Abstract
In the present work, the removal of Cr (VI), Cd (II) and Pb (II) at 50 mg/L of each metal ion concentration was investigated by Microbacterium paraoxydans strain VSVM IIT(BHU). The heavy metal binding on the bacterial cell surface was confirmed through X-ray photoelectron spectroscopy and energy dispersive X-ray. X-ray photoelectron spectroscopy analysis also confirmed the reduction of Cr (VI) to Cr (III). Heavy metal removal dynamics was investigated by evaluating dimensionless, and the value of Nk (9.49 × 10–3, 9.92 × 10–3 and 1.23 × 10–2 for Cr (VI), Cd (II) and Pb (II) ions) indicated that the removal of heavy metals by bacterial isolate was mixed diffusion and transfer controlled. It was found that both the experimental and predicted values for isolated bacterial strain coincided with each other with a good R2 value in the L-M Algorithm range of 0.94–0.98 for the ternary metal ion system. The bacterial isolate presented a maximum heavy metal ion removal efficiency of 91.62% Cr (VI), 89.29% Pb (II), and 83.29% Cd (II) at 50 mg/L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are used in pigment, paint, battery, steel, coal, chrome plating and tannery industries (Sarangi et al. 2008). The effluents from these industries cause heavy metal contamination in the aquatic ecosystem (Singh et al. 2021a, 2020). Human beings are exposed to heavy metals by the intake of contaminated water, food and contact with skin, which can lead to a variety of cancers, nephrological and neurological disorders (Balali-Mood et al. 2021). Therefore, it is imperative to remove toxic heavy metals, such as Cr (VI), Cd (II) and Pb (II) ions from the water (Garg et al. 2012). The traditional heavy metal removal techniques, including membrane filtration, oxidation, precipitation, adsorption and ion exchange (Singh et al. 2021b) have been extensively practised in the past. These methods have limitations like insufficient removal of heavy metals, high operating cost and generation of secondary chemical sludge after wastewater treatment (Goksungur et al. 2005). Thus, it is demanding to find a suitable heavy metal removal methods for wastewater treatment (Singh et al. 2020). Biomass has been widely used as a bio-remediating agent to remove heavy metals (Elahi et al. 2020). Using living biomass results in the generation of minimum or no secondary chemical sludge and lower operation costs (Hedayatkhah et al. 2018). Additionally, they are widely and easily available across the globe (Bar-On et al. 2018). Among the living biomass, bacteria are highly resistant to toxic metals and accumulate heavy metal ions in their intracellular space (Jacob et al. 2018). Bacterial strains such as Pseudomonas (El-Naggar et al. 2020), Klebsiella (Tekerlekopoulou et al. 2013), Microbacterium (Humphries et al. 2005) and Bacillus (Li et al. 2020) have been used for heavy metal removal. Additionally, bacterial mediated heavy metal removal is considered a cost-effective and eco-friendly option (Ibrahim et al. 2012).
Due to their tolerance to heavy metals, bacteria may flourish in environments with high concentrations of heavy metal ions (Liu et al. 2012). Liu et al (2012) reported that Microbacterium spp. can tolerant up to 4.08 mM of chromate ions. The antioxidant system in the bacteria reduces the toxicity of heavy metals (Garg et al. 2013; Kubrak et al. 2010). Antioxidants are expressed in the cell when stress is created due to metal toxicity. These antioxidants capture reactive oxygen species (ROS) and minimize toxicity (Kumar et al. 2013). The expression of antioxidants participates in the detoxification of heavy metals with their subsequent intracellular bioaccumulation (Joutey et al. 2015).
The present work aims at the removal of the ternary metal ion complex of Cr (VI), Pb (II) and Cd (II) by a bacterial isolate. The reduction of Cr (VI) into Cr (III) by bacterial isolate was also investigated. The effects of heavy metal concentrations on bacterial cell development and morphological alterations were studied. Artificial Neural Network (ANN) study was conducted to predict optimum conditions for removing heavy metal ions. The contrivance of bacterium-mediated bioremediation was investigated in terms of antioxidant production, and dimensionless numbers were calculated to describe the mechanism of heavy metal removal.
Materials and methods
Characterization of bacterial isolate
Heavy metal resistant bacterium specie was isolated from the drain (Baliya Nala, Singrauli, Madhya Pradesh, India) situated in the vicinity of coal washery units (Singh and Mishra, 2021a). The bacterial isolate was isolated by serial dilution followed by spreading on agar plate. 1 mL of wastewater was mixed in 9 mL of normal saline water and was serially diluted up to 10–10 dilution. 0.2 mL of each dilution was poured on Luria–Bertani (LB) agar plates containing 100 mg/L heavy metal ions. The plates were incubated for 24 h at 37 °C. The most tolerant heavy metal resistant pure bacterial culture was identified by DNA isolation followed by 16S rRNA gene sequencing and Basic Local Alignment Search Tool (BLAST) analysis. Obtained 16S rRNA gene sequence was submitted to NCBI GenBank and accession number was obtained (Singh and Mishra, 2021a).
Surface morphology of control and heavy metal exposed bacterial cells were analysed through a scanning electron microscope (SEM) (ZEISS EVO, Carl Zeiss Microscopy make, Germany). Samples for SEM–EDX were prepared by the procedure mentioned in Singh and Mishra (2021a). The elemental composition, including Cr (VI), Cd (II) and Pb (II) in control and in heavy metal exposed bacterial cells was investigated by energy dispersive X-ray (EDX) (EDAX Inc. make, USA) and X-ray photoelectric spectroscopy (XPS) (Thermo Fisher Scientific make, USA). The surface functional groups on the bacterial cell surface were identified by the Attenuated Total Reflectance-Fourier Transform Infrared spectrophotometer (ATR-FTIR) (NICOLET spectrophotometer (iS5) make, USA). Samples for ATR-FTIR and XPS were prepared by modifying the method of Shao et al (2019).
Effect of heavy metal concentration on the growth of isolated bacterial strain
The fresh bacterial pre-inoculum was prepared in 20 mL of LB broth in 100 mL conical flask. The stock solution of Cr (VI), Cd (II) and Pb (II) were prepared by adding predetermined amount of Potassium Dichromate (K2Cr2O7), Cadmium Nitrate Tetrahydrate (Cd(NO3)2.4H2O) and Lead (II) Nitrate (Pb(NO3)2) in 1 L of distilled water, respectively. The effect of Cr (VI), Pb (II) and Cd (II) concentration was observed on the bacterial growth between 50 and 200 mg/L of each metal in the ternary metal ion complex system. The LB broth medium containing heavy metals and control was inoculated with 1% of fresh bacterial inoculum and flask was placed in the incubator for 24 h at 37 °C and 180 rpm. The bacterial cells were grown in 20 mL LB broth in 100 mL flask in control and in heavy metal containing growth medium. The sample was collected at every 2 h up to 24 h. The all experiments were performed in the triplicate and average of the readings were recorded. Bacterial growth in the liquid broth was determined in the form of cell density by measuring absorbance at 600 nm.
Estimation of heavy metals
Cr (VI), Cd (II) and Pb (II) removal was investigated at 50 and 100 mg/L of each. Bacterial cells were grown in LB broth medium using 1% of bacterial inoculum (0.5–0.6 optical density) at 180 rpm, pH 7 and 37 °C for 1 to 5 days. After incubation, the sample was withdrawn, centrifuged and cell pellet was discarded. The remaining heavy metal ion concentration in the supernatant was estimated by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES), Perkin Elmer, Optima 7000 DV make, USA. The heavy metal removal from the aqueous phase was calculated by Eq. 1.
where, Ci and Ce are the initial and equilibrium concentrations of heavy metals (mg/L).
Antioxidants activity
The activity of antioxidants activity was observed in the bacterial isolate exposed to 100 mg/L of ternary heavy metal complex of Cr (VI), Pb (II) and Cd (II). The culture was incubated for 24 h at 37 °C and 180 rpm. The samples were collected at stationary phase (population density was 1.63 ± 0.08 OD at 600 nm). A homogenized solution of bacterial cell pellet in PBS buffer was centrifuged at 10,000 rpm for 12 min and the supernatant was collected. The activities of antioxidants, including glutathione S-transferase (GST), superoxide dismutase (SOD), peroxidase (POX) and catalase were analyzed in the supernatant by methods described in Habig et al. (1974); Beers Jr and Sizer (1952); Ewing and Janero (1995); Reuveni et al. (1992); and Singh and Mishra (2021a).
Derivation of dimensionless numbers
Heavy metal bio-sorption on the bacterial surface is controlled by rearrangement, film, bulk diffusion, and intra-particle diffusion (Imaga and Abia 2015). Dimensionless numbers have been deduced and explained in Section S1.1 of the supplementary information.
ANN
An output experimental data pattern can be predicted when relevant input experimental data is given (Yildiz 2018). Regarding data analysis, the Levenberg–Marquardt (LM) algorithm is commonly used. In most of the attempts, it has been picked due to its ease of use and high level of training capability (Singh and Mishra 2020a, 2020b). In the present work, contact time, pH and temperature were used as input constraints.
Statistical analysis
All experiments were carried out in triplicate (n = 3), and the average values were used to generate the graphs. The experimental errors (± standard deviation) were determined and displayed in graphs as error bars.
Result and discussion
Characterization
The metal-resistant bacterial strain was isolated from coal mining effluent and identified as Microbacterium paraoxydans strain VSVM IIT(BHU). The bacterial strain was submitted to NCBI GenBank under accession no. MN650647.
Identification of bacterial isolate by 16S rRNA gene sequencing
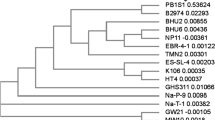
Identification and phylogenetic analysis of bacterial isolate has been done in our previous study (Singh and Mishra, 2021a). Genomic DNA of bacterial isolate was extracted and 16S rRNA gene was amplified by polymerase chain reaction (PCR). Amplification of 16S rRNA gene was confirmed through gel electrophoresis which showed DNA band between 1200 and 1600 bp (Singh and Mishra, 2021a). The bacterial isolate 16S rRNA sequence showed maximum similarity with Microbacterium paraoxydans. The 16S rRNA gene sequence of bacterial isolate was submitted to NCBI GenBank under the bacterial name Microbacterium paraoxydans strain VSVM IIT(BHU) with accession number (MN650647) (Singh and Mishra, 2021a). The phylogenetic analysis of bacterial isolate is shown in Fig. 1.
Evolutionary phylogenetic tree of Microbacterium paraoxydans strain VSVM IIT (BHU) (accession number MN650647) and other similar bacterial strain (Singh and Mishra, 2021a)
Microbacterium paraoxydans strain VSVM IIT(BHU) (accession no. MN650647) showed monophyletic grouping with Microbacterium paraoxydans strain D2O3 which indicated handy evolution relationship between both the strains. Microbacterium paraoxydans strain VSVM IIT (BHU) (accession no. MN650647) showed grouping with other strains. An external strain Microbacterial barkeri strain DSM 20145 confirmed this grouping, located outside of the entire tree.
Fan et al. 2018 isolated heavy metal tolerant bacterial strain from coal mining area and identified it by 16S rRNA sequencing followed by phylogenetic analysis. Marzan, et al. 2017 isolated heavy metal resistant bacterial isolates from tannery effluent. Authors identified bacterial isolates as Gemella sp., Micrococcus sp. and Hafnia sp. by 16S rRNA gene sequence analysis. Jiang et al. 2017 isolated heavy metal resistant bacterial strains from ramie rhizosphere soil present around mine refinery and identified it by 16S rRNA sequencing followed by phylogenetic analysis.
SEM and EDX
The surface morphology and elemental analysis of the bacterial isolated are presented in Fig. 2. It became evident from Fig. 2a that the bacterial cells were rod-shaped with a smooth surface. Bacterial cells secrete a sticky extracellular polymeric substance (EPS) that later accumulated on the exterior surface of the cells giving bacteria their rough texture (Das et al. 2013). The EPS mainly consist of polysaccharides and proteins (Zeng et al. 2020). Sodhi et al. (2020) studied the heavy metal reduction by Alcaligenes sp. MMA and analysed surface morphology using SEM. Shao et al. (2019) investigated the morphological changes in Bacillus sp. exposed to Pb (II) and Cr (VI), and observed that the surface of the cells was rough. Reis-Mansur et al. (2019) isolated Microbacterium sp. LEMM J01 from Antarctic soil and reported elongated and rod-shaped bacterial structures. Gao et al. (2013) carried out the isolation of M. neimengense sp. from the rhizosphere of the maize and observed a similar kind of bacterial morphology.
EDX analysis confirmed the presence of those elements which are component of the bacterial cell wall. It also indicated towards the binding heavy metal ions on the surface of bacterial cells. C, O, Na, Mg, Al, Si and Ca were reported in the EDX of bacterial cells (Fig. 2b). Si, C and O available in the majority were responsible for the biosorption of heavy metals on the bacterial surface (Singh et al. 2020). The presence of C on the bacterial cells makes them suitable for the biosorption of heavy metals (Labied et al. 2018). Si has a robust heavy metal binding affinity, forming a complex structure with heavy metals (Oh et al. 2007). Both O and Si combine to form a mesoporous amorphous structure on the bacterial cell surface, which creates a suitable environment for the biosorption of heavy metals (Bois et al. 2003). Cr (VI), Pb (II) and Cd (II) also appeared on the surface of bacterial cells grown in a medium spiked with the ternary metal heavy-ion system (Fig. 2b), confirming their biosorption. Syed and Chinthala (2015) investigated Cu (II), Pb (II) and Cd (II) bioremediation by Bacillus sp. isolated from Solar Salterns. Authors performed EDX analysis for the heavy metal exposed bacterial species and reported successful biosorption of Cd (II), Pb (II) and Cu (II) on the surface of bacterial cells. Goswami et al (2017) confirmed the biosorption of heavy metal ions on the Rhodococcus opacus bacterial cells in SEM–EDX results. Shamim et al. (2013) investigated Pb (II) removal by Aeromonas caviae strain KS-1. In the EDX analysis, the authors observed Pb (II) binding on the bacterial cells.
XPS
The control and cells grown in 100 mg/L concentration of each metal ion were incubated at 37 °C for 24 h. The results of XPS of control and heavy metal exposed bacterial isolate are shown in Fig. 3.
The C and O elements were present on the bacterial cell in majority (Fig. 3a). The N was also observed in the XPS analysis in the bacterial cells. The XPS indicated that Pb (II), Cr (VI) and Cd (II) were bound to the bacterial cells (Fig. 3b). Heavy metals interact with surface functional groups (i.e., amino, hydroxyl, and carboxyl groups) present on the bacterial cell surface (Hossan et al. 2020). Both surface adsorption and bioaccumulation get involved when heavy metal interact with living bacterial cell. Heavy metal adsorbs on the surface of the bacterium through surface functional groups and enters into the bacterial cell through cell surface receptor (Singh and Mishra 2021a). The presence of Cr (VI), Cd (II) and Pb (II) was detected in the binding energy range of 579 to 575 eV, 417 to 410 eV and 145 to 141 eV for Cr2p3, Cd3d3 and Pb4f5, respectively (Fig. 3b). Zhao et al (2021) investigated Cd (II) removal by sulphate-reducing bacteria. Authors reported Cd (II) binding on the bacterial surface as a Zn–S–Cd complex. Kim et al (2021) performed XPS analysis of Sporosarcina pasteurii exposed to Zn, Pb, Cu, Cd and observed the presence of divalent cations in XPS.
ATR-FTIR
The surface functional groups on bacterial cells were investigated through ATR-FTIR. Results indicated a shifting in the functional groups in the cells exposed to heavy metals. The ATR-FTIR of bacterial cells grown in control and heavy metals is shown in Fig. 4.
Transmittance peaks at 1300–1400 and 1,600–1,700 cm−1 indicated C=C and C–H stretching, respectively (Singh et al. 2020). ATR-FTIR peaks at 1,050 − 1,210 cm−1 confirmed C–O and C–N groups of aliphatic compounds (Saha et al. 2013; Kibami et al. 2017). Shifting in FTIR spectra in the range of 1000–1400 cm−1 region revealed that bacterial cells were exposed to heavy metals. Changes in the peaks between 1300 and 1400 cm−1 reflected the involvement of C=C groups. Shifting the ATR-FTIR spectra at 1050 cm−1, 1210 cm−1 reflected C–O and C–N groups. Bacterial cells secrete extracellular polymeric substance (EPS) consisting of polysaccharides and proteins. These extracellular polymeric substances are involved in the binding of heavy metal ions on bacterial cell surface (Zeng et al. 2020). A comparison of both types of spectra represented Cd (II), Pb (II) and Cr (VI) binding on the bacterial cell through both non-covalent and covalent interactions (Singh et al. 2020). Wang et al (2018) investigated Pb (II) biosorption in the Arthrobactor strain GQ-9 and substantiated the participation of functional groups present on the bacterial surface, such as –OH, –NH, C=O and C–N in the Pb (II) binding.
Bacterial growth at various heavy metal concentrations
The effect of the ternary metal complex on bacterial growth was observed at 50, 100, 150 and 200 mg/L of each metal. The microbial growth in control and heavy metal exposed growth medium are shown in Fig. 5.
It became apparent from Fig. 5 that maximum and minimum growth was observed in control and at 200 mg/L concentration of ternary metal ions complex. Bacterial cells were grown in 50 mg/L Cr (VI)-Pb (II)-Cd (II) and showed a better growth rate after the control. The bacterial growth parameters such as growth rate (µ) and generation time (g) are listed in Table 1.
The microbial growth rate gradually decreased with an increase in concentration of heavy metals due to heavy metal toxicity. Heavy metal ions generate ROS in the bacteria, inhibiting the expression of several bacterial intracellular proteins, including growth factors (Briffa et al. 2020). ROS production significantly increases in the bacterial cells with the rise in heavy metal concentration in the growth medium. ROS-mediated stress in bacterial cells damages the cell or inhibits bacterial growth (Lazarova et al. 2014).
Nath et al (2019) isolated Bacillus megaterium strain GCCSO1 from the contaminated soil sample that showed resistance against Cd (II), Pb (II), Cu (II) and Fe (II). It showed a maximum growth in the presence of copper and lead and minimum growth in the presence of cadmium. Dabir et al (2019) isolated M. oxydans CM3 and Rhodococcus sp. AM1 from coal and aluminium mines and observed bacterial growth inhibition in the presence of Cd (II) and Pb (II) at 400 mg/L.
Removal of heavy metals
Microorganisms utilize trace amounts of heavy metals for their metabolic activities (Tian et al. 2019). They acquire a resistance against heavy metal ions, which helps in their bioaccumulation (Voica et al. 2016). Metal resistance property is based on the antioxidant expression and expression of metal-binding protein within the bacterial cells (Ianeva 2009; Voica et al. 2016). Heavy metal enters into the cytoplasm of bacterial cell through several cell surface receptors. Heavy metal induces the production of oxidative stress in the cell which causes damage in cell proteins and DNA. Metal binding protein minimizes the heavy metal toxicity and enhances the storage of heavy metals into the bacterial cell (Diep et al. 2018). Metallothioneins are the well-known metal binding protein which play an important role in the supply of essential metals ions into the cell and bioaccumulation of toxic heavy metal ions into the cell. Metallothioneins, small protein of about 300 amino acids, are located in the cytoplasm. Metallothioneins mainly consist aromatic (10%) and high proportion of cysteine residues (15–35%) (Balzano et al. 2020).
Moreover, protein folding, intracellular enzymatic activity and ionization rate are the key players in heavy metal bioremediation (Zhang and Li 2011). Bioremediation of Heavy metal also depends on the initial concentration of heavy metals in the growth medium, reaction time, pH, temperature, agitation rate, inoculum dose, types and composition of growth medium, bacterial strain and bacterial resistance (Tang et al. 2021). When heavy metal ions enter into the bacterial cell, heavy metal mediated oxidative stress is generated inside the cell. This oxidative stress is detrimental to the cell. The antioxidants present in the bacterial cell neutralizes this mediated stress and enhance the uptake or bioremediation capacity of bacterial cell (Shao et al. 2019). Optimized pH and temperature for Microbacterium paraoxydans strain VSVM IIT(BHU) were 7 and 37 °C (Singh and Mishra 2021a). In the present study, the removal of ternary metal complex of Cd (II), Cr (VI) and Pb (II) was investigated in the range of 50 to 100 mg/L of metals at pH 7 and 37 °C. The removal efficiency of a bacterial isolate is shown in Fig. 6.
Heavy metal removal in ternary metal ion system at 50 mg/L (a) and 100 mg/L (b). The experimental errors (± standard deviation) were in the range of ± 1.27–7.65 and are shown as error bars. The statistical analysis were performed using Paired T-test using (Graphpad Prism 9.00). (*p≤0.05; **p≤0.01; ***p≤0.005)
The Cr (VI), Pb (II) and Cd (II) removal were recorded as 91.62%, 89.29% and 83.29% at 50 mg/L, respectively (Fig. 6a). It was observed that the removal efficiency decreased when the bacterial isolate was grown in 100 mg/L of metal ions. 75.51% (Cr), 73.84% (Pb) and 68.51% (Cd) removal was reported at 100 mg/L (Fig. 6b). Bacterial strain followed the heavy metal removal pattern as Cr (VI) > Pb (II) > Cd (II). The least removal of Cd (II) was reported as compared other two heavy metals owing to its higher toxicity.
Membrane proteins mediate the bioaccumulation of toxic heavy metals in the bacterial cells (channels and receptors). Intracellular heavy metal ions bind with intracellular proteins like metallothionein. The Cr (VI) is reduced into Cr (III) in the intracellular cell compartment, a process that depends on a number of enzymatic reactions. The Cr (III) accumulates in the bacterial cells (Chojnacka 2010). Huang et al (2001) investigated the Cd (II), Pb (II) and Cr (VI) removal by E. coli and B. subtilis at 50 mg/L initial metal ions concentration. E. coli and B. subtilis were able to remove 63.39% and 69.90% Cd (II), 68.51% and 67.36% Pb (II) and 60.26% and 54.56% Cr (VI). Sharma et al (2022) isolated Pb (II) and Ni (II) tolerant bacterial strain from the metal contaminated site of Haryana, Chandigarh and Mohali, India. Authors reported that 39 bacterial isolate were tolerant to 500 mg/L Pb (II) at pH 7. Rajivgandhi et al (2022) investigated Pb (II) removal by Bacillus cereus RMN 1 (MK521259) isolated from metal contaminated sites (Thoothukudi city, the southern Part of India). Authors investigated Pb (II) and Cu (II) removal at 300 mg/L, pH 7 for 60 min. Authors reported 85.2% and 60.2% removal of Pb (II) and Cu (II), respectively. Khadim et al (2019) investigated Cd (II) and Ni (II) bioremediation by ureolytic bacteria isolated from barn horse soil. Authors claimed that bacterial isolate was able to removal 96% Cd (II) and 89% Ni (II) at 500 mg/L, pH 7 and incubation for 48 h. Dabir et al (2019) isolated M. oxydans CM3 and Rhodococcus sp. AM1 from coal and aluminium mines and recorded Pb (II) removal efficiency of M. oxydans CM3 and Rhodococcus sp. AM1 as 58 and 39% at 400 mg/L.
Intracellular heavy metal ions generate ROS which damage the cell. Antioxidant enzymes can neutralise this heavy metal-mediated stress (Banerjee et al. 2015). These antioxidants actively participate in the heavy metal bioremediation by bacteria. The Cr (VI) is reduced into Cr (III) in the bacterial cell. The surface functional groups on the bacterial cell and intracellular protein such as chromate reductase participate in the reduction of Cr (VI).
Expression of antioxidants
The antioxidants protect cells from oxidative damage (Ge et al. 2011). The expression of antioxidants has been analyzed in the control and heavy metal exposed bacterial cells (Fig. 7).
Expression of antioxidants in the bacterial cells grown in control and ternary metal complex of Pb (II), Cd (II) and Cr (VI). The experimental errors (± standard deviation) were in the range of ± 2.00–13.76 and are shown as error bars. The statistical analyses are with reference to control. *p≤0.05; **p≤0.01; ***p≤0.005 (Paired T-test using Graphpad Prism 9.00)
The antioxidant activity increased in the bacterial cells when grown in the medium containing 100 mg/L each metal ion (Fig. 7). The samples of heavy metal exposed bacterial culture was collected after 24 h at stationary phase and population density was 1.63 ± 0.08 OD at 600 nm. The bacterial isolate exposed to heavy metals showed higher antioxidants expression than control. The antioxidants expression in the metal exposed bacterial cells enhanced up to 36.02% (GST), 54.49% (catalase), 45.13% (SOD) and 96.32% (POX). The enhanced expression of antioxidants in the heavy metal exposed bacterial cells showed bioaccumulation and toxicity of heavy metals in the intracellular space.
Elahi et al (2019) conveyed that the activity of antioxidants in the Cr (VI) exposed M. testaceum B-HS2 increased considerably. Liao et al (2020) re-counted Cr (VI) reduction by Pannonibacter phragmitetus and observed that the expression of antioxidants plays an essential role in Cr (VI) reduction. Banerjee et al (2015) evaluated the heavy metal removal efficiency of Enterobacter cloacae B1 and testified that SOD activity enhanced in the Cd (II) and Pb (II) exposed bacterial cells. The catalase activity also increased in the Cd (II) exposed bacterial cells. Steunou et al (2020) explored the Cd (II) metal ion mediated stress in bacterial cells. The authors observed that SOD activity in the bacterial cells increased when cells were exposed to Cd (II).
Metal bioremediation mechanism
Heavy metal uptake dynamics
Passive diffusion is responsible for most of the uptake of Cd (II), Pb (II) and Cr (VI) by bacteria. Passive diffusion is the simplest and unregulated technique of transporting a chemical molecule/ ion across a membrane (Arnot et al. 2010). When molecules are transported from one area to another, the driving force is concentration gradient. The present work explored the dimensionless numbers in the ternary metal ion complex. The dimensionless numbers are tabulated in Table 2.
The mixed diffusion and transfer control was observed as rate-controlling step (Nk between 10–3 and 101 in all cases) in the ternary metal ion biosorption together with thorough surface coverage and minimized surface tension (φ and λ between 10–2 to 104 and 10–12 to 108) (Joos and Serrien 1989; Ferri and Stebe 2000). Heavy metal ions in the aqueous medium cross the plasma membrane and enter the bacterial cell's intracellular space. Passive diffusion is a simple mechanism in which small molecules cross the plasma membrane. The small molecules dissolve in the phospholipid bilayer, cross the bacterial plasma membrane, and enter the intracellular of the bacterial cell (Ma et al. 2009a, b). Singh and Mishra (2020a) conducted a similar investigation on nickel removal by composite material and observed that nickel ion adsorption on composites was mostly regulated by diffusion. Additionally, Singh and Mishra (2020b) identified an adsorption mechanism based on dimensionless numbers for removing zinc, nickel and copper ions from residual neem twig ash and stated that the process was diffusion controlled. According to Singh and Mishra (2021b) Zn, Ni, and Cu adsorption on composite material was diffusion-controlled during their simultaneous removal. Gupta and Diwan (2016) reported heavy metal uptake mechanism in the bacteria. Authors reported that heavy metal enter into the bacterial through passive diffusion (metabolic independent) and active transport (metabolic dependent). Ma et al (2009a, b) reported that heavy metal ions enter into bacterial cells through passive diffusion. The transport of heavy metal into the cytoplasm also depends on concentration gradient of heavy metal ions in the cell across membrane (Ma et al. 2009a, b).
Reduction of Cr (VI) into Cr (III)
Cr (VI) is several times more toxic than Cr (III). M. paraoxydans strain VSVM IIT(BHU) showed the ability to reduce Cr (VI) into Cr (III). The reduction of Cr (VI) into Cr (III) is shown in Fig. 8.
XPS spectra of heavy metal exposed bacterial cells were collected from the Cr2p domain. Cr (III) presence was observed at binding energy 577.0—579.0 and 586.5—588.0 eV, corresponding to Cr2p3/2 and Cr2p1/2 orbital, respectively (Park et al., 2007). The minor Cr (VI) peak was observed at 579.0—581.0 and 588.5—590.0 eV (Park et al., 2007). These results indicated that chromium bound to the M. paraoxydans strain VSVM IIT(BHU) was in a trivalent oxidation state mostly, which revealed reduction of Cr (VI) into Cr (III).
Aranda-Garcia and Cristiani-Urbina (2020) investigated Cr (VI) biosorption by Quercus crassipes shell in a continuous up-flow fixed-bed column, and demonstrated that most of the chromium adsorbed on the biosorbent was in the trivalent state. Dave and Bhatt (2018) investigated Cr (VI) reduction by a novel bacterial consortium and observed that Cr (VI) was reduced into Cr (III) by bacterial consortium. Nancharaiah et al (2010) examined Cr (VI) immobilization and its reduction into Cr (III) by a mixture of microbes. Gautam et al. (2021) also observed Cr (VI) reduction by Alkalihalobacillus clausii CRA1 in the aqueous phase.
ANN
Contact time, pH and temperature were provided as network inputs, with the optical density of the sample being used as the target. The feed-forward back-propagation network type was applied in conjunction with the L-M algorithm to predict the output function. The network was trained until the smallest number of epochs were recorded. Thereafter, the experimental data was merged with the network simulation. The experimental findings were compared with the predicted output function. The mean square error (MSE) of the ANN model for the Cd (II), Cr (VI), and Pb (II) ions in the ternary metal-ion system is depicted in Fig. 9.
The L-M algorithm produced the lowest MSE through data training, testing, and validation (encircled point). The biosorption of the ternary metal complex has been shown as the regression between experimental and model values (Fig. 10).
The coloured lines in the plot represent predicted values produced from ANN whereas the circles represent experimental data. The experimental and theoretical results appeared to coincide indicating a strong regression coefficient (R2 = 0.94–0.99). It was confirmed that the L-M algorithm was effective in accurately expecting the output function with the minimum MSE at epoch 6 and maximum validation performance in 10 neurons at 0.0069958 for Pb (II), Cr (VI) and Cd (II) ions in the ternary metal ion system.
The correlation plot between experimental and model values revealed the highest regression of 0.96 with a minimal deviation of 0.003% between the experimental and model values (Fig. 11).
Figure 11 demonstrates the applicability of the L-M algorithm. Ghosh and Sinha (2015) employed ANN modelling to optimize the reduction of copper by Stenotrophomonas maltophilia PD2 biomass and found R2 of 0.958 from the trained network. Similarly, Talib et al (2019) used ANN to investigate the removal of Cr (VI) by Acinetobacter radioresistens strain NS-MIE and came up with an R2 of 0.9991. Additionally, Ahmad et al. (2014) observed R2 of 0.997 between the experimental data and model output, when was used ANN to predict the biosorption efficiency of immobilised Bacillus subtilis for removing Cd (II) ions. After executing ANN for modelling biosorption of Pb (II) ions, Khan et al (2017) obtained R2 in the range of 0.95–0.99.
Comparative study of heavy metal removal
The comparison of heavy metal removal efficiency of bacterial isolate with other microorganisms is shown in Table 3. It is evident from Table 3 that the heavy metal removal efficiency of M. paraoxydans strain VSVM IIT(BHU) (accession no. MN650647) is substantially higher (Cr (VI) (91.62%), Cd (II) (83.29%) and Pb (II) (89.29%)) as compared to other bacterial strains. Though these readings have been derived in different environmental conditions, yet they show the importance and possible application of bacterial isolate on a larger scale.
Conclusion
Microbacterium paraoxydans strain VSVM IIT(BHU) accession no. MN650647 showed maximum removal of Pb (II), Cr (VI) and Cd (II) at 50 mg/L. The bacterial isolate showed high heavy metal tolerant capacity and it can grow at 200 mg/L heavy metal concentration. The Cr (VI) was also reduced into Cr (III) by M. paraoxydans strain VSVM IIT(BHU). The growth of bacterial cells decreased with the increase in heavy metal concentration from 50 to 200 mg/L. Antioxidants activities increased in the heavy metal exposed bacterial isolate, which showed heavy metal toxicity and its bioaccumulation into intracellular space. The heavy metal removal dynamics results revealed that metal removal was regulated by the mix diffusion and transfer process. The smallest MSE (0.0069958) and the largest R2 values (0.98) were obtained with the L-M Algorithm to predict the optical density of heavy metal ions. The bacterial isolate identified in the present study has significant potential in removing toxic metal ions from the liquid phase.
References
Abbas SZ, Rafatullah M, Ismail N, Lalung J (2014) Isolation, identification, and characterization of cadmium resistant pseudomonas sp. M3 from industrial wastewater. J Waste Manage 2014:160398
Ahmad MF, Haydar S, Bhatti AA, Bari AJ (2014) Application of artificial neural network for the prediction of biosorption capacity of immobilized Bacillus subtilis for the removal of cadmium ions from aqueous solution. Bioch Eng J 84:83–90
Aranda-Garcia E, Cristiani-Urbina E (2020) Hexavalent chromium removal and total chromium biosorption from aqueous solution by Quercus crassipes acorn shell in a continuous up-flow fixed-bed column: influencing parameters, kinetics, and mechanism. PLoS ONE 15:e0227953
Arnot JA, Arnot MI, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P (2010) Molecular size cutoff criteria for screening bioaccumulation potential: fact or fiction? Integr Environ Assess Manage 6:210–224
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:227–245
Balzano S, Sardo A, Blasio M, Chahine TB, Dell AF, Sansone C, Brunet C (2020) Microalgal metallothioneins and phytochelatins and their potential use in bioremediation. Front Microbiol 11:517
Banerjee G, Pandey S, Ray AK, Kumar R (2015) Bioremediation of heavy metals by a novel bacterial strain enterobacter cloacae and its antioxidant enzyme activity, flocculant production, and protein expression in presence of lead, cadmium, and nickel. Water Air Soil Pollut 226:91
Bar-On YM, Phillips R, Milo R (2018) The biomass distribution on Earth. Proc Natl Acad Sci USA 115:6506–6511
Batta N, Subudhi S, Lal B, Devi A (2013) Isolation of a lead tolerant novel bacterial species, Achromobacter sp. TL-3: assessment of bioflocculant activity. Indian J Exp Biol 51:1004–1011
BeersJr RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bhakta JN, Munekage Y, Ohnishi K, Jana BB (2012) Isolation and identification of cadmium- and lead-resistant lactic acid bacteria for application as metal removing probiotic. Int J Environ Sci Technol 9:433–440
Bois L, Bonhomme A, Ribes A, Pais B, Raffin G, Tessier F (2003) Functionalized silica for heavy metal ions adsorption. Colloids Surf A 221:221–230
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Chojnacka K (2010) Biosorption and bioaccumulation-the prospects for practical applications. Environ Int 36:299–307
Dabir A, Heidari P, Ghorbani H, Ebrahimi A (2019) Cadmium and lead removal by new bacterial isolates from coal and aluminum mines. Int J Environ Sci Technol 16:8297–8304
Das S, Mishra J, Das SK, Pandey S, Rao DS, Chakraborty A, Sudarshan M, Das N, Thatoi H (2013) Investigation on mechanism of Cr(VI) reduction and removal by Bacillus amyloliquefaciens, a novel chromate tolerant bacterium isolated from chromite mine soil. Chemosphere 96:112–121
Dave S, Bhatt N (2018) Biotransformation of Cr (VI) by newly invented bacterial consortium SN6. J Pure Appl Microbiol 12:1375–1384
Diep P, Mahadevan R, Yakunin AF (2018) Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front Bioeng Biotechnol 6:157
Elahi A, Ajaz M, Rehman A, Vuilleumier S, Khan Z, Hussain SZ (2019) Isolation, characterization, and multiple heavy metal-resistant and hexavalent chromium-reducing Microbacterium testaceum B-HS2 from tannery effluent. J King Saud Univ Agric Sci 31:1437–1444
Elahi A, Arooj I, Bukhari DA, Rehman A (2020) Successive use of microorganisms to remove chromium from wastewater. Appl Microbiol Biotechnol 104:3729–3743
El-Naggar NE, El-Khateeb AY, Ghoniem AA, El-Hersh MS, Saber WIA (2020) Innovative low-cost biosorption process of Cr6+ by Pseudomonas alcaliphila NEWG-2. Sci Rep 10:14043–14061
Ewing JF, Janero DR (1995) Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem 232:243–248
Fan M, Liu Z, Nan L, Wang E, Chen W, Lin Y, Wei G (2018) Isolation, characterization, and selection of heavy metal-resistant and plant growth-promoting endophytic bacteria from root nodules of Robinia pseudoacacia in a Pb/Zn mining area. Microbiol Res 217:51–59
Ferri JK, Stebe KJ (2000) Which surfactants reduce surface tension faster? A scaling argument for diffusion-controlled adsorption. Adv Colloid Interface Sci 85:61–97
Gao M, Wang M, Zhang YC, Zou XL, Xie LQ, Hu HY, Xu J, Gao JL, Sun JG (2013) Microbacterium neimengense sp. nov., isolated from the rhizosphere of maize. Int J Syst Evol Microbiol 63:236–240
Garg SK, Tripathi M, Srinath T (2012) Strategies for chromium bioremediation of tannery effluent. Rev Environ Contam Toxicol 217:75–140
Garg SK, Tripathi M, Singh SK, Singh A (2013) Pentachloro phenol dechlorination and simultaneous Cr6+ reduction by Pseudomonas putida SKG-1 MTCC (1050): characterization of PCP dechlorination products, bacterial structure, and functional groups. Environ Sci Pollut Res 20:2288–2304
Ge W, Zamri D, Mineyama H, Valix M (2011) Bioaccumulation of heavy metals on adapted Aspergillus foetidus. Adsorption 17:901–910
Ghosh A, Sinha K (2015) Optimization of reduction of copper using Stenotrophomonas maltophilia PD2 biomass and artificial neural network modeling. Environ Eng Manage J 14:37–44
Goksungur Y, Uren S, Guven U (2005) Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour Technol 96:103–109
Goswami L, Manikandan NA, Pakshirajan K, Pugazhenthi G (2017) Simultaneous heavy metal removal and anthracene biodegradation by the oleaginous bacteria Rhodococcus opacus. 3 Biotech 7:37–45
Gupta P, Diwan B (2016) Bacterial Exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep (amst) 13:58–71
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hedayatkhah A, Cretoiu MS, Emtiazi G, Stal LJ, Bolhuis H (2018) Bioremediation of chromium contaminated water by diatoms with concomitant lipid accumulation for biofuel production. J Environ Manage 227:313–320
Hossan S, Hossain S, Islam MR, Kabir MH, Ali S, Islam MS, Imran KM, Moniruzzaman M, Mou TJ, Parvez AK, Mahmud ZH (2020) Bioremediation of hexavalent chromium by chromium resistant bacteria reduces phytotoxicity. Int J Environ Res Public Health 17:6013
Huang M, Pan J, Zheng L (2001) Removal of heavy metals from aqueous solutions using bacteria. J Shanghai Jiaotong Univ (sci) 5:253–259
Humphries AC, Nott KP, Hall LD, Macaskie LE (2005) Reduction of Cr (VI) by immobilized cells of Desulfovibrio vulgaris NCIMB 8303 and Microbacterium sp. NCIMB 13776. Biotechnol Bioeng 90:589–596
Ianeva OD (2009) Mechanisms of bacteria resistance to heavy metals. Mikrobiol Z 71:54–65
Ibrahim ASS, El-Tayeb AM, Elbadawi BY, Al-Salamah AA, Antranikian G (2012) Hexavalent chromate reduction by alkaliphilic Amphibacillus sp. KSUCr3 is mediated by copper-dependent membrane-associated Cr (VI). Extremophiles 16:659–668
Imaga CC, Abia AA (2015) Adsorption kinetics and mechanisms of Ni2+ sorption using carbonized and modified sorghum (Sorghum bicolor) hull of two pore sizes (150 µm and 250 µm): a comparative study. Int J Chem Stud 2:59–68
Jacob JM, Karthik C, Saratale RG, Kumar SS, Prabakar D, Kadirvelu K, Pugazhendhi A (2018) Biological approaches to tackle heavy metal pollution: a survey of literature. J Environ Manage 217:56–70
Jiang J, Pan C, Xiao A, Yang X, Zhang G (2017) Isolation, identification, and environmental adaptability of heavy-metal-resistant bacteria from ramie rhizosphere soil around mine refinery. 3 Biotech 7:5
Joos P, Serrien G (1989) Adsorption kinetics of lower alkanols at the air/water interface: effect of structure makers and structure breakers. J Colloid Interface Sci 127:97–103
Joutey NT, Sayel H, Bahafid W, El Ghachtouli N (2015) Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev Environ Contam Toxicol 233:45–69
Khadim HJ, Ammar SH, Ebrahim SE (2019) Biomineralization based remediation of cadmium and nickel contaminated wastewater by ureolytic bacteria isolated from barn horses soil. Environ Technol Innovation 14:100315
Khan T, Mustafa MRU, Isa MH, Manan TSBA, Ho YC, Lim JW, Yusof NZ (2017) Artificial Neural Network (ANN) for modelling adsorption of lead (Pb (II)) from aqueous solution. Water Air Soil Pollut 228:426
Kibami D, Pongerner C, Rao KS, Sinha D (2017) Surface characterization and adsorption studies of Bambusa bulgaris-a low cost adsorbent. J Mech Eng Sci 8:2494–2505
Kim Y, Kwon S, Roh Y (2021) Effect of divalent cations (Cu, Zn, Pb, Cd, and Sr) on microbially induced calcium carbonate precipitation and mineralogical properties. Front Microbiol 12:763–774
Kubrak OI, Lushchak OV, Lushchak JV, Torous IM, Storey JM, Storey KB, Lushchak VI (2010) Chromium effects on free radical processes in goldfish tissues: comparison of Cr (III) and Cr (VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp Biochem Physiol C: Toxicol Pharmacol 152:360–370
Kumar MS, Praveenkumar R, Ilavarasi A, Rajeshwari K, Thajuddin N (2013) Biochemical changes of fresh water cyanobacteria Dolichospermum flos-aquae NTMS07 to chromium-induced stress with special reference to antioxidant enzymes and cellular fatty acids. Bull Environ Contam Toxicol 90:730–735
Labied R, Benturki O, Hamitouche AYE, Donnot A (2018) Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): kinetic, equilibrium, and thermodynamic study. Adsorpt Sci Technol 36:1066–1099
Lazarova N, Krumova E, Stefanova T, Georgieva N, Angelova M (2014) The oxidative stress response of the filamentous yeast Trichosporon cutaneum R57 to copper, cadmium and chromium exposure. Biotechnol Biotechnol Equip 28:855–862
Li MH, Gao XY, Li C, Yang CL, Fu CA, Liu J, Wang R, Chen LX, Lin JQ, Liu XM, Lin JQ, Pang X (2020) Isolation and identification of chromium reducing Bacillus cereus species from chromium-contaminated soil for the biological detoxification of chromium. Int J Environ Res Public Health 17:2118
Liao Q, Tang J, Wang H, Yang W, He L, Wang Y, Yang Z (2020) Dynamic proteome responses to sequential reduction of Cr(VI) and adsorption of Pb(II) by Pannonibacter phragmitetus BB. J Hazard Mater 386:121988–121997
Liu Z, Wu Y, Lei C, Liu P, Gao M (2012) Chromate reduction by a chromate-resistant bacterium Microbacterium sp. World J Microbiol Biotechnol 28:1585–1592
Ma Z, Jacobsen FE, Giedroc DP (2009a) Metal transporters and metal sensors: how coordination chemistry controls bacterial metal homeostasis. Chem Rev 109:4644–4681
Ma Z, Jacobsen FE, Giedroc DP (2009b) Coordination chemistry of bacterial metal transport and sensing. Chem Rev 109:4644–4681
Marzan LW, Hossain M, Mina SA, Akter Y, Chowdhury AMMA (2017) Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: bioremediation viewpoint. Egyp J Aquat Res 43:65–74
Nancharaiah YV, Dodge C, Venugopalan VP, Narasimhan SV, Francis AJ (2010) Immobilization of Cr(VI) and its reduction to Cr(III) phosphate by granular biofilms comprising a mixture of microbes. Appl Environ Microbiol 76:2433–2438
Nath S, Paul P, Roy R, Bhattacharjee S, Deb B (2019) Isolation and identification of metal-tolerant and antibiotic-resistant bacteria from soil samples of Cachar district of Assam. India SN Appl Sci 1:727
Oh JY, Song H, Shin WS, Choi SJ, Kim YH (2007) Effect of amorphous silica and silica sand on removal of chromium (VI) by zero-valent iron. Chemosphere 66:858–865
Oyetibo GO, Ilori MO, Obayori OS, Amund OO (2013) Chromium (VI) biosorption properties of multiple resistant bacteria isolated from industrial sewerage. Environ Monit Assess 185:6809–6818
Park D, Lim SR, Yun YS, Park JM (2007) Reliable evidences that the removal mechanism of hexavalent chromium by natural biomaterials is adsorption-coupled reduction. Chemosphere 70:298–305
Rahman A, Nahar N, Nawani NN, Jass J, Hossain K, Saud ZA, Saha AK, Ghosh S, Olsson B, Mandal A (2015) Bioremediation of hexavalent chromium (VI) by a soil-borne bacterium, Enterobacter cloacae B2-DHA. J Environ Sci Health Part A 50:1136–1147
Rajivgandhi G, Ramachandran G, Chackaravarthi G, Maruthupandy M, Quero F, Chelliah CK, Manoharan N, Alharbi NS, Kadaikunnan S, Khaled JM, Li WJ (2022) Metal tolerance and biosorption of Pb ions by Bacillus cereus RMN 1 (MK521259) isolated from metal contaminated sites. Chemosphere 308(Pt 1):136270
Reis-Mansur MCPP, Cardoso-Rurr JS, Silva JVMA, de Souza GR, Cardoso VS, Mansoldo FRP, Pinheiro Y, Schultz J, Balottin LBL, da Silva AJR, Lage C, dos Santos EP, Rosado AS, Bea A (2019) Carotenoids from UV-resistant Antarctic Microbacterium sp. LEMMJ01. Sci Rep 9:9554
Reuveni R, Shimoni M, Karchi Z, Kuc J (1992) Peroxidase activity as a biochemical marker for resistance of muskmelon Cucumis melo to Pseudopernospora cubensis. Phytopathology 82:749–753
Saha R, Mukherjee K, Saha I, Ghosh A, Ghosh SK, Saha B (2013) Removal of hexavalent chromium from water by adsorption on mosambi (Citrus limetta) peel. Res Chem Intermed 39:2245–2257
Sarangi A, Krishnan C (2008) Comparison of in vitro Cr (VI) reduction by CFEs of chromate resistant bacteria isolated from chromate contaminated soil. Bioresour Technol 99:4130–4137
Shamim K, Naik MM, Pandey A, Dubey SK (2013) Isolation and identification of Aeromonas caviae strain KS-1 as TBTC- and lead-resistant estuarine bacteria. Environ Monit Assess 185:5243–5249
Shao W, Li M, Teng Z, Qiu B, Huo Y, Zhang K (2019) Effects of Pb(II) and Cr(VI) stress on phosphate-solubilizing bacteria (Bacillus sp. Strain MRP-3): oxidative stress and bioaccumulation potential. Int J Environ Res Public Health 16:2172
Sharma R, Jasrotia T, Umar A, Sharma M, Sharma S, Kumar R, Alkhanjaf AAM, Vats R, Beniwal V, Kumar R, Singh J (2022) Effective removal of Pb(II) and Ni(II) ions by Bacillus cereus and Bacillus pumilus: an experimental and mechanistic approach. Environ Res 212(Pt B):113337
Singh J, Mishra V (2020a) Modeling of adsorption flux in nickel-contaminated synthetic simulated wastewater in the batch reactor. J Environ Sci Health Part A 55:1059–1069
Singh J, Mishra V (2020b) Simultaneous removal of Cu2+, Ni2+ and Zn2+ ions using leftover Azadirachta indica twig ash. Biorem J 25:48–71
Singh V, Mishra V (2021a) Microbial removal of Cr (VI) by a new bacterial strain isolated from the site contaminated with coal mine effluents. J Environ Chem Eng 9:106279
Singh J, Mishra V (2021b) Development of sustainable and ecofriendly metal ion scavenger for adsorbing Cu2+, Ni2+ and Zn2+ ions from the aqueous phase. Sep Sci Technol 57:354–371
Singh V, Singh MP, Mishra V (2020) Bioremediation of toxic metal ions from coal washery effluent. Desalin Water Treat 197:300–318
Singh V, Singh S, Mishra V (2021a) Sorption kinetics of an eco-friendly and sustainable Cr (VI) ion scavenger in a batch reactor. J Environ Chem Eng 9:105125–105166
Singh V, Singh S, Mishra V (2021b) Development of a cost-effective, recyclable and viable metal ion doped adsorbent for simultaneous adsorption and reduction of toxic Cr (VI) ions. J Environ Chem Eng 9:105124–105137
Sodhi KK, Kumar M, Singh DK (2020) Multi-metal resistance and potential of Alcaligenes sp. MMA for the removal of heavy metals. SN Appl Sci 2:1885
Steunou AS, Babot M, Bourbon ML, Tambosi R, Durand A, Liotenberg S, Krieger-Liszkay A, Yamaichi Y, Ouchane S (2020) Additive effects of metal excess and superoxide, a highly toxic mixture in bacteria. Microbial Biotechnol 13:1515–1529
Syed S, Chinthala P (2015) Heavy metal detoxification by different Bacillus species isolated from solar salterns. Scientifica (cairo) 2015:1–8
Talib NSR, Halmi MIE, Ghani SSA, Zaidan UH, Shukor MYA (2019) Artificial neural networks (ANNs) and response surface methodology (RSM) approach for modelling the optimization of chromium (VI) reduction by newly isolated Acinetobacter radioresistens strain NS-MIE from agricultural soil. Biomed Res Int 2019:5785387
Tang X, Huang Y, Li Y, Wang L, Pei X, Zhou D, He P, Hughes SS (2021) Study on detoxification and removal mechanisms of hexavalent chromium by microorganisms. Ecotoxicol Environ Saf 208:111699
Tekerlekopoulou AG, Tsiflikiotou M, Akritidou L, Viennas A, Tsiamis G, Pavlou S, Bourtzis K, Vayenas DV (2013) Modelling of biological Cr (VI) removal in draw-fill reactors using microorganisms in suspended and attached growth systems. Water Res 47:623–636
Tian Y, Zhang H, Zheng L, Li S, Hao H, Yin M, Cao Y, Huang H (2019) Process analysis of anaerobic fermentation exposure to metal mixtures. Int J Environ Res Public Health 16:2458
Voica DM, Bartha L, Banciu HL, Oren A (2016) Heavy metal resistance in halophilic bacteria and archaea. FEMS Microbiol Lett 363:1–9
Wang T, Yao J, Yuan Z, Wang F, Chen H (2018) Isolation of lead-resistant Arthrobactor strain GQ-9 and its biosorption mechanism. Environ Sci Pollut Res 25:3527–3538
Yildiz S (2018) Artificial neural network approach for modeling of Ni (II) adsorption from aqueous solution by peanut shell. Ecol Chem Eng S 25:581–604
Yu X, Zhao JT, Liu X, Sun L, Tian J, Wu N (2021) Cadmium pollution impact on the bacterial community structure of arable soil and the isolation of the cadmium resistant bacteria. Front Microbiol 12:2099–2109
Zeng W, Li F, Wu C, Yu R, Wu X, Shen L, Liu Y, Qiu G, Li J (2020) Role of extracellular polymeric substance (EPS) in toxicity response of soil bacteria Bacillus sp. S3 to multiple heavy metals. Bioprocess Biosyst Eng 43:153–167
Zhang K, Xue Y, Zhang J, Hu X (2020) Removal of lead from acidic wastewater by bio-mineralized bacteria with pH self-regulation. Chemosphere 241:125041
Zhao C, Yang Q, Chen W, Teng B (2012) Removal of hexavalent chromium in tannery wastewater by Bacillus cereus. Can J Microbiol 58:23–28
Zhao Q, Li X, Xiao S, Peng W, Fan W (2021) Integrated remediation of sulfate reducing bacteria and nano zero valent iron on cadmium contaminated sediments. J Hazard Mater 406:124680
Acknowledgements
The authors of this manuscript are thankful to the IIT (BHU), University of Allahabad and Poznan University of Technology for their necessary support during this study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, experiments, data collection and analysis were performed by VS, JS, NS, MKV, MV, VS, MSC, SNR, MB and VM. The first draft of the manuscript was written by VS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.”
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, V., Singh, J., Singh, N. et al. Simultaneous removal of ternary heavy metal ions by a newly isolated Microbacterium paraoxydans strain VSVM IIT(BHU) from coal washery effluent. Biometals 36, 829–845 (2023). https://doi.org/10.1007/s10534-022-00476-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-022-00476-4