformation measures is predictive of a subsequent decrease in LVEF, with the most predictive measure being global longitudinal strain (GLS) [5, 11]. GLS has been shown to be the most sensitive predictor of early systolic dysfunction [28]. Studies have found a decrease of GLS between 10 and 15% was predictive of cardiotoxicity [11, 29]. Strain imaging software is not yet widely available but should be used to detect and predict cardiotoxicity if possible.
Stress echo may be used to evaluate presence of ischemia for patients who have been treated with certain chemotherapy agents including fluorouracil, bevacizumab, sorafenib, and sunitinib. These agents are associated with a higher incidence of post-treatment coronary artery disease and ischemia [29].
The major limitations with echo include its moderate reproducibility and interobserver variability. One way to improve reproducibility is to have the same reader interpret each echo in serial evaluation. However, this may not always be practical. 3D echo has better reproducibility than 2D echo, but its availability is limited, and its accuracy is still dependent on image quality and the experience of the operator [4].
Diastolic function, based on global index such as mitral valve Doppler flow, E/A ratio and others are used with the hope to detect cardiotoxicity at an early stage. The ability to global diastolic function to predict systolic failure due to cancer drugs is inconsistent [32,33,34].
Cardiac magnetic resonance imaging
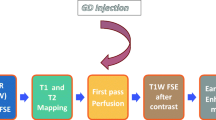
Cardiac MRI (CMR) has emerged in recent years as the gold standard for detecting cardiotoxicity due to its accuracy, reproducibility, and ability to detect subtle changes in cardiac function that may be predictive of cardiotoxicity [5, 10]. In addition to detecting early, subclinical decline in LVEF, CMR also has the ability to detect subtle changes in cardiac structure and to help identify the specific cause of LVEF. It is also useful for evaluating the pericardium, and late gadolinium enhancement (LGE) can help detect scarring or fibrosis, which can contribute to LVD [5]. Several techniques can be used with CMR to help identify various indicators of cardiotoxicity. Cine imaging is used to evaluate the cardiac structure and morphology, phase-contrast imaging is used to assess valvular function, LGE is used to detect focal myocardial fibrosis or scar tissue, and stress-perfusion is used to evaluate for cardiac ischemia [10]. However, CMR currently has limited availability and higher costs than echo and MUGA.
The use of cardiovascular imaging in cardiotoxic surveillance and early detection is usually directed to left ventricular systolic function. Assessment of ejection fraction is being recommended for patients using chemotherapy and/or radiotherapy [35•, 36] (Fig.1). Cardiac magnetic resonance (CMR) is an accurate and reproductive imaging method to determine cardiac structure and function with the advantage of no ionizing radiation and low inter- and intraobserver variability, becoming the gold standard modality for left ventricular volume and function [37, 38] (Fig. 1).
Importantly, CMR is a reliable method to access perfusion during rest or stress and tissue characterization [39]. All the variables derived by a CMR exam can be examined to comprehensively diagnose and quantify cardiac adverse effects of chemotherapy or radiotherapy. When compared to 2D echo, CMR have a better accuracy to detect global and regional function abnormalities in anthracycline and/or radiotherapy treatments [40]. The reliability of measurements and diagnosis strategies (use of T1- and T2-weighted images coupled with gadolinium for perfusion at rest and stress and myocardial/pericardial enhancement) make CMR recognized by the ACC/AHA as a method to screen for chemotherapy-related cardiotoxicity [38].
CMR has been able to detect early changes in global or regional function and provide tissue characterization in cancer patients undergoing different chemotherapy agents. In a pilot study, CMR detected early changes in myocardial enhancement that was later associated with a slight decline in function of patients receiving anthracyclines [41]. Late gadolinium enhancement using CMR could be a novel way of detecting early changes in the myocardium due to trastuzumab-induced cardiotoxicity [42]. In a rat model, it was demonstrated that changes in signal intensity within the LV myocardium on T1-weighted gadolinium-enhanced images could identify animals that develop future adverse cardiac events, primarily a fall in LVEF after doxorubicin [43]. This finding was later replicated in 65 patients, most of them are breast cancer receiving antraciclines. T1-weighted increased slightly as LVEF declines after 3 months but not T2-weighted edema images. These indicate that T1 images could serve as an early marker of injury that predicts future LV dysfunction [44].
Beyond the data obtained by traditional resonance sequences, it is also possible to derive strain measurements and aortic stiffness, both with the potential to contribute for early detection of cardiotoxicity. In 46 long-term childhood cancer survivors with anthracycline use with normal baseline LV function, strain, and myocardial characteristics were abnormal, depicting the potential to detect subtle myocardial damage associated with chemotherapy [45]. In 42 middle-aged patients, 10 (25%) developed trastuzumab-related cardiotoxicity. Tissue velocity and strain were able to detect preclinical changes leading to discontinuation of the drug as a mid-myocardial portion lateral LV-delayed enhancement and a decrease in LVEF was detected [46]. In a study with 10 subjects with hematologic malignancy treated with chemotherapy including anthracyclines, 3-month post-treatment global circumferential strain, but not global longitudinal strain, was significantly decreased compared with pretreatment values [47].
Our group prospectively followed newly diagnosed breast cancer patients with no prior cardiovascular disease treated with anthracycline or trastuzumab. The patients were evaluated at baseline, during cancer treatment, 2 weeks, and 6 months after chemotherapy. The LV remodeling response to chemotherapy was characterized by a dilated/eccentric ventricular geometry and a declining (5%) but preserved LVEF. These changes do not appear to be associated with laboratory or clinical evidence of increased risk for heart failure, which questioned whether early transitory changes in heart function will necessarily lead to cardiotoxicity and heart failure [1].
Long-term follow-up of cancer treatment survivors
In a cross-sectional study of 114 adult survivals (median age = 39; range 22–53 years) of childhood cancer, 16 individuals had a LVEF <50% (prevalence of low EF by CMR was 14%). Echocardiography appears to overestimate the LVEF in that study [47, 48].
A small study included 30 pediatric patients (mean age, 15.2 years) with normal LV function at baseline. After 2 years or more of anthracycline use, increased extracellular volume (ECV—to estimate myocardial fibrosis) fraction correlated with surrogate endpoints such as decreased left ventricular mass and wall thickness, lower peak oxygen uptake on cardiopulmonary stress test, and higher anthracycline cumulative dose [49].
Associations of heart abnormalities with ECV were found in another small single-center study this time in adults (42 subjects with a mean age, 55 years). There was a median time interval of 84 months following anthracycline therapy. Important associations were diastolic dysfunction and increased left atrial volumes with increased ECV [50].
CMR in radiotherapy: cardiac and pericardial consequences
In the treatment of several types of cancer, such as breast cancer, gastrointestinal cancer, and lymphoma, radiotherapy is widely administered. It is estimated that almost half of all cancer patients used radiotherapy at some time during the treatment. Acute or subacute manifestations such as myocarditis or pericarditis after exposure to radiation are infrequent [51].
Lately, radiotherapy is associated with cardiovascular fibrosis which can lead to diverse clinical manifestations including heart failure (diastolic or systolic dysfunction), proximal proliferation/fibrosis of the coronaries, valve disease, arrhythmias and pericardial effusion or constrictive pericarditis [52, 53]. A prospective study indicated that late (3–6 years) perfusion defects after radiotherapy for breast cancer are frequent but do not appear to have functional or clinical relevance [54].
Cardioprotective data
Clinical trials testing the effects of drugs on cardioprotection include the use of beta-blockers, ACE inhibitors, and angiotensin receptor antagonists, or their combination using functional data as endpoints. Several agents have the potential to prevent cardiotoxicity.
The PRADA trial used CMR to show that candesartan (but not metoprolol or the association) has a small but significant less decline in left ventricular function in breast cancer patients treated with antracicline regiments with or without trastuzumab and radiation [55]. However a trial using MUGA have failed to find a protective effect of Candesartan in early breast cancer patients treated with trastuzumab [56].
Cardiovascular masses
The evaluation of cardiac masses by CMR offers a unique opportunity to evaluate and to comprehensively integrate data to reach an accurate diagnosis and prognosis. Mass characteristics on cine, T1-weighted turbo spin echo (T1w-TSE) and T2-weighted turbo spin echo (T2w-TSE), contrast first-pass perfusion (FPP), post-contrast inversion time (TI) scout, and late gadolinium enhancement (LGE) complement itself to characterize cardiac masses. CMR allows to differentiate between cardiac thrombi from tumors, and is important for the distinction of benign versus malignant masses [57]. Characteristics such as location, size, mobility, infiltration, first pass perfusion, size, homogeneity, T1 and T2 weighted intensity and late gadolinium enhancement help to distinguish between different type of masses.
Cardiac tumors are rare and, when visualized, are more frequently secondary to extra cardiac cancer (lung, breast, esophagus, blood and melanoma) and can valve mimic or pericardial disease [57]. The most frequent benign primary cardiac tumors are in decrescendo order mixoma, fibroelastoma, fibroma and lipomas. Malign cardiac tumors are extremely uncommon but mostly found are sarcomas and lymphomas.
Cardiac CT
Cardiovascular CT can be complementary or an attractive alternative to Cardiac MR in approaching cardiac masses when there are contraindications to resonance imaging [58]. Cardiac CT is a fast ECG gated examination with advantages of evaluation of calcified masses, the global assessment of the chest and lung tissue and corresponding vascular structures, and the exclusion of obstructive coronary artery disease or assessment of involvement the coronary arteries by tumors and good visualization of mass mobility. Pitfalls include radiation exposure, contrast allergies or nephropathy and lower resolution for soft tissue when compared to MR.
As with echocardiography and Cardiac MR, several aspects of cardiovascular function are also viable in post processing of Cardiac CT that have the potential to be useful in the evaluation of neoplasms, and complications from chemotherapy or radiotherapy (Fig. 1) [59].
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Avelar E, Truong Q, Inyangetor D et al. (Published Ahead of Print Journal of Thoracic Imaging 2017). Effect of adjuvant chemotherapy on left ventricular remodeling in newly diagnosed primary breast cancer women: a pilot prospective longitudinal cardiac magnetic resonance imaging study.
Murphy SL, Kochanek KD, Xu JQ, Arias E. Mortality in the United States, 2014. NCHS data brief, no 229. Hyattsville, MD: National Center for Health Statistics. 2015.
Lozano R, et al. "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010" (PDF). Lancet. Dec 2012;380:2095–128. doi:10.1016/S0140-6736(12)61728-0.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300.
Zamorano JL 2016 ESC Position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. 2016;37(36):2768–2801 doi: 10.1093/eurheartj/ehw211
Kumar S, Marfatia R, Tannenbaum S, Yang C, Avelar E. Doxorubicin-induced cardiomyopathy 17 years after chemotherapy. Tex Heart Inst J. 2012;39(3):424–7.
Thomy LB, Theobald K. Cardiotoxicity related to anti-cancer drug treatment: a literature review. Aust J Cancer Nurs. 2015;16:4–11.
Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2014;27:911–39.
Curigliano G, Cardinale D, Suter T, Plataniotis G, De Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol. 2012; suppl 7 vii 155-66 doi: 10.1093/annonc/mds293
Tamene AM, Masri C, Konety SH. Cardiovascular MR imaging in cardio-oncology. Magn Reson Imaging Clin N Am. 2015;23:105–16.
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–68.
Vejpongsa P, Yeh ETH. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938–45.
Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr. 2010;4:407–33. doi:10.1016/j.jcct.2010.01.010.
Kara B, Nayman A, Guler I, Gul EE, Koplay M, Paksoy Y. Quantitative assessment of left ventricular function and myocardial mass: a comparison of coronary CT angiography with cardiac MRI and echocardiography. Pol J Radiol. 2016;81:95–102.
de Geus-Oei LF, Mavinkurve-Groothuis AMC, Bellersen L, et al. Scintigraphic techniques for early detection of cancer treatment-induced cardiotoxicity. Journal of NuclearMedicine. 2011;52:560–71.
Walker CM, Saldaña DA, Gladish GW, Dicks DL, Kicska G, Mitsumori LM, Reddy GP. Cardiac complications of oncologic therapy. Radiographics. 2013;33(6):1801–15.
Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br J Cancer. 2002;86:1697–700.
Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–79.
Groch MW, DePuey EG, Belzberg AC, Erwin WD, Kamran M, Barnett CA, Hendel RC, Spies SM, Ali A, Marshall RC. Planar imaging versus gated blood-pool SPECT for the assessment of ventricular performance: a multicenter study. J Nucl Med. 2001;42:1773–9.
Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracycline cardiotoxicity: a prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol. 2002;13:699–709.
Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J Clin Oncol. 2008;26:1201–3.
Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–9.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14.
Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–21.
Mor-Avi V, Lang RM. Is echocardiography reliable for monitoring the adverse cardiac effects of chemotherapy? J Am Coll Cardiol. 2013;61(1):85–7.
Yingchoncharoen T, Agarwal S, Popovic ZB, et al. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26(2):185–91.
Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107(9):1375–80.
Poterucha JT, Kutty S, Lindquist RK, et al. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25(7):733–40.
Pizzino F, Vizzari G, Qamar R, et al. Multimodality imaging in cardio-oncology. J Oncol. 2015;2015:263950.
Galema TW, Van De Ven ART, Soliman OII, et al. Contrast echocardiography improves interobserver agreement for wall motion score index and correlation with ejection fraction. Echocardiography. 2011;28(5):575–81. doi:10.1111/j.1540-8175.2010.01379.x.
•• Hoffmann R, Barletta G, Von Bardeleben S, et al. Analysis of left ventricular volumes and function: a multicenter comparison of cardiac magnetic resonance imaging, cine ventriculography, and unenhanced and contrast-enhanced two-dimensional and three-dimensional echocardiography. J Am Soc Echocardiogr. 2014;27(3):292–301. doi:10.1016/j.echo.2013.12.005. This article adds to others in the field comparisons of different imaging modalities in more than one center.
Stoddard MF, Seeger J, Liddell NE, et al. Prolongation of isovolumetric relaxation time as assessed by Doppler echocardiography predicts doxorubicin-induced systolic dysfunction in humans. J Am Coll Cardiol. 1992;20(1):62–9.
Stoodley PW, Richards DA, Boyd A, et al. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur Heart J Cardiovasc Imaging. 2013;14(3):228–34.
Dorup I, Levitt G, Sullivan I, et al. Prospective longitudinal assessment of late anthracycline cardiotoxicity after childhood cancer: the role of diastolic function. Heart. 2004;90(10):1214–6.
• Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal - Cardiovascular Imaging. 2014;15(10):1063–93. doi:10.1093/ehjci/jeu192. This consensus provides opinions of leaders in the field of imaging and cardio-oncology of how to best apply imaging modalities.
Lancellotti P, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(9):1013–32.
Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. J Cardiovasc Magn Reson. 2004;6:727–65.
Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nu-clear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–97.
Pennell DJ. Cardiovascular magnetic resonance: twenty-first century solutions in cardiology. Clin Med. (Lond) 2003;3(3):273–8.
Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Nes KK, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonanc. 2012;30(23):2876–2884.
Wassmuth R, Lentszch S, Erdbruegger U, Schulz-Menger J, Doerken B, Dietz R, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging—a pilot study. Am Heart J. 2001;141:1007–13.
Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in traztuzumab induced cardiomyopathy. J Cardiovasc Mag Res. 2008;10:5.
Lightfoot JC, D’Agostino Jr RB, Hamilton CA, Jordan J, Torti FM, Kock ND, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3:550–8.
Jordan JH, D’Agostino Jr RB, Hamilton CA, Vasu S, Hall ME, Kitzman DW, Thohan V, Lawrence JA, Ellis LR, Lash TL, Gregory Hundley W. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2014;7:872–9.
Toro-Salazar OH, Gillan E, O’Loughlin MT, Burke GS, Ferranti J, Stainsby J, Liang B, Mazur W, Raman SV, Hor KN. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ Cardiovasc Imaging. 2013;6:873–80.
Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, Grenier D, Jassal DSJ. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;31; 57(22):2263–70.
Wassmuth R, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging—a pilot study. Am Heart J. 2001;141(6):1007–13.
Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Daniel Donovan F, Metzger ML, Arevalo A, Durand JB, Joshi V, Hudson MM, Robison LL, Flamm SD. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30(23):2876–84. doi:10.1200/JCO.2011.40.3584.
Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15(1):48.
Neilan TG, Coelho-Filho OR, Shah RV, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111(5):717–22.
Delaney G, Jacob S, Featherstone C, et al. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence based clinical guidelines. Cancer. 2005;104(6):129–37.
Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95(3):252–8.
Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiol Res Pract doi:10.4061/2011/317659.
Prosnitz RG, Hubbs JL, Evans ES, et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer. 2007;110:1840–50.
Gulati G, Heck SL, Hoffmann P, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37(21):1671–80.
Boekhout AH, Gietema JA, Milojkovic Kerklaan B, et al. Angiotensin II–receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.1726.
Pazos-López P, Pozo E, Siqueira ME, García-Lunar I, Cham M, Jacobi A, Macaluso F, Fuster V, Narula J, Sanz J. Value of CMR for the differential diagnosis of cardiac masses. J Am Coll Cardiol Img. 7(9):896–905.
Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr. 2010;4:407–33. doi:10.1016/j.jcct.2010.01.010.
Kara B, Nayman A, Guler I, Gul EE, Koplay M, Paksoy Y. Quantitative assessment of left ventricular function and myocardial mass: a comparison of coronary CT angiography with cardiac MRI and echocardiography. Pol J Radiol. 2016;81:95–102.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Erick Avelar, Caitlin R. Strickland, and Guido Rosito each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Imaging
Appendix
Appendix
Cardiotoxicity risk assessment form
Section 1: general risk factors | ||
Patient characteristics | ||
>65 years old | YES | NO |
Current smoker | YES | NO |
BMI ≥ 25 | YES | NO |
FH cardiac disease—immediate relatives: | ||
MI | YES | NO |
CHF | YES | NO |
CAD | YES | NO |
HTN | YES | NO |
Hyperlipidemia | YES | NO |
*Two or more “YES” answers to above section ➔ HIGH RISK
*One or fewer “YES” answers to above section➔ Regular risk
Section 2: specific risk factors | ||
PMH cardiac disease | ||
MI | YES | NO |
CHF | YES | NO |
CAD | YES | NO |
HTN | YES | NO |
Hyperlipidemia | YES | NO |
Current or past cardiotoxic chemotherapy | ||
Antracyclines: daunorubicin, doxorubicin (adriamycin, doxil), epirubicin, idarubicin, valrubicin | YES | NO |
Trastuzumab (herceptin) | YES | NO |
Anthracycline + trastuzumab, cyclophosphomide, or paclitaxel | YES | NO |
*One or more “YES” answers to above section ➔ HIGH RISK.
* Zero “YES” answers to above section ➔ Regular risk.
If the patient is calculated to be HIGH RISK in either of the two sections, then the patient should be classified as HIGH RISK.
Rights and permissions
About this article
Cite this article
Avelar, E., Strickland, C.R. & Rosito, G. Role of Imaging in Cardio-Oncology. Curr Treat Options Cardio Med 19, 46 (2017). https://doi.org/10.1007/s11936-017-0546-2
Published:
DOI: https://doi.org/10.1007/s11936-017-0546-2