Abstract
Inspired by the paper platforms for 3-D cell culture, a paper-based microfluidic device containing drug concentration gradient was designed and constructed for investigating cell response to drugs based on high throughput analysis. This drug gradient generator was applied to generate concentration gradients of doxorubicin (DOX) as the model drug. HeLa cells encapsulated in collagen hydrogel were incubated in the device reservoirs to evaluate the cell viability based on the controlled release of DOX spatially. It was demonstrated that drug diffusion through the paper fibers created a gradient of drug concentration, which influenced cell viability. This drug screening platform has a great opportunity to be applied for drug discovery and diagnostic studies with simultaneous and parallel tests of drugs under various gradient concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
For traditional drug screening, multiple toxicity tests with different drug types, dosages and schedules are necessary to evaluate their treatment efficacy and effective dose for therapeutics on a specific disease (Agresti et al. 2010). Some existing platforms testing drug concentration gradient are based on the multi-well plate cell culture platform, which suffers from the lengthy and tedious processing, lower diagnostic reliability and large reagent consumption (Fernandes et al. 2009; Sundberg 2000). Such technical challenges have hindered the drug discovery and development (Kang et al. 2008). Hence, there exists an urgent demand of more rapid and accurate methods to analyze drug candidates. Ideally, a desirable drug screening system features (I) tiny volume of analytes and reagents to minimize the screening cost; (II) screening capability both at the molecular and cellular levels; (III) the ability of multi-step liquid handling particularly for screenings with a complicated procedures (Du et al. 2013). For high-throughput drug screening, cell soring and biosensing, microfluidic systems have exhibited impressive advantages due to their capacity to minimize analytes and reagent consumptions, and the integrated automation as an analytical device (Chung et al. 2007; Hong et al. 2009). Microfluidic platforms such as gradient-generation devices and micro-well arrays have been particularly popular for drug screening studies (Kim et al. 2012; Yang et al. 2011).
Concentration gradient plays an important role in cell-based drug screening (Du et al. 2009; Seidi et al. 2011a). Many drug screening tests have been miniaturized using gradient-generating microfluidic devices, where the cellular responses are evaluated conveniently under the multiple doses generated. For hosting the cell culture, 3-dimensional (3D) matrix materials (i.e. paper, hydrogel) could be a new and capable substrate for gradient generation and drug screening. In fact, paper-based microfluidic devices have become a novel platform for point-of-care disease diagnostics, environmental monitoring and food inspection, owing to their unique advantages of low cost, rapid and tiny volume analysis, and convenience of disposal after single use (Martinez et al. 2007). Filter paper has been extensively used for fabricating paper-based devices because of its outstanding wicking ability (Fenton et al. 2009; Martinez et al. 2010; Li et al. 2010). It has been demonstrated that reagent dilution, mixing, separation and hydrodynamic focusing can be achieved on various paper devices based on capillary wetting through porous fiber network, which is a convenient passive transport mechanism without traditional pumps and mixing actuators as used in conventional microfluidic devices (Osborn et al. 2010). In addition, colorimetric, electrochemical, chemiluminescence and eletrochemiluminescence detections have been realized for analyzing trace amount of reagents on paper-based microfluidic devices (μPAD) (Li et al. 2012). Moreover, compared to other popular materials such as polystyrene and glass, paper is an attractive fiber-based substrate and can be used in both 2D and 3D cell culture applications (Derda et al. 2009, 2011; Juvonen et al. 2013).
The 2D monolayer cell culture for drug screening studies has a major limitation that it cannot mimic the native 3D physiological environment and cellular response in vivo, where the extracellular matrix (ECM) is critical in maintaining the cell physiological function based on sophisticated signaling mechanism (Daley et al. 2008). Hence, 3D cell culture systems are more desirable to study the authentic cellular behaviors in response to various stimuli. Due to the coherent composition and controllable properties, 3D hydrogel was able to retain the native cell functions and differentiation potency during cell culture (Cushing and Anseth 2007). Therefore 3D cell culture in hydrogel has attracted growing attention in recent years (Choi et al. 2007; Wong et al. 2008). As a very popular biomaterial, collagen has been widely used in constructing 3D ECM scaffold for tissue engineering applications (Hwang and Lyubovitsky 2011). Thus, the combination of microfluidic technique for gradient concentration and drug-embedded 3D paper fiber enables the analysis of effects under various concentration of drug on the cell behavior in 3D cell culture system.
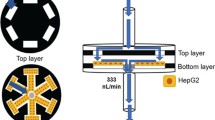
In this work, we present a concentration gradient generator based on a paper-based microfluidic device coupled with cell culture microarrays for high throughput drug screening (Fig. 1). The device was fabricated using traditional photolithography. The concentration gradient of various reagents was verified with readable color gradient. Doxorubicin (DOX) was used as a model anti-cancer drug to study the efficacy under gradient concentrations on this device. HeLa cells, a human epithelial cell line derived from cervical carcinoma, were cultured in the coupled microarray filled with collagen hydrogel as a 3D scaffold. The efficacy of DOX was characterized after the HeLa cells were exposed to the drug gradient for up to 8 h. It was found that the viability of these tumor cells was inhibited in the presence of gradient drug loading through the paper chip. Moreover, the inhibition effect is highly dependent on the gradient concentration, indicating the good potential of this convenient platform for high-throughput drug screening.
2 Materials and methods
2.1 Reagents
Whatman chromatography paper #1 and collagen I (rat protein, tail) was purchased from Thermo Fisher Scientific Pte Ltd., Singapore. Cochineal red food dye was purchased from Gim Hin Lee Pte Ltd., Singapore. Prestoblue cell viability reagent, DMEM medium, Fetal Bovine Serum (FBS), streptomycin and penicillin were purchased from Life Technology, Singapore. SU-8 was obtained from Microchem, USA. PDMS was purchased from Dow Corning Co., USA. Doxorubicin hydrochloride (DOX) was provided by Dalian Meilun Biotech Co., China. Horseradish Peroxidase (HRP, ~150 U mg−1), o-Phenylenediamine (OPD, >98 %) and hydrogen peroxide (H2O2) was obtained from Sigma-Aldrich, Singapore. Deionized water with a resistivity of 18.2 MΩ·cm was collected from Millipore Synthesis A10 (Molsheim, France). 1 × PBS was purchased from First Base, Singapore.
2.2 Chip fabrication
The microfluidic paper-based drug screening chip was designed in two dimensions using AutoCAD2012. Diameter of inlets and outlets is 6 mm. Width of channels is 2 mm. The entire chip has a dimension of 49 mm × 47 mm. The procedure of fabricating paper-based microfluidic chip is as follows. Whatman chromatography paper #1 was impregnated with negative photoresist SU-8 3010, and a spin coating was conducted at 2000 rpm for 30 s to soak the polymer within the paper homogeneously. Afterwards, the photoresist-soaked paper was baked on a hot plate at 95 °C for 10 min. Then the paper was compressed tightly with a predesigned film mask (Fig. 1b) and exposed to a UV laser (wavelength 365 nm, intensity 17 mW cm−2) for 30 s. After baking on the same hot plate at 95 °C for 1 min, non-polymerized photoresist was removed by immersing the device into acetone for 1 min and rinsing with DI water. Finally, the obtained chip was dried in vacuum oven overnight and was ready for use. The photoresist-eluted region on the chip is hydrophilic, whereas the region with polymerized photoresist is hydrophobic.
2.3 Device assembling
The microfluidic paper-based drug screening device comprised five layers, including a top and bottom plastic fixer, two layers of crosslinked PDMS and one piece of paper chip in the center (Fig. 1a, c). Holes were punched through the top plastic fixer and the top PDMS layer as medium inlets and cell culture array, which were aligned precisely with the inlets and outlets of the paper chip. These five layers were tightly assembled by screw fastening (Fig. 1c).
2.4 Colorimetric test
Two types of colorimetric tests were performed on this device. Briefly, 500 μL of Cochineal red food dye (10 % v/v dissolved in DI water) was introduced into one inlet of device. Simultaneously, 500 μL of DI water was dropped into the other inlet. After the liquid reagents completely filled the five paper outlets below the culture array, the device was disassembled and the image of the paper chip was captured by a digital scanner. The color image were processed and analyzed by Image J 1.46r. For the second colorimetric test, the red food dye was replaced by 500 μL of H2O2 (4 mM). After the reagents flow into the outlets, 50 μL of O-phenylenediamine (10 mM) and HRP (0.2 mg mL−1) were introduced into the five wells of cell culture array. The reaction was allowed to proceed for 15 min followed by image processing of the paper chip.
2.5 Generation of drug gradient
The general procedure for generating drug gradients involves the injection of two distinct solutions (drug and dilution buffer) into two individual inlets of the device. Doxorubicin (DOX) was utilized as the model drug to create the concentration gradients. Briefly, 500 μL 1 × PBS solution containing 2 mg mL−1 DOX was injected into drug inlet, while another 500 μL 1 × PBS solution without DOX was injected into the buffer inlet. After a stable gradient was obtained in the paper chip, the five paper outlet reservoirs were sliced from the chip for quantitative analysis of the drug release from each reservoir. The profile of released DOX was characterized by soaking the drug-loaded paper reservoirs into 25 mL of PBS (0.01 M, pH = 7.4) respectively and incubating at 37 °C for 90 min under magnetic stirring. At each consecutive time point, 2 mL of release medium was collected from the container and another 2 mL of fresh PBS was introduced to replenish the releasing system. The amount of released DOX was determined based on a fluorescence spectra (Ex =480 nm, Em = 590 nm) based on a standard calibration curve using a florescent spectrometer (PerkinElmer, Waltham, MA, USA).
2.6 Cell culture
HeLa cells were cultured in a T-flask containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % of fetal bovine serum (FBS), penicillin (100 U mL−1) and streptomycin (100 μg mL−1) in a humidified atmosphere at 37 °C with 5 % CO2. Nonadherent cells were washed away after 48 h and the adherent cells were subcultured until reaching confluence. Cells were harvested by centrifugation at 1000 rpm for 5 min and then resuspended in culture medium. The number density of cells was determined using a Petroff-hausser cell counting chamber.
2.7 Cell viability assay
A conventional cytotoxicity assay was conducted to determine the toxic effect of DOX under different concentrations and time of treatment on cell viability. Briefly, HeLa cell suspension were mixed with neutralized collagen type I and the mixture was pipetted into a 96-well plate at density of 104 cells per well. Then the hydrogel loaded with cells was incubated with 200 μL of fresh culture medium containing DOX with various gradient concentrations (10 μg mL−1, 50 μg mL−1, 100 μg mL−1 and 200 μg mL−1) in a humidified atmosphere at 37 °C. After further incubation for various duration (2 h, 4 h and 8 h), the cells were stained with Calcein AM/PI for live/dead cell imaging. For quantitative evaluation of cytotoxicity, cell viability was further determined by Prestoblue cell viability assay.
2.8 Drug release from gradient paper reservoirs to cell culture array
HeLa cell suspension were mixed with neutralized collagen type I and then pipetted into 5 wells of the cell culture array that had been loaded with gradient drugs at cell density of 104 per well. Then 200 μL of fresh culture medium was added in each culture well and the entire chip device was incubated in a humidified atmosphere at 37 °C for 8 h. Cell viability was calculated based on the results from Prestoblue cell viability assay as described previously.
2.9 Statistical analysis
One-way analysis of variance (ANOVA) served as the method for data analysis using OriginPro 8.5.1 (OriginLab, MA, USA). Statistically significant was determined based on a p-value of less than 0.05 (*p < 0.05, n = 4). Data are presented as the mean ± standard deviation (SD).
3 Results and discussion
The fabrication of the paper chip was achieved based on traditional photolithography technique. In the final assembled chip (Fig. 1c), the paper microfluidic channels starting from two inlets are featured by a series of serpentine channel network, enhancing the mixing of the drug and buffer by diffusion. At the downstream terminals of this network, the flows are branched into five drug reservoirs with concentrations gradient. The curved serpentine channel network can efficiently enhance the fluid mixing in the paper device compared to the straight channel design (Rezk et al. 2012). The width of microchannel was determined as 2 mm considering a few factors: the patterning resolution of photoresist, mixing efficiency and flow rate. We found that the patterning resolution of paper chip using photoresist SU-8 was about 1 mm due to light refraction through porous paper medium during photolithography, which implied that microchannel narrower than 1 mm could not be fabricated on the paper chip using this method. On the other hand, the liquid flow and mixing process are much slower in the narrower channel due to higher flow resistance. For example, the wetting and mixing in the entire chip takes about 40 min if using 1 mm channel width, while it takes only 10 min if using 2 mm channel width. Therefore, we chose channel width of 2 mm that can be easily fabricated and achieve faster mixing in the paper chip.
A critical issue for paper-based microfluidic devices is the liquid evaporation during the capillary flow through the paper fibers, which could cause uncontrollable concentration of the drug and irregular flows inside the paper channels. Many approaches have been used to prevent the undesirable evaporation in paper-based microfluidic devices. For instance, a covered dish containing a water source was used to enclose the “loaded” paper to minimize the evaporation effect (Fu et al. 2011). However, this design cannot effectively prevent the evaporation during the application. Alternatively, an adhesive tape was applied to seal the bioactive channel (Yu et al. 2011), whereas it was hard to disassemble the setup for further analysis. To address these major issues, we used two thin layers of elastic PDMS to seal the paper chip forming the “sandwich” structure (Fig. 1a). The PDMS layer is inherently hydrophobic which does not influence the capillary flow inside the paper channels. Moreover, PDMS is featured by excellent biocompatibility and has been extensively used for on-chip cell and tissue cultures (Peterson et al. 2005). Therefore, the cell-based assay can be safely performed on this paper-PDMS hybrid device.
The drug concentration gradient generator was firstly evaluated using colorimetric assays. Red food dye was injected into this device to visualize the concentration gradient generated in the five outlet reservoirs on the paper chip (Fig. 2a), which took about 10 min to achieve after initial loading of reagents. The color of the edge inside the outlet reservoir was slightly darker than the central region, which was attributed to the liquid evaporation and dye accumulation at the edge. Therefore, the circular area with diameter of 2 mm in the center of each reservoir was selected for quantitative reading of grayscale intensity. As expected, the quantitative results (Fig. 2b) revealed the decrease of dye concentration in the paper reservoirs from one end to the other of the array, which was consistent to the observation simply based on the color intensity change (Fig. 2a).
The grayscale intensity gradient can also be achieved based on color development reaction in this device. H2O2 was introduced into the inlet, diffused and mixed with buffer solution through the paper channel networks. OPD and HRP were added into the outlet reservoirs where the H2O2 concentration gradient was formed. The chemical reduction of H2O2 due to the hydrogen donors from HRP caused the color change of oxidized OPD. Because the color intensity is highly dependent on the amount of H2O2 in each outlet reservoir, the gradual variation of color in five outlets (Fig. 3a) and the quantified color intensity using grayscale (Fig. 3b) indicated successful generation of H2O2 concentration gradient. These results also implied that this device could be used for colorimetric bioassays.
DOX was used as a model drug to generate concentration gradient for simulated chemotherapy in vitro. After DOX with gradient concentrations were generated and loaded in each outlet reservoir, the paper reservoirs were sliced and tested to characterize their drug release profile in vitro. As shown in Fig. 4, it was typical that most of the loaded DOX (>95 %) were released within the first hour followed by a slow release at an extremely low rate, similar to other burst release behavior on paper chip (Huang and Brazel 2001). The total amount of released DOX from each outlet reservoir within 90 min are 36.5 μg, 28.6 μg, 18 μg, 8.2 μg and 0.8 μg respectively, indicating the gradient concentration that showed a consistent trend compared with those obtained by colorimetric assays.
Live/dead cell viability assay based on a conventional multi-well plate was conducted to investigate the effects of DOX concentration and treatment time on cell viability. Live HeLa cells exhibited vivid green fluorescence while the dead cells exhibited red fluorescence. Figure 5a showed that more dead HeLa cells appeared under higher drug concentration and prolonged incubation time. The green circles in the fluorescence images represent the presence of gas bubbles created during the gel formation in the cell culture reservoir, which did not affect the experimental results. Such chemo-cytotoxicity was also verified quantitatively using traditional Prestoblue cell viability assay (Fig. 5b).
Drug effect on cell viability. a Fluorescence images of HeLa cells cultured in 96-well cell culture plate and incubated with gradient concentrations of DOX under various incubation times (Scale bar = 200 μm); b Cell viability in multi-well cell culture system; c Cell viability quantifications on paper reservoirs with a gradient concentration of DOX at 8 h
The inhibition effect of DOX release from gradient paper reservoirs on HeLa cells during on-chip culture were studied using Prestoblue viability assay. Based on the results of drug release, the concentrations of drug in different paper outlet reservoirs was calculated as 182.5 μg mL−1, 143 μg mL−1, 90 μg mL−1, 41 μg mL−1 and 4 μg mL−1, respectively. The cells encapsulated in the hydrogel were incubated in the culture array on the chip at 37 °C for 8 h and subjected to the released DOX from the paper reservoirs at the bottom of the array. Figure 5c revealed the lowest cell viability under the highest DOX concentration, or a reverse trend of cell viability change compared to DOX gradient concentration.
Some prior studies have developed drug screening by generating gradient drug concentrations using microfluidic devices (He et al. 2010; Ostrovidov et al. 2012; Seidi et al. 2011b), where the cells were directly exposed to the diffused drug solution without any release regulation. Furthermore, the drug gradient effect was only examined on 2D cell culture, which was still lacking in the native 3D physiological environment. While in this study, we presented the combination of a paper-based microfluidic device and a 3D cell culture array that could offer a convenient tool to examine the drug gradient on cell culture in 3D hydrogel scaffold. Of course, the mixing efficiency of drug and buffer in the porous paper channels are usually lower than in the regular hollow channels. The mixing efficiency plays an important role in establishing desirable drug concentration with linear gradients. To address this limitation of the paper devices, there are many methods that could improve the mixing efficiency on a paper microfluidic chip, especially using active mixing methods such as surface acoustic waves (SAW) (Rezk et al. 2012). SAW actuation can provide rapid, more uniform and predictable mixing compared to passive mixing within paper fibers.
In the literature, many PDMS-based microfluidic devices are also used to create drug gradient for high-throughput drug screening and efficacy test. In PDMS microchips, laminar flows of drug and buffer are separately introduced into the microchannel network with carefully controlled flow rates (Chung et al. 2005; Pihl et al. 2005; Dertinger et al. 2001; Holden et al. 2003; Zhou et al. 2009; Walker et al. 2005). Then the streams meet at a particular location, such as a T- or Y-junction, where drug molecules diffuse between the streams and the mixing effect depends on the regulation of relative flow rates. Multiple PDMS microdevices such as “Christmas tree” and diffusion-based gradient generators have been designed for creating various concentration gradients (Toh et al. 2004). However, highly stable flow rate is necessary to maintain a constant concentration gradient, otherwise flow disturbance will occur and compromise the established concentration gradient. Additionally, the reagent consumption is costly to maintain constant flow rates in PDMS devices for prolonged use. Meanwhile, in the paper-based microdevices, the flow behavior is mainly dependent on the capillary force in the porous paper channels. No additional instruments such as a pump is required to drive the fluid flow during the operation. Moreover, the concentration gradient in a paper-based device is very stable once being established. Therefore, no continuous supply of reagent is required after generating the gradient concentrations and reagent consumption is much reduced in these paper devices.
In summary, this paper-based microdevice offers unique advantages for drug screening applications compared to conventional methodologies. Firstly, the gradient drug concentration is generated automatically through paper fluidic networks instead of requiring individual loading into multi-wells. Secondly, the mass-produced paper chips are more cost effective, disposable and environmental friendly than using plastic well plates. Thirdly, low consumption of drug solution (average 100 μL per cell culture reservoir) allows for gradient generation within 10 min.
4 Conclusions
In this study, a paper-based concentration gradient generator coupled with 3D cell culture array was designed and fabricated for monitoring the drug toxicity on the tumor cells in response to gradient drug release on a chip device. This microfluidic paper chip was able to create the gradient concentrations of DOX by diffusion and mixing within the paper fibers. Then the gradient drug dosages were gradually released in the culture array containing cells encapsulated in 3D hydrogel, where the cell viability under various drug stimulations were monitored in parallel and in real time. The imbedded drug gradient generator through paper fluidic network can reduce the reagent consumption, automate the drug gradient generation procedure, and thereby improve the efficiency while reduce the cost of traditional cell-based bioassays using multi-well plates. This new hybrid lab-on-a-chip device combining paper microfluidic chip and cell culture array shows a promising potential for future drug discovery and high-throughput drug screening.
References
J. J. Agresti, E. Antipov, A. R. Abate, K. Ahn, A. C. Rowat, J. C. Baret, M. Marquez, A. M. Klibanov, A. D. Griffiths, D. A. Weitz, Proc. Natl. Acad. Sci. U. S. A. 107, 4004 (2010)
N. W. Choi, M. Cabodi, B. Held, J. P. Gleghorn, L. J. Bonassar, A. D. Stroock, Nat. Mater. 6, 908 (2007)
B. G. Chung, L. A. Flanagan, S. W. Rhee, P. H. Schwartz, A. P. Lee, E. S. Monuki, N. L. Jeon, Lab Chip 5, 401 (2005)
B. G. Chung, L. F. Kang, A. Khademhosseini, Expert. Opin. Drug Discov. 2, 1653 (2007)
M. C. Cushing, K. S. Anseth, Science 316, 1133 (2007)
W. P. Daley, S. B. Peters, M. Larsen, J. Cell Sci. 121, 255 (2008)
R. Derda, A. Laromaine, A. Mammoto, S. K. Y. Tang, T. Mammoto, D. E. Ingber, G. M. Whitesides, Proc. Natl. Acad. Sci. U. S. A. 106, 18457 (2009)
R. Derda, S. K. Y. Tang, A. Laromaine, B. Mosadegh, E. Hong, M. Mwangi, A. Mammoto, D. E. Ingber, G. M. Whitesides, PLoS One 6 (2011)
S. K. W. Dertinger, D. T. Chiu, N. L. Jeon, G. M. Whitesides, Anal. Chem. 73, 1240 (2001)
Y. Du, J. Shim, M. Vidula, M. J. Hancock, E. Lo, B. G. Chung, J. T. Borenstein, M. Khabiry, D. M. Cropek, A. Khademhosseini, Lab Chip 9, 761 (2009)
G. S. Du, J. Z. Pan, S. P. Zhao, Y. Zhu, J. M. J. den Toonder, Q. Fang, Anal. Chem. 85, 6740 (2013)
E. M. Fenton, M. R. Mascarenas, G. P. Lopez, S. S. Sibbett, ACS Appl. Mater. Interfaces 1, 124 (2009)
T. G. Fernandes, M. M. Diogo, D. S. Clark, J. S. Dordick, J. M. S. Cabral, Trends Biotechnol. 27, 342 (2009)
E. L. Fu, S. Ramsey, P. Kauffman, B. Lutz, P. Yager, Microfluid. Nanofluid. 10, 29 (2011)
J. K. He, Y. A. Du, J. L. Villa-Uribe, C. M. Hwang, D. C. Li, A. Khademhosseini, Adv. Funct. Mater. 20, 131 (2010)
M. A. Holden, S. Kumar, E. T. Castellana, A. Beskok, P. S. Cremer, Sensor. Actuat B-Chem. 92, 199 (2003)
J. Hong, J. B. Edel, A. J. deMello, Drug Discov. Today 14, 134 (2009)
X. Huang, C. S. Brazel, J. Control. Release 73, 121 (2001)
Y. J. Hwang, J. G. Lyubovitsky, Anal. Methods 3, 529 (2011)
H. Juvonen, A. Maattanen, P. Lauren, P. Ihalainen, A. Urtti, M. Yliperttula, J. Peltonen, Acta Biomater. 9, 6704 (2013)
L. F. Kang, B. G. Chung, R. Langer, A. Khademhosseini, Drug Discov. Today 13, 1 (2008)
J. Kim, D. Taylor, N. Agrawal, H. Wang, H. Kim, A. Han, K. Rege, A. Jayaraman, Lab Chip 12, 1813 (2012)
X. Li, J. F. Tian, G. Garnier, W. Shen, Colloid Surf. B-Biointerfaces 76, 564 (2010)
X. Li, D. R. Ballerini, W. Shen, Biomicrofluidics 6 (2012)
A. W. Martinez, S. T. Phillips, M. J. Butte, G. M. Whitesides, Angew. Chem.-Int. Edit. 46, 1318 (2007)
A. W. Martinez, S. T. Phillips, G. M. Whitesides, E. Carrilho, Anal. Chem. 82, 3 (2010)
J. L. Osborn, B. Lutz, E. Fu, P. Kauffman, D. Y. Stevens, P. Yager, Lab Chip 10, 2659 (2010)
S. Ostrovidov, N. Annabi, A. Seidi, M. Ramalingam, F. Dehghani, H. Kaji, A. Khademhosseini, Anal. Chem. 84, 1302 (2012)
S. L. Peterson, A. McDonald, P. L. Gourley, D. Y. Sasaki, J. Biomed. Mater. Res. Part A 72A, 10 (2005)
J. Pihl, J. Sinclair, E. Sahlin, M. Karlsson, F. Petterson, J. Olofsson, O. Orwar, Anal. Chem. 77, 3897 (2005)
A. R. Rezk, A. Qi, J. R. Friend, W. H. Li, L. Y. Yeo, Lab Chip 12, 773 (2012)
A. Seidi, H. Kaji, N. Annabi, S. Ostrovidov, M. Ramalingam, A. Khademhosseini, Biomicrofluidics 5 (2011a)
A. Seidi, M. Ramalingam, I. Elloumi-Hannachi, S. Ostrovidov, A. Khademhosseini, Acta Biomater. 7, 1441 (2011b)
S. A. Sundberg, Curr. Opin. Biotechnol. 11, 47 (2000)
A. G. G. Toh, Z. P. Wang, C. Yang, N. T. Nguyen, Microfluid. Nanofluid. 16, 1 (2004)
G. M. Walker, J. Q. Sai, A. Richmond, M. Stremler, C. Y. Chung, J. P. Wikswo, Lab Chip 5, 611 (2005)
A. P. Wong, R. Perez-Castillejos, J. C. Love, G. M. Whitesides, Biomaterials 29, 1853 (2008)
C. G. Yang, Y. F. Wu, Z. R. Xu, J. H. Wang, Lab Chip 11, 3305 (2011)
J. Yu, S. Wang, L. Ge, S. Ge, Biosens. Bioelectron. 26, 3284 (2011)
Y. Zhou, Y. Wang, T. Mukherjee, Q. Lin, Lab Chip 9, 1439 (2009)
Acknowledgments
This work was supported by a Tier 2 Academic Research Fund (ARC 22/13) and a Tier 1 Academic Research Fund (RG 37/14) from the Ministry of Education of Singapore awarded to Y.K. The Ph.D. scholarship from Nanyang Technological University awarded to P.X. is gratefully acknowledged. P.X. also thanks Yuli Kang for assistance in paper chip fabrication and setup.
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Hong and P. Xue contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hong, B., Xue, P., Wu, Y. et al. A concentration gradient generator on a paper-based microfluidic chip coupled with cell culture microarray for high-throughput drug screening. Biomed Microdevices 18, 21 (2016). https://doi.org/10.1007/s10544-016-0054-2
Published:
DOI: https://doi.org/10.1007/s10544-016-0054-2