Abstract
Metallic pollution in aquatic ecosystems has expanded dramatically due to the astonishing growth in industrial activity, posing a threat to the safety and health of the environment. Bacterial bioremediation offers promising solutions for decontaminating a polluted environment in a variety of situations. As a result, the tolerance and toxicity levels of the quaternary metal in bacteria isolated from the electroplating effluent were evaluated. The best tolerable strains were Bacillus megaterium, Sphingobacterium ginsenosidimutans, and Kocuria rhizophila, chosen for potential biosorption and bioaccumulation at high concentrations and maximum sorption rate and time. The mechanisms of bioremediation were verified by Scanning electron microscopy energy-dispersive x-ray analysis and Fourier transform infrared spectroscopy. The results showed that the maximum biosorption was 83.73% Ni and 75.49% quaternary during 6 h, while the Cu and Ni accumulation levels were 0.291 mg/g at 24 h and 0.159 mg/g at 12 h in B. megaterium,, respectively. The consortium achieved high biosorption with individual metals and quaternary ranging from 75.68 to 90.79%, and the highest accumulating amount of Cu was 0.399 mg/g during 12 h and 0.374 mg/g Ni during 6 h. During the exponential phase and using the bacterial consortium, the best metal bioremediation outcomes were found. It was observed that metal binding changes cell morphology and FTIR spectra identified the dominant groups involved in the biosorption of metals on the surfaces of bacteria. The study shed some light and offered more knowledge of the interactions of metal-tolerant bacteria during bioremediation processes and their practical applicability to mineral processing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The complexity of wastewater has grown as a result of rapid industrial development. Industrial water discharged into bodies of water is regarded as one of the primary sources of environmental contamination that causes water quality alterations (Aniyikaiye et al. 2019). The degree of environmental deterioration caused by diverse human activities in aquatic ecosystems is typically reflected in their quality (Bhat et al. 2018). Surface water has been severely damaged by effluents from industrial processes such as the electroplating sector (Aniyikaiye et al. 2019). This is because they may be included in significant amounts of copper, nickel, chromium, and zinc (Xiao et al. 2019).
Heavy metals are elements having a density greater than 5 g/cm3 and metals or metalloids with an atomic mass greater than 4000 kg m3, or five times that of water (Paschoalini and Bazzoli 2021). Metallic ions from diverse industrial operations are a serious concern due to their toxicity to all living organisms, accelerated rates of movement or transportability in the environment, and influence on ecosystem balance (Ali et al. 2019). Furthermore, the problem is that these pollutants are persistent elements with accumulating properties and non-biodegradability, which causes significant environmental concern and has a high water dispersal capability. As a result, under the appropriate conditions, it may be bioavailable for uptake by other aquatic species (Elbasiouny et al. 2021; Aniyikaiye et al. 2019). Therefore, remediation measures must be implemented to prevent heavy metals from entering terrestrial and aquatic environments and to reduce metallic pollution (Yan et al. 2020; Hasan et al. 2019).
Today, numerous conventional approaches are employed to reduce pollution and remove heavy metals from industrial effluent. The methods include layer detachment, particle trade, ultrafiltration, filtration, sedimentation, electrodialysis, photocatalysis, flocculation/coagulation, and adsorption (Meepho et al. 2018). Most of these traditional techniques require complex operations, are expensive and consume more energy. It was confirmed that despite their use and prevalence, they may face certain defects such as low efficiency at low metal concentration, high cost, and toxic by-products (Nugroho et al. 2021; Zhang 2014).
Biosorption is a simple, economical and environmentally friendly process that removes pollutants by binding them to a material of biological origin or biomass. Advances in this field have reinforced the interest in this technique to solve environmental pollution problems (Torres 2020). Furthermore, biosorption has advantages over traditional metal removal methods, such as lower biomass production costs, efficient heavy metal removal, multiple uptake, large wastewater treatment, no chemical additions, function under varying conditions, easy metal desorption, and reduced waste volume or toxic material production (Shamim 2018). Therefore, adsorption turns out to be the best strategy with great potential (Rajendran et al. 2022). Various methods based on biosorption have currently been evaluated (Qin et al. 2020). It is the removal of heavy metals or other pollutants from solutions, either by surface adsorption or infiltration into the middle of the cell. In the first process, metals are linked with extracellular polymers, while in the second process, intracellular accumulation occurs. Surface adsorption occurs quickly in the first phase and can be followed by high metal accumulation in the middle of the cell (Tarekegn et al. 2020).
Bacteria are one of the microbes that possess excellent adsorption capacity due to their high surface-to-volume ratio and the good number of active chemical adsorption sites in their cells (Kushwaha and Kashyap 2021). According to Tarekegn et al. (2020), various bacteria isolated from metal-contaminated environments and their efficacy compared to isolated species in this study are shown in Table 1. The microbial species B. megaterium, S. ginsenosidimutans and K. rhizophila have been reported to have a potential role in bioremediation. The biosorption capacity of several bacterial species, including B. megaterium was found to remove several heavy metal ions (Tarekegn et al. 2020). As well, heavy metals tested on K. rhizophila showed multi-metal resistance ability, genetic analysis of K. rhizophila revealed that it contains genes associated with tolerance to different concentrations of heavy metals (50–500 mg/L) (Afridi et al. 2021).
However, in one of the studies it was shown that Sphingobacterium spp can thrive in high concentrations of metals, including Cu and Cr (Mgbodile et al. 2022). More evidence is needed on the ability of S. ginsensidmutans to grow and resist multiple metals. There is still room for S. ginsensidmutans to be discovered with greater capabilities and trapping traits. Moreover, there is a need to investigate and compare the multiple uptake capacities of various indigenous biosorbents isolated from contaminated sites, as well as which stage of bacterial growth can achieve the best sorption.
Since the two previous species are Gram positive and more metals are uptaken by Gram positive bacteria due to the presence of glycoproteins, this supported their selection to study metal remediation. While it was reported that less metal uptake by Gram negative bacteria is observed due to phospholipids and lipopolysaccharides (LPS). Therefore, there is a need to verify the capabilities of these isolates (Shamim 2018). The complete comparison of the bioremediation efficacy of three isolates with different forms in multi-metal solutions compared with single metals is the basis and uniqueness of the study. In general, the effects of the simultaneous presence of multiple chemicals have not received as much attention as the effects of single metals. The effects of multiple chemicals are more important than single effects and should not be neglected (Adnan et al. 2021). Although these conditions are representative of the real-life environment, there is not enough data for combined toxicological interactions for heavy metals, and there is not much literature indicating the interactions of heavy metals with the biosorbent in the composite mixtures. It was reported that the sorption of metal ions by biological materials is limited to single-metal solutions; multi-metal solutions such as binary, ternary, and quaternary solutions are extremely rare; and evaluation methodologies make it difficult to draw meaningful conclusions (Costa and Tavares 2018).
One of the first experiments was to evaluate the combined toxicity of a quaternary mixture comprising the metals Cu, Zn, Ni and Cr at the highest concentrations of this mixture. Based on their highest tolerance levels and lowest toxicity, three bacterial isolates (B. megaterium, S. ginsenosidimutans, and K. rhizophila) were selected for a comprehensive comparison of the efficiency of the isolates in removing individual metals and quaternary from aqueous solutions. In addition, to determine the living biomass capacity of three isolates as singles and consortiums in metal sorption, as well as the maximum time and rate of sorption. The biosorbents were investigated using FTIR and SEM–EDX spectroscopy to elucidate the diverse reaction mechanisms. Potential bioremediation mechanisms, including those for biosorption and bioaccumulation mechanisms of bacterial species, can be disclosed by measuring their sorption capacities during periods of metallic stress to which they were particularly exposed throughout the growth phases.
Materials and methods
Bacteria and culture media
The bacterial isolates employed in the present study were isolated from electroplating industrial effluents. Bacterial isolates were initially obtained from nutrient agar plates (Oxoid, Lab-Lemco Powder) incorporated with 10, 50, and 100 mg/L concentrations of Cu, Zn, Ni, and Cr as individual metals and as a quaternary solution at 37 °C for 24 h. The isolates that were able to tolerate and grow at high concentrations were chosen for further study. They were maintained on nutrient agar slants at 4 °C for further use. Based on the 16S rDNA data, bacterial isolates were identified and confirmed to belong to the following genera and species: Microbacterium paraoxydans (NR_025548.1), Streptomyces werraensis (NR_112390), Microbacterium arabinogalactanolyticum (NR_0449321), Staphylococcus haemolyticus (NR_036956.1), Bacillus paramycoides (NR_1577341), Bacillus megaterium (NR_117473.1), Sphingobacterium ginsenosidimutans (NR_108689.1), Kocuria rhizophila (NR_026452.1), and Sphingobacterium detergens (NR_118238). Bacillus megaterium, Sphingobacterium ginsenosidimutans, and Kocuria rhizophila were selected based on the tolerance levels and toxicity limits achieved by the isolates. To evaluate the bioremediation potential of Cu, Zn, Ni and Cr individually and in quaternary over intervals of 1–48 h. As well as examining the maximum sorption rate and required time in multi-metal resistant bacteria.
Preparation of standardized cultures from bacterial isolates
The suspensions of nine isolates were grown to exponential phase in nutrient broth on a rotary incubator (PROTECH, Model CSI 100) at 150 rpm and 37 °C, while pH and temperature were at 7.0 and 37 °C, respectively. Thereafter, the cultures were harvested by centrifugation (Hettich Zentrifuge, Universal 320) at 4000 rpm for 10 min. The supernatant was discarded, and the harvested cells or pellets were washed twice using sterilized saline water to avoid any residual nutrients (Hoseini et al. 2020). The washed cells were resuspended in sterilized saline water, and turbidity was adjusted to give an optical density of 0.6 at 600 nm using a spectrophotometer (Genesys 20, Thermo Spectronic). Bacterial suspensions have been used as inoculum in measurements of biosorption and bioaccumulation. All experiments were performed using standardized cultures (OD = 0.6) in order to equalize the enzyme activity. They were carried out twice, and the mean values for each experiment were recorded (Marzan et al. 2017; Sharma and Dwivedi 2017).
Inoculum preparation for consortium
Adopting a modified method from Nwanyanwu et al. (2017), approximately 1% of the isolated bacteria were cultured overnight in nutrient broth on a rotating shaker (150 rpm) at 37 °C. The cells were then extracted by centrifugation at 4000 rpm for 10 min. Cells were harvested, and washed twice in sterile double-distilled water before being suspended in it. In a sterile Erlenmeyer flask, equal quantities (1 mL) of each standardized cell suspension were combined together to form a bacterial consortium of three isolates. To obtain the bacterial consortium employed in this study, the optical density for all cell suspensions was adjusted to 0.6 at 600 nm by dilution of the bacterial suspension with sterile double-distilled water.
Preparation of heavy metal solutions
Stock solutions of 1000 mg/L concentrations of Zn,Cu, Cr, and Ni were prepared by dissolving zinc sulfate (ZnSO4), copper sulfate (CuSO4), potassium chromate (K2Cr2O7) (R&M Chemical), and nickel chloride (NiCl2) (HmbG Chemical) in double-distilled water. Thereafter, the solutions were shaken for 15 min and then left to stand for 24 h to obtain complete dissolution. Metal solutions were sterilized by a membrane filter and stored at 4 °C (Abd El Hameed et al. 2015).
Heavy metals tolerance and growth of bacterial isolates
Overnight isolates with an O.D = 0.6 were inoculated in a nutrient broth containing sterile heavy metals of Zn, Cu, Cr, and Ni (10, 50, and 100 mg/L). In each culture medium flask, the metals were added separately and quaternary in a 250-mL Erlenmeyer flask and incubated at 37 °C for 24 h. The degree of tolerance of bacterial isolates was determined by measuring the optical density at 600 nm with metal-free bacterial culture.
Estimation of metals toxicity and maximum tolerance
The degree of bacterial resistance and toxicity of metals were also determined at high concentrations (100, 300, and 500 mg/L) of quaternary mixed metals by measuring the OD at 600 nm with metal-free bacterial cultures. The most tolerant and least toxic isolates were selected for quantifying the metal sorption rate during their growth intervals under metallic stress. The following equation was used as described by Sannasi et al. (2010):
where Y is the bacterial growth rate at metal concentrations (mg/L); C and a are the growth rates without metal in the control; the B variable factor (inverse concentration; L/mg) is an indicator of metal toxicity to bacteria at metal concentrations. An analysis of variance (ANOVA) and the Tukey test (p ≤ 0.05) (SPSS version 25) were used to determine the significance of the difference for selected heavy metals on tolerance levels and toxicity within isolates at high concentrations.
Assays of biosorption, bioaccumulation and the maximum time and sorption rate
Standard cultures were prepared from three bacterial isolates (OD = 0.6), including B. megaterium, S. ginsenosidimutans, and K. rhizophila, individually and in consortium. Aliquots of a 1 mL overnight suspension of the isolate bacterial culture were inoculated in 100 mL of nutrient broth medium containing 100 mg/L concentrations of Zn2+, Cu2+, Cr2+, and Ni2+ as a primary and quaternary solution in a 250 mL Erlenmeyer flask (Jaafar et al. 2015; Fadel et al. 2015; Murthy et al. 2014). The Erlenmeyer flasks were incubated at 37 °C at 150 rpm for 24 h. Aliquots of 5 mL were extracted from the same flask each time and at the established intervals T1, T6, T12, T24, and T48 h. After that, each extracted sample was centrifuged at 5000 rpm for 15 min to separate the suspensions into supernatant and pellets. Each component was treated independently in order to determine the maximum time as well as the biosorption and bioaccumulation rates of the tested bacteria.
Determination of biosorption
The metal-amended cultures were centrifuged to separate the supernatant and precipitate, and the residual metal concentration in the supernatant liquids was estimated based on the difference between the initial and final metal concentrations which was considered as a metal bound biosorption material. The procedure was as follows: The supernatants were collected and digested using a double volume of 67% HNO3 and 30% H2O2 v/v. The mixtures were heated to 100 °C on a hotplate stirrer to accomplish acid digestion until the final volume decreased to the initial supernatant volume. Then, the extracts were filtered through filter paper (Whatman, 42) to remove any insoluble substances and gathered into a volumetric flask, where they were diluted (Jaafar et al. 2015). The residual metal concentrations in the extracts were analyzed by ICP-MS, and the observations of supernatants were conducted within the time periods 1, 6, 12, 24, and 48 h. To distinguish the maximum sorption rates achieved between these time periods with each isolate, all experiments were conducted in triplicate. The amount of metal bound by the biosorbent was determined as follows:
where: I = initial metal concentration, F = final metal concentration (Olukanni et al. 2014).
Determination of bioaccumulation
The pellets were washed twice with sterile acidified water (100 mL of 0.1 M HCl in double distilled water) to remove any metal precipitated or adsorbed and residues of growth medium on the cell surface. Thus, any trace of metal on the surface may be removed, leaving just the metal accumulating in the cells that can be quantified. Thereafter, the pellets were resuspended in 1 mL of distilled water and dried in an oven (memmert) at 65 °C for 20 h before being weighted. The dried cells were placed into a 100-mL beaker, and 5 mL of concentrated nitric acid was added for 5 min on medium heat. The heat was raised until the vapors appeared for a short time and a white residue was formed. The beaker was allowed to cool for 2 min before restarting digestion with 2 mL of concentrated nitric acid. After the brown fumes stopped appearing, the beaker was cooled again for 2 min before adding 2 mL of 1:1 hydrochloric acid (37%) (acid mixture concentrated HCl /H2O). The mixture was heated at a medium rate for 3 min. After that, it was cooled to room temperature and made up to 10 mL with distilled water. According to Jaafar et al. (2015), the metal content of the bacterial cells was determined after acid dissolution of the cells. Metal concentrations were measured by ICP-MS. The metal accumulation was then calculated using this equation:
where E. Con = the concentration of heavy metals in sample (mg/g), A initial concentration of heavy metals, B final concentration of sample (mg/L), D dry weight of sample (gm).
Lyophilization (freeze-drying) of bacterial isolates
Aliquots of 10 g of dry skim milk (Himedia™) were mixed with 100 mL of distilled water and sterilized by autoclaving. The bacterial isolates from the exponential phase were centrifuged at 10,000 rpm for 5 min and washed three times with sterile double distilled water. Then the supernatants were discarded, whereas the bacterial pellets were resuspended with 0.5 mL of double -distilled water and an equal volume of 10% skim milk solution. Resuspended bacteria combined with skim milk were separated into clear Amber glass bottles, then the tubes were transferred to a freezer at − 80 °C (ESCO LEXICON ULT. Model: UUS-552A-1-SS), thereafter lyophilized in a Labconco freeze dryer (Model: Flourtop type) then stored at − 20 °C until further use (Xu et al. 2021).
Fourier transform infrared (FTIR) spectroscopy observation
Overnight bacterial cultures modified with individual and quaternary metals at 100 mg/L plus the unmodified cultures were centrifuged at 10,000 rpm for 5 min, and pellets were lyophilized (Sodhi et al. 2020). Sample disks were made from 2 mg of the bacterial pellets in 200 mg of KBr powder and analyzed using FTIR (Li et al. 2018). Fourier Transform Infrared Spectroscopy (FTIR spectra) was used to provide a qualitative and preliminary characterization of the primary functional chemical groups found in living biomass and responsible for heavy metal biosorption. Functional groups that were responsible for metal absorption in the metal-laden and unladen bacteria (B. megaterium, S. ginsenosidimutans, and K. rhizophila) were analyzed on a Perkin Elmer (FT-IR) spectrometer in the region of 400–4000 cm−1 (Perkin Elmer Spectrum 100 FTIR, Watham, MA, USA).
Scanning electron microscopy energy-dispersive X-ray spectroscopy (SEM–EDX)
The bacterial species B. megaterium, S. ginsenosidimutans, and K. rhizophila were cultured in 100 mg/L of Cu2+, Cr6+, Ni2+, and Zn2+ individually and quaternary with a control to examine the change in morphology on the surface after metallic stress. After 24 h, the cultures were centrifuged at 10,000 rpm for 5 min, and the supernatants were discarded, whereas the formed pellet was washed with phosphate buffer saline (PBS, 0.1 M) to remove the heavy metal from the suspension. The pellet was fixed overnight with 2.5% glutaraldehyde (25%; Q R C®) QREC, (ASIA) SDM BHD, then washed three times with PBS (pH 7.2), centrifuged, and collected. Pellet dehydration by serial dilution with 30%, 50%, 70%, 90%, and 100% ethyl alcohol, with each ethyl alcohol concentration incubated for 10 min while the last two concentrations were incubated for 20 min. The supernatant was decanted and left in the sample microfuge tube with the cells overnight in a desiccator to air-dry at room temperature. The dried cells were then mounted on a SEM sample stub with double-sided sticky tape. To detect changes in cell morphology caused by metal ion binding, the sample was sputtered with a gold sputter coater (Ion sputter coater, Hitachi E-1010) and examined in a SEM (SEM HITACHI SU-1510). The elemental analysis and ion distribution mapping of the investigated samples were carried out using EDX (EDX Oxford Xplore 30) (Sodhi et al. 2020).
Statistical analysis
The data obtained on the bioaccumulation and biosorption of single and quaternary metals at 100 mg/L concentration and with different time intervals, by single bacterial isolates and consortium were subjected to statistical analysis using the SPSS program (SPSS Version 25). The data were analyzed through an analysis of variance (ANOVA) and a post hoc Tukey test to analyze the differences in the biosorption and bioaccumulation rates within isolates. To detect the statistical significance of differences (P \(\le 0.05)\) between means.
Potential sources of error in the experiments included measurement error, sampling error, and systematic error. To ensure the accuracy of the results and the validation of the assumptions, the first steps were careful experiment planning, the use of more accurate instruments, and the careful calibration of instruments before making measurements to avoid a problem in the experimental setup or procedure, which represents one of the systematic errors, and careful control of external variables such as temperature changes or vibrations. Taking many measurements, averaging them, using larger sample sizes, and repeating sampling to ensure that the sample is representative of the bacterial culture.
Quality control (QC) and quality assurance (QA)
Standard quality control (QC) and quality assurance (QA) procedures were followed throughout the laboratory experiments to ensure the reliability and accuracy of the laboratory tests and the results obtained. As a result, certified reference materials, blanks, laboratory reagent blanks, calibration standards, and analysis replications were employed as needed. The following is a detailed description of the QC/QA techniques used. The initial step was to soak all laboratory glassware and centrifuge tubes in a 10% v/v HNO3 bath overnight, followed by three rinses with double-distilled water. Furthermore, all chemical solutions and dilutions were prepared using analytical grade quality chemicals and double-distilled water. Each heavy metal analysis measurement was performed in triplicate, and the mean values were approved if the error was less than 10%. Instrument accuracy was confirmed using standard reference materials to avoid/minimize instrumental error.
Results and discussion
In this study, bacteria were isolated from the electroplating wastewater of electroplating factories in Serdang City, Selangor State, Malaysia. The isolates were tested in terms of their ability to grow, tolerate to multiple metals, including Cu, Zn, Ni, and Cr as quaternary metals, and the degree of toxicity that occurs after exposure to high concentrations of metals, which affects the activity and survival of bacteria. Bacterial growth potential and metal sorption are critical tests for the successful deployment of these living bacteria as effective bioremediation tools for the removal of metal contamination. The earliest investigations to choose the most tolerant isolates involved evaluating the growth, tolerance limits and toxicity levels of bacterial isolates.
Bacterial growth rate at different concentrations of heavy metals
Measuring the optical density per mL (OD = 600) was used to measure the viability of isolates under the action of single and quaternary metals. That is because it is fast, inexpensive, and does not cause any harm to the tested bacteria (Sutton 2011). The bacterial growth was compared with a blank, free-metal nutrient growth medium at different concentrations during the 24 h of incubation or the end of the exponential phase. The results of the effect of metal concentrations of 10, 50, and 100 mg/L individually and quaternary on viability are presented in Table 2. The isolates exhibited higher densities at 10 mg/L, but there were relatively low optical density values for all metals with increased metal concentrations compared to the state where no metal is present, which showed that the growth of bacteria was reduced as a result of that.
The growth was performed in triplicate, and the findings were expressed as the mean ± standard deviation (SD). The results showed a low standard deviation in the data sets, indicating that the data points are generally close to the mean or median value. Where there was less variance in the data points and dispersion below the mean.
Toxicity level and tolerance limits of metal quaternary
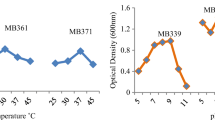
The results for tolerance and toxicity at high concentrations of quaternary metals are shown in Fig. 1a, b. Heavy metal toxicity levels (B) and tolerance limits (1/B) were investigated in nine isolates; the highest B values indicated the highest toxicity. Toxicity values B are inversed to reflect the highest theoretical concentration of metal ions that bacteria can withstand (Sannasi et al. 2010). All isolates showed the ability to tolerate quaternary metals, especially at 100 mg/L compared to the other concentrations. The highest tolerance and lowest toxic effect at 100 mg/L quaternary were, respectively, 1.919 mg/L in B. megaterium, 1.499 mg/L in K. rhizophila and 1.451 mg/L in S. ginsenosidimutans. While tolerance levels were reduced at 300 mg/L concentrations with the isolates, respectively, to 0.732 mg/L, 0.726 mg/L, and 0.746 mg/L. At the highest meal concentration of 500 mg/L, these isolates were also more tolerant than others (0.732 mg/L, 0.683 mg/L, and 0.746 mg/L), as illustrated in Fig. 1a. While the toxicity values presented in Fig. 1b showed that quaternary toxicity increased significantly when metal concentrations exceeded 100 mg/L, all isolates were able to grow in all tested concentrations of quaternary, demonstrating their ability to resist multiple metals. Relatively, the isolates of B. megaterium, S. ginsenosidimutans, and K. rhizophila were the most tolerant and had the least toxicity. Figure 2a–c depicted microscopic images of these isolates using Gram Stain, 1000 × magnification and a light microscope (Omax-Microscope).
The important factor affecting the toxicity and tolerance in bacterial cells is the concentration of the metal. The growth rate of bacteria is strongly influenced by the concentration of metal ions, and the growth is often inversely proportional to the toxicity of heavy metals. It was confirmed that different types of microbic cells respond differently to metallic stresses (Zhang et al. 2022). Furthermore, bacterial tolerance to heavy metals varies depending on the type of bacteria and metals. Thus, the efficacy of microbial remediation varies depending on the type of microbe, resistance, nature, level, and synergistic toxicity of heavy metals (Kapahi and Sachdeva 2019; De Sliva et al. 2012).
The results of the statistical analysis of tolerance and toxicity levels at 100 mg/L quaternary were significantly different (p ≤ 0.05) from 300 and 500 mg/L. The concentrations of 300 mg/L were also significantly different (p ≤ 0.05) from those of 500 mg /L. There were significant differences in tolerance limits and toxicity levels in the isolates when exposed to all the high concentrations of the quaternary. All bacterial isolates were able to grow and tolerate high quaternary concentrations at different levels. The results obtained support the fact that microbes exposed to a diverse mixture may have synergistic, antagonistic, or collective effects on cell existence and activity (Nweke et al. 2017).
Biosorption assay of single bacteria and consortium
Live cellular biomass is actively used to remove heavy metals through biosorption (Priyadarshanee and Das 2020). Regardless of whether the biomass is dead or alive, biosorption is a surface phenomenon that allows heavy metals to be absorbed at the cellular surface. Mechanisms of biosorption include surface adsorption as a result of a physical reaction (electrostatic reaction or van der Waals reaction), a chemical reaction (ion exchange displacement of attached metal cations), complexation, diffusion, or precipitation (Mustapha and Halimoon 2015). In the biosorption process, heavy metals are passively adsorbed on the surface without needing energy expenditure until equilibrium is attained (Velásquez and Dussan 2019; Jeyakumar et al. 2023).
The isolates that achieved the highest tolerance and lowest toxicity were selected to measure the biosorption of copper, zinc, nickel and chromium individually and quaternary during the time periods 1, 6, 12, 24 and 48 h. The bacterial capacity of three isolates: B. megaterium, S. ginsenosidimutans and K. rhizophila was assessed at the highest initial concentration of 100 mg/L, because it has been reported that it is always necessary to determine the maximum saturation of the biosorbent to be tried with the highest possible initial metal concentration (Zango et al. 2020). The maximum biosorption time and rate at different growth stages were also studied.
The results revealed that the isolates had a good ability to absorb heavy metals. As a result, the cellular surface normally has a negative charge that attracts heavy metals aggressively, whereas in certain situations, the cellular surface may consist of a mucus or polysaccharide layer that significantly adsorbs heavy metals through physical interactions. Figure 3a–e shows the biosorption of a single bacteria and a consortium.
Furthermore, bacterial cell surfaces contain functional groups such as phosphates, sulfates, amides, hydroxyls, or negatively charged proteins that exchange ions with metal ions, resulting in strong ionic interaction and allowing heavy metal reduction (Kanamarlapudi et al. 2018; Jeyakumar et al. 2023). In addition, the results showed that biosorption depends on the type of metal and also on time. The complexation mechanism has been reported to occur due to the aggregation of two or more metal species and functional groups on the cell surface. Monodentate and polydentate complexes are two types of complexes most commonly involved in biosorption (Kanamarlapudi et al. 2018). In monodentate complexes, the metal ion makes covalent bonds in the center with the ligands, whereas multiple metal ions bind to the ligands of polydentate complexes at several places (Jeyakumar et al. 2023).
Metal biosorption differences were minor between individual isolates, with increased biosorption of particular metals occurring after 6 h in all single isolates. In general, B. megaterium achieved higher biosorption rates, which were 83.73% Ni, 72.69% Zn, 72.27% Cr, 75.49% quaternary, and 67.25% Cu. Cells in the logarithmic phase were absorbed at a faster rate (6–12 h) than cells in the stationary phase (24–48 h). The exponential (log) phase was reported to be the most active part of the cell growth curve, in which cells multiply rapidly. This is the ideal stage of growth since all components of the cell are growing at the same rate, enzymes are available, and they are at their peak of activity (Sakthiselvan et al. 2019). In contrast, depletion of nutrients and accumulation of toxic metabolic products are the most characteristic features of the stationary phase which leads to decreased bacterial growth compared to the previous logarithmic phase (Shaifali 2021).
Isolates achieved the highest biosorption with Ni, Zn, and quaternary compared to Cu and Cr. Although the synergistic effect of multi-metal, which could be due to the increases in the total metal ion concentration compared to the single metal ion systems, leads to a significant difference in concentration between the cell surface and the metal solution, the latter could be a strong driving force for further metal uptake in multi-metal systems (Mishra and Malik 2013). Furthermore, isolates in the exponential phase outperformed cells in the stationary phase in terms of biosorption. A consortium of isolates also showed higher biosorption in the exponential phase than single isolates. These results can be attributed to the other fact that microbial cells at different stages of growth can have different membrane lipid structures, cell metabolisms, and cell wall structures, which may directly affect their susceptibility to toxic chemicals. Thus, these changes may be one of the contributing factors influencing the biosorption rate of toxic pollutants (Fan et al. 2014). The results showed that the rate of biosorption was faster in the early phases of growth, which might be attributed to the abundance of metal ions and unsaturated or vacant metal binding sites on the biosorbent. Then the other phase is slower, which may be due to saturation of the metal binding sites. Metal absorption increases with time and reaches a maximum at equilibrium. At this time, the absorption value remains constant (Jaafar et al. 2015).
The biosorption capacity varies according to the type of metal, single or quaternary, as well as the form of the bacteria. The duration of maximum biosorption for the individual metals and quaternary by the consortium was 6 h, while for the quaternary it was 12 h for all the single isolates. It was reported that the microbial consortium has become an important technology because it degrades pollutants more effectively than a single strain (Liu et al. 2021). Moreover, it was reported that in the natural environment, bioremediation is often carried out by a microbial consortium rather than by individual species, and various species perform distinct functional roles. Co-cultivation of the microbial consortium is more effective than single microbes, destroys contaminants quicker, and can greatly improve metal remediation (Zhang et al. 2021; Varjani et al. 2021).
As shown in Fig. 3a–e, statistical analysis revealed a significant difference in metal biosorption (p ≤ 0.05); the Tukey test determined that the biosorption of consortium was significantly different from that of single bacteria, while there was no significant difference (p ≤ 0.05) between single bacteria.
Bioaccumulation assay of single bacteria and consortium
Bioaccumulation is a natural and active metabolic process in living biomass. The process is more complicated since it involves energy-dependent respiration in the cytoplasm via the cell metabolic cycle. Bioaccumulation occurs when the rate of contaminant intake exceeds the rate of loss (Pham et al. 2022). The metabolic activity in this process allows for the active transport of contaminants over the membrane into the cell interior, where they can be sequestered by proteins. Pollutants can collect inside the cell in this manner (bioaccumulation). That is because enzyme activity is conserved in live biomass, differing enzymatic activities may affect the condition of the pollutant (biodegradation and biotransformation). Using living biomass as a biosorbent will allow for greater removal of contaminants, which is a significant advantage when using this type of biomass (Torres 2020).
Since concentrations of 100 mg/L were used to determine the biosorption rate, the same concentrations were used to determine the total amounts of metals transported into the cell. Thus, bound amounts to the cell surface in the first biosorption phase can be compared to accumulated amounts in the second phase (bioaccumulation phase), where the bioaccumulation process includes two stages.
The results of metal accumulation revealed that bacterial isolates accumulated quantities of single and quaternary metals over time. Isolates accumulated the most during the logarithmic phase and then declined during the stationary period. Single isolates accumulated the greatest metals in time intervals (24–48 h), while a consortium took 12–24 h. Metal accumulation in single isolates rose in parallel with increasing the time to 12 h for Ni, 24 h for Cr, and 48 h for quaternary. Cu and Zn accumulation occurred in single isolates between 24 and 48 h. In contrast, accumulation with the consortium took the shortest period of 12 h for Cu, Ni, and Cr and 24 h for Zn and the quaternary. The rate of accumulation differed slightly between isolates; for example, B. megaterium was able to accumulate the most Cu and Ni, which were, respectively 0.291 mg/g and 0.159 mg/g at 12 h in comparison to 0.105 mg/g and 0.222 mg/g for Cu and 0.137 mg/g and 0.148 mg/g at 12 h for Ni with both S. ginsenosidimutans and K. rhizophila. Figure 4a–e shows the results in more detail.
At 24 h, the single isolates were able to accumulate high amounts of Cr in K.rhizophila and B. megaterium, respectively, 0.155mg/g and 0.149mg/g. Zn accumulated the least among metals, with the maximum concentrations in B. megaterium reaching 0.131 mg/g after 48 h. The single isolates were likewise able to acquire equal amounts of quaternary, with B. megaterium accumulating the most at 0.119 mg/g. The varied rates of accumulation in isolates could be attributed to different types of metals and their toxicity to bacterial isolates, or to chemical metal characteristics that cause uneven metal tolerance and accumulation among isolates.
Metal accumulation by bacterial isolates was the highest in the consortium and decreased with increasing time. This is due to reaching a saturation point, and hence the accumulation reduces. During 12 h, the consortium achieved the maximum Cu accumulation of 0.399 mg/g and the highest Ni accumulation of 0.374 mg/g. Furthermore, the consortium displayed good accumulation capability with Cr and quaternary, with 0.287 mg/g at 12 h and 0.266 mg/g at 24 h, respectively. Metal ion binding and transport into living cells are two phases in the bioaccumulation process, making it more time-consuming and complex than biosorption. A consortium of bacterial species is more effective than a single species for performing complex bioaccumulation processes. Combining microorganisms to produce microbial consortiums with varying removal capabilities allows the benefits of each strain to be combined to achieve high efficacy in pollution remediation. When compared to a single microbial strain, the consortium exhibits good contamination tolerance and synergistic activity to reduce the accumulation of intermediate products, which boost the metabolic activity of the consortium and hence generate better contaminant uptake (Zhang et al. 2022).
The analysis of variance of bioaccumulation of metals between single isolates and consortium, Fig. 4a–e, using one way Anova and Tukey HSD tests showed that there were significant differences (p \(\le 0.05)\) in bioaccumulation of individual metals and quaternary by single isolates (B. megaterium, S. ginsenosidimutans, and K. rhizophila) and consortium.
Fourier transform infrared (FTIR) spectroscopy observation
The (FTIR) spectra of metal laden and metal free isolates were obtained in the range of 400–4000 cm−1. FTIR spectra can be used to identify functional groups on the bacterial cell surface and assess their interactions with single metals and quaternary compounds. FTIR spectra of metal laden biomass B. megaterium, S. ginsenosidimutans, and K. rhizophila resulted in prominent peaks, the large peaks was 3426 cm−1 and the lowest was 544 cm−1. Figures 5a–c depict the results of infrared (IR) spectra obtained from FTIR for the three isolates, which show a number of absorption peaks.
a FT-IR spectra the absorbance of Bacillus megaterium in the absence of metal and in metals laden cells (100 mg /L). b FT-IR spectra the absorbance of S. ginsenosidimutans in the absence of metal and in metals laden cells (100 mg/ L). c FT-IR spectra the absorbance of K rhizophila in the absence of metal and in metals laden cells (100 mg/ L)
The results in three isolates showed that the peaks corresponded to absorption caused by N–H, C–H, and O–H single bonds in the range of (4000–2500 cm−1), which are able to interact with cations of metals. There were distinct peaks in the range of 2000–1500 cm−1 which correspond to absorption caused by double bonds such as C=O, C=N and C=C. Add to the large number of absorption peaks that account for a large variety of single bonds, C–C, C–N and C–O, these peaks were in the range (1500–400 cm−1). Figure 5a-c shows a number of absorption peaks for bacterial isolates. The FTIR absorption wavelengths of each peak and corresponding functional groups are presented in Table 3 for B. megaterium, S. ginsenosidimutans, and K. rhizophila,, respectively.
The results of the spectra showed that there were clear shifts in the bands and a decrease in intensity in the bands. FTIR peaks were identified in all isolates with single metals and quaternary, and they displayed data on functional groups, chemical bonds, and the structure of substances responsible for the interaction. (Nandiyanto et al. 2019). Through figures of FTIR spectra, the changes in the absorption peak frequencies can be seen due to the fact that metal binding causes a change in the biosorption frequencies. These transformations in the biosorption observed indicate metallic bond processes that take place at the active sites on the cell surface. FTIR spectra analysis showed the presence of ionized functional groups capable of reacting with metal ions. This means that these functional groups can be an effective tool for removing metal ions.
The FTIR spectra of three isolates, B. megaterium, S. ginsenosidimutans, and K. rhizophila, were recorded with and without metals. Broad spectra bands were observed with three isolates in the range between 3300 and 3500 cm−1, indicating the presence of a bonded hydroxyl group and the stretching bond of the –NH from an amino group (Li et al. 2018; Fan et al. 2014). The spectra had the tendency to shift to higher frequencies after contact with individual metals and quaternary at the range 2800–2900 cm−1 which corresponded to asymmetrical CH stretching vibration in lipids (CH2, CH3) (Suriya et al. 2013; Li et al. 2018). The band between 1500 and 1800 cm−1 showed shifts for three isolates, which were considered to be due to the existence of amide II (NH) and carboxylic groups (Nandiyanto et al. 2019) and carboxylic groups (Chowdhury et al. 2012). The peaks between 1640 and 1540 cm−1 could be assigned to amide groups in proteins (Masoumi et al. 2016). The band between 1500 and 1000 cm−1 were showed both minor and higher shifts in isolates, which represent C–O stretching of bacteria under different metals (Xu et al. 2021). In addition, in the band that ranged between 1400 and 500 cm−1 there were significant shifts that might be caused by the organic phosphates (P=O stretch). It was reported that carbohydrates are responsible for a board stretch of 1000 and 1125 cm−1 (C–O–C, C–O). The presence of uronic acid and o-acetyl ester linkage bonds, in particular, is determined by the peaks between 1000 and 1125 cm−1 (Ramya et al. 2022). In addition, The peaks at 1000–500 cm−1 appear to be changes at this band that might be caused by aliphatic organohalogen compounds, aliphatic chloro compounds, C–Cl stretch, or aromatic C–H stretch.
In summary, after metal adsorption occurred, the overall FTIR spectra analysis indicated the involvement of functional groups such as hydroxyl and carboxyl groups of saccharides, amino and amide groups of proteins, phosphate groups, and sulfate groups in the interaction of metals with bacteria. Moreover, the FTIR spectra for metal-free and metal-loaded cells clearly showed the difference, possible interactions between individual metals, quaternary and functional groups on the cell wall, and the potential for adsorption. The FTIR spectra of non-metal and metal-laden bacteria revealed that metallic stress induction in the microbial sample caused functional groups and chemical alteration. The spectral data thus confirms the presence of amine, hydroxyl, carbonyl, carboxyl, phosphate, sulfate, and compounds of aromatic C-H and aliphatic groups (CH2 and CH3) in the bacterial isolates that were responsible for biosorption and the binding of metals to cells.
Scanning electron microscopy (SEM–EDX) analysis
Scanning electron microscopy was utilized to examine the surface features and morphological changes of three bacterial isolates: B. megaterium, S. ginsenosidimutans, and K. rhizophila, all of which were subjected to metallic stress. The isolates were exposed to high concentrations of 100 mg/L of single metals and quaternary, in comparison with the same isolates when they were far from metallic stress conditions. SEM images of the biomasses with and without metal sorption at 10,000 × magnification and a tension of 15 kV are shown in Figs. 6, 7 and 8. The most prominent morphological changes seen after metal contact were aggregations and tight interconnection between cells, which were substantially more frequent in all heavy metal-laden isolates compared to metal-unladen isolates.
This is due to the fact that increasing the surface area creates more sites for metal absorption, which is considered an influencing factor of metal biosorption by bacteria and is proportional to metabolic and growth rates (Campbell 2002; Shamim 2018).
The metal stress caused changes in the appearance or shape of the cells, and the cells became swollen or thicker, which was more evident in B. megaterium. The surface became rougher, and the deposition of metal ions occurred on the cell surface, which was largely the case in S. ginsenosidimutans and K. rhizophila, especially when they were under the influence of quaternary stress. The deposited ions were associated with chemical functional groups on cell walls as a result of the ion exchange process. The changes in cell shape that occurred are a consequence of the bacterial response to metal stress and a protective mechanism against metal toxicity. The aggregation and precipitation on the cells were caused by metal ions binding to extracellular polysaccharides. Thus, these stressed cells can survive and tolerate the metal stress in addition to carrying out their usual metabolic activities (Ramya and Thatheyus 2018).
The determination of the elemental composition of biomass was performed using EDX. The presence of exopolysaccharides and proteins in biomass can be determined by the signals of carbon, nitrogen, and oxygen in EDX spectra. Unladen biomass showed no single metal or quaternary signals, while these could be detected in metal-laden biomasses through the EDX spectra. However, EDX spectra could only determine the presence or absence of metals in the biomass qualitatively, not quantitatively (Li et al. 2018). The nickel ions have more affinity and have achieved higher biosorption compared to other metals. It was reported that Ni has a high metabolic effect on living cells because it is a significant component in many enzymes. As well, various microbial cells can bind Ni by using different uptake mechanisms, including intracellular accumulation, straight biosorption on cell surfaces (through physical adsorption, complexation, or ion-exchange), and extracellular precipitation (Lusa et al. 2021). Bacillus megaterium has the highest affinity for nickel, which is supported by its high metal sorption capacity and resistance to multiple metals. Due to Ni-specific response genes like nccA and smtAB, Ni removal has been correlated with these genes (Fierros-Romero et al. 2016, 2019).
Nickel ions had a closer affinity with the functional groups on the cell surface of the bacterial isolates. Therefore, only the reaction results for nickel and biomass were shown during the SEM–EDX analysis instead of the other metals, as shown in Fig. 9a–f. At elevated concentrations of 100 mg/L, the percentage of absorbed metals was not high. When metal concentrations are high, free binding sites diminish, resulting in a drop in biosorption rate. Because of the presence of metal ions, all active sites on the cell surface are occupied as the metal concentration increases. As a result, there are no extra binding sites to attract additional metal ions, and these sites are not available for additional metal ion biosorption. Add to that the fact that functional groups capable of binding to heavy metals differ in their affinity and specificity for metal binding and that ionic size is a factor leading to differences in biosorption among metals (Khan et al. 2016; Abbas et al. 2014; Díaz et al. 2022).
Moreover, it was found that some elements were initially available in metal-unladen biomass but were not observed in metal-laden biomass, and some of them decreased their percentage compared to the control. This could be explained by certain ions replacing others that were previously present on the biomass surface. In addition, it was also clarified that some elements decreased in the metal-laden biomass, which indicates that the mechanism of ion exchange plays a role in the sorption of metal ions.
Practical implications, challenges and recommendations
This research focuses on biological approaches to heavy metal removal. It additionally offers an extensive examination of the role of naturally occurring bacteria as a strategy that requires little effort, is less labor-intensive, inexpensive, environmentally beneficial, economical, and relatively simple to execute. To tackle the serious and rising industrial contamination of aquatic habitats and to treat and restore polluted environments. In this study, it was remarkable that a consortium of indigenous bacteria was able to improve and accelerate the bioremediation process. However, heavy metals might exert hazardous effects on living bacterial cells, as the high metal concentration used and the potential success of bioremediation are the most critical difficulties of the present research. Despite the importance of bacterial bioremediation, there are a number of future challenges that may be faced, the majority of which are related to its slow and time-consuming nature, the difficulty of treating some inorganic pollutants and thus determining whether the pollutants have been eliminated or not, and the toxicity of metals to bioprocessors when they are in their live forms. As well, bioremediation performance evaluation might also be difficult because there is no accepted end point. New research is needed to improve bioremediation methods and identify new biological solutions for bioremediation of heavy metal contamination in various ecosystems. Improving or optimizing an environment for growth in order to boost the efficacy of bacterial bioremediation. Thus, the volume of biomass increases, overcoming the slowness and death of bioremediators and allowing the treatment to be completed in the least amount of time with the least amount of damage to the activity and existence of living cells.
Conclusion
The purpose of this study was to assess the metal treatment capabilities of multi-metal-resistant bacterial species isolated from electroplating effluents based on their highest tolerance and lowest toxicity to the metal mixture. To assess the rate of metal sorption in single isolates and consortiums at high concentrations of individual metals and quaternary metals during their growth phases, biosorption and bioaccumulation mechanisms were examined. Fourier Transform Infrared (FTIR) spectroscopy and scanning electron microscopy (SEM) were used to further evaluate the biosorbents. The results showed that isolates as living biomasses could tolerate, absorb, and accumulate both single and quaternary metals. Maximum time and sorption rate were influencing factors in the efficiency and speed of the metal sorption. The best bioremediation outcomes were obtained during the exponential period and with the bacterial consortium. The findings supported the potential benefits of employing the living biomasses of three isolates, B. megaterium, S. ginsenosidimutans, and K. rhizophila, for heavy metal uptake and efficient pollutant removal from effluent. SEM and FTIR revealed that metal stress causes morphological alterations in bacterial isolates, including aggregations, interconnections, and cell shape changes. EDX probes revealed that bacterial isolates had a high affinity for nickel and attained higher biosorption than other metals, while FTIR spectra revealed functional group and biosorption frequencies. The presence of at least amide, sulfate, phosphate, hydroxyl, carboxylic acid, amine, aliphatic compounds, methylene, carbonyl, and others on the surface of multimetal-tolerant bacteria was discovered. The findings of this study demonstrated that isolated bacterial species can be used as an effective and low-cost biosorbent for eliminating multimetal pollution.
References
Abd El Hameed AH, Eweda WE, Abou-Taleb KAA, Mira HI (2015) Biosorption of uranium and heavy metals using some local fungi isolated from phosphatic fertilizers. Ann Agric Sci 60(2):345–435. https://doi.org/10.1016/j.aoas.2015.10.003
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Technol 3(4):74–102
Adnan NA, Halmi MIE, Abd-Gani SS, Zaidan UH, Abd-Shukor MY (2021) Comparison of joint effect of acute and chronic toxicity for combined assessment of heavy metals on Photobacterium sp.NAA-MIE. Int J Environ Res Public Health 18:6644. https://doi.org/10.3390/ijerph18126644
Afridi MS, Van Hamme JD, Bundschuh J, Khan MN, Salam A, Waqar M, Chaudhary HJ (2021) Biotechnological approaches in agriculture and environmental management-bacterium Kocuria rhizophila 14ASP as heavy metal and salt-tolerant plant growth-promoting strain. J Biol 76(10):3091–3105
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem 4:1–14. https://doi.org/10.1155/2019/6730305
Aniyikaiye TE, Oluseyi T, Odiyo JO, Edokpayi JN (2019) Physico-chemical analysis of wastewater discharge from selected paint industries in Lagos, Nigeria. Int J Environ Res Public Health 16(7):1235. https://doi.org/10.3390/ijerph16071235
Bhat B, Parveen S, Hassan T (2018) Seasonal assessment of physicochemical parameters and evaluation of water quality of river Yamuna, India. Adv Environ Technol 4(1):41–49
Campbell PG, Errécalde O, Fortin C, Hiriart-Baer VP, Vigneault B (2002) Metal bioavailability to phytoplankton–applicability of the biotic ligand model. Comp Biochem Physiol C Toxicol Pharmacol 133(1–2):189–206. https://doi.org/10.1016/s1532-0456(02)00104-7
Chowdhury A, Bhowal A, Datta S (2012) Equilibrium, thermodynamic and kinetic studies for moval of copper (II) from aqueous solution by onion and garlic skin. Water 4:37–5. https://doi.org/10.14294/WATER.2012.4
Costa F, Tavares T (2018) Biosorption of multicomponent solutions: a state of the art of the understudy case. In: Derco J (Ed) Biosorption, pp. 51–68. IntechOpen.68261. https://doi.org/10.5772/intechopen.72179.
De Silva AAL, de Carvalho MA, de Souza SA, Dias PM, da Silva Filho RG, de Meirelles Saramago CS, de Melo Bento CA, Hofer E (2012) Heavy metal tolerance (Cr, Ag AND Hg) in bacteria isolated from sewage. Braz J Microbiol 43(4):1620–1631. https://doi.org/10.1590/S1517-838220120004000047
Díaz A, Marrero J, Cabrera G, Coto O, Gómez JM (2022) Biosorption of nickel, cobalt, zinc and copper ions by Serratia marcescens strain 16 in mono and multimetallic systems. Int Biodeterior 33(1):33–43. https://doi.org/10.1007/s10532-021-09964-9
Elbasiouny H, Darwesh M, Elbeltagy H et al (2021) Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: a review. Environ Monit Assess 193:449. https://doi.org/10.1007/s10661-021-09236-2
Fadel M, Hassanein NM, Elshafei MM, Mostafa AH, Ahmed MA, Khater HM (2015) Biosorption of manganese from groundwater by biomass of Saccharomyces cerevisiae. HBRC J 13(1):106–113
Fan J, Okyay TO, Rodrigues DF (2014) The synergism of temperature, pH and growth phases on heavy metal biosorption by two environmental isolates. J Hazard Mater 279:236–243
Fierros-Romero G, Gómez-Ramírez M, Arenas-Isaac GE, Pless RC, Rojas-Avelizapa NG (2016) Identification of Bacillus megaterium and Microbacterium liquefaciens genes involved in metal resistance and metal removal. Can J Microbiol 62:505–513. https://doi.org/10.1139/cjm-2015-0507
Fierros-Romero G, Gómez-Ramírez M, Sharma A, Pless RC, Rojas-Avelizapa NG (2019) czcD gene from Bacillus megaterium and Microbacterium liquefaciens as a potentialnickel–vanadium soil pollution biomarker. J BasicMicrobiol. https://doi.org/10.1002/jobm.201900323
Gheethi AA, Efaq AN, Mohamed RM, Abdel-Monem MO, Abdullah AH, Amir Hashim M (2017) Bio-removal of nickel ions by Sporosarcina pasteurii and Bacillus megaterium, a comparative study. In: IOP conference series: materials science and engineering, vol. 226(1), p. 012044. IOP Publishing.
Haq F, Butt M, Ali H, Chaudhary HJ (2016) Biosorption of cadmium and chromium from water by endophytic Kocuria rhizophila: equilibrium and kinetic studies. Desalin Water Treat 57(42):19946–19958. https://doi.org/10.1080/19443994.2015.1109561
Hasan MM, Uddin MN, Ara-Sharmeen I, Alharby HF, Alzahrani Y, Hakeem KR, Zhang L (2019) Assisting phytoremediation of heavy metals using chemical amendments. Plants (Basel) 8(9):295. https://doi.org/10.3390/ijms20143412
Hoseini AA, Kaboosi H, Ahmady-Asbchin S, Ghorbanalinezhad E, Ghadikolaii FP (2020) Binary biosorption of Cadmium(II) and Nickel(II) onto Planococcus sp. isolated from wastewater: kinetics, equilibrium and thermodynamic studies. Ind Biotechnol 16:386–393. https://doi.org/10.1089/ind.2020.0021
Jaafar R, Al-Sulami A, Al-Taee A, Aldoghachi F, Napes S (2015) Biosorption and bioaccumulation of some heavy metals by Deinococcus Radiodurans isolated from soil in Basra Governorate- Iraq. J Biotechnol Biomater 5:190. https://doi.org/10.4172/2155-952X.100019
Jeyakumar P, Debnath C, Vijayaraghavan R, Muthuraj M (2023) Trends in bioremediation of heavy metal contaminations. Environ Eng Res 28(4):22063. https://doi.org/10.4491/eer.2021.631
Kanamarlapudi SL, KumarChintalpudi V, Muddada S (2018) Application of biosorption for removal of heavy metals from wastewater. In: Derco J, Vrana B (eds) Biosorption, vol 69. IntechOpen, London
Kapahi M, Sachdeva S (2019) Bioremediation options for heavy metal pollution. J Health Pollut 9(24):191203. https://doi.org/10.5696/2156-9614-9.24.191203
Khan Z, Rehman A, Hussain SZ, Nisar MA, Zulfiqar S, Shakoori AR (2016) Cadmium resistance and uptake by bacterium, Salmonella enterica 43C, isolated from industrial effluent. AMB Express 6:54. https://doi.org/10.1186/s13568-016-0225-9
Kushwaha P, Kashyap PL (2021) A review of advances in bioremediation of heavy metals by microbes and plants. J Nat Resour Conserv Manag 2(1):65–80. https://doi.org/10.51396/ANRCM.2.1.2021.65-80
Li X, Li D, Yan Z, Ao Y (2018) Biosorption and bioaccumulation characteristics of cadmium by plant growth-promoting rhizobacteria. RSC Adv 8(54):30902–30911. https://doi.org/10.1039/c8ra06270f
Liu Z, Zhou A, Wang S, Cheng S, Yin X, Yue X (2021) Quorum sensing shaped microbial consortia and enhanced hydrogen recovery from waste activated sludge electro-fermentation on basis of free nitrous acid treatment. Sci Total Environ 766:144348. https://doi.org/10.1016/j.scitotenv.2020.144348
Lusa M, Bomberg M (2021) Microbial community composition correlates with metal sorption in an ombrotrophic boreal bog: implications for radionuclide retention. Soil Syst 5:19. https://doi.org/10.3390/soilsystems5010019
Marzan LW, Hossain M, Mina SA, Akter Y, Chowdhury AM (2017) Isolation and biochemical characterization of heavy-metal resistant bacteria. Egypt J Aquat Res 43(1):65–74. https://doi.org/10.1016/j.ejar.2016.11.002
Masoumi F, Khadivinia E, Alidoust L, Mansourinejad Z, Shahryari S, Safaei M, Mousavi A, Salmanian AH, Zahiri HS, Vali H, Noghabi KA (2016) Nickel and lead biosorption by Curtobacterium sp. FM01, an indigenous bacterium isolated from farmland soils of northeast Iran. J Environ Chem Eng 4:950–957. https://doi.org/10.1016/j.jece.2015.12.025
Meepho M, Sirimongkol W, Ayawanna J (2018) Samaria-doped ceria nanopowders for heavy metal removal from aqueous solution. Mater Chem Phys 214:56–65. https://doi.org/10.1016/j.matchemphys.2018.04.083
Mgbodile CF, Otutu U, Onuoha S, Eze U, Ugwuoji T, Nnabuife O, Nwagu TNT (2022) Hydrolases secreting, heavy metal-resistant halophilic bacteria isolated from metal dumpsites. Asian J Trop Biotechnol 19(1):11–19
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43(11):1162–1222. https://doi.org/10.1080/10934529.2011.627044
Murthy S, Bali G, Sarangi SK (2014) Effect of lead on growth, protein and biosorption capacity of Bacillus cereus isolated from industrial effluent. J Environ Biol 35(2):407–411
Mustapha MU, Halimoon N (2015) Microorganisms and biosorption of heavy metals in the environment: a review paper. J Microb Biochem Technol 7:253–256. https://doi.org/10.4172/1948-5948.1000219
Nandiyanto A, Oktiani R, Ragadhita R (2019) How to read and interpret FTIR spectroscope of organic material. Indones J Sci Technol 4(1):97–118. https://doi.org/10.17509/ijost.v4i1.15806
Nugroho AP, Butar ESB, Priantoro EA et al (2021) Phytoremediation of electroplating wastewater by vetiver grass (Chrysopogon zizanoides L.). Sci Rep 11:14482. https://doi.org/10.1038/s41598-021-93923-0
Nwanyanwu CE, Adieze IE, Nweke CO, Nzeh BC (2017) Combined effects of metals and chlorophenols on dehydrogenase activity of bacterial consortum. Inter Res J Biol Sci 6(4):10–20
Nweke CO, Mbachu IAC, Opurum CC, Mbagwu CL (2017) Toxicity of quaternary mixtures of metals to aquatic microbial community. Int J Environ Res 6(3):30–37
Olukanni DO, Agunwamba JC, Ugwu EI (2014) Biosorption of heavy metals in industrial wastewater using micoorganisms (Pseudomonas aeruginosa). Am J Sci Ind Res 5(2):81–87
Paschoalini AL, Bazzoli N (2021) Heavy metals affecting Neotropical freshwater fish: a review of the last 10 years of research. Aquat Toxicol 237:105906. https://doi.org/10.1016/j.aquatox.2021.105906
Pham VHT, Kim J, Chang S, Chung W (2022) Bacterial biosorbents, an efficient heavy metals green clean-up strategy: prospects, challenges, and opportunities. Microorganisms 10(3):610. https://doi.org/10.3390/microorganisms10030610
Priyadarshanee M, Das S (2020) Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: a comprehensive review. J Environ Chem Eng. https://doi.org/10.4172/1948-5948.1000219
Qin H, Hu T, Zhai Y, Lu N, Aliyeva J (2020) The improved methods of heavy metals removal by biosorbents: a review. Environ Pollut 258:113777. https://doi.org/10.1016/j.envpol.2019.113777
Rajendran S, Priya AK, Senthil Kumar P et al (2022) A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-a review. Chemosphere 303(Pt 2):135146. https://doi.org/10.1016/j.chemosphere.2022.135146
Ramya D, Thatheyus JA (2018) Microscopic investigations on the biosorption of heavy metals by bacterial cells: a review. Sci Int 6:11–17
Ramya D, Thatheyus AJ, Juliana SJB, Kiruba NJM, Selvam ADG (2022) Physical characterization and kinetic studies of Zn (II) biosorption by Morganella morganii ACZ05. Water Sci Technol 85(4):970–986. https://doi.org/10.2166/wst.2022.031. (PMID: 35228348)
Roșca M, Silva B, Tavares T, Gavrilescu M (2023) Biosorption of hexavalent chromium by Bacillus megaterium and Rhodotorula sp. inactivated biomass. Processes 11(1):179. https://doi.org/10.3390/pr11010179
Sakthiselvan P, Meenambiga SS, Madhumathi R (2019) Kinetic studies on cell growth. In: Cell growth. IntechOpen, London.
Sannasi P, Salmijah S, Kader J (2010) Effect of heavy metals to bacterial culture and the removal of heavy metals from an industrial effluent. Biosci Biotech Res 7(2):543–557
Shaifali CKG (2021) Microbial remediation of heavy metals. Int J Chem Stud 9(2):92–107. https://doi.org/10.22271/chemi.2021.v9.i2b.11706
Shamim S (2018) Biosorption of heavy metals. Biosorption 2:21–49. https://doi.org/10.5772/intechopen.72099
Sharma N, Dwivedi A (2017) Bioremediation of dairy wastewater for nitrate reduction. World J Pharm Pharm Sci 3(1):375–384
Sodhi KK, Kumar M, Singh DK (2020) Multi-metal resistance and potential of Alcaligenes sp. MMA for the removal of heavy metals. SN Appl Sci 2:1885. https://doi.org/10.1007/s42452-020-03583-4
Suriya J, Bharathiraja S, Rajasekaran R (2013) Biosorption of heavy metals by biomass of Enterobacter cloacae isolated from metal-polluted soils. Int J ChemTech Res 5(3):1229–1238
Sutton S (2011) Measurement of microbial cells by optical density. J Valid Technol 17(1):46–49
Tarekegn MM, Salilih FZ, Ishetu AI (2020) Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food Agric 6:1783174
Torres E (2020) Biosorption: a review of the latest advances. Processes 8(12):1584. https://doi.org/10.3390/pr8121584
Varjani S, Pandey A, Upasani VN (2021) Petroleum sludge polluted soil remediation: integrated approach involving novel bacterial consortium and nutrient application. Sci Total Environ 763:142934. https://doi.org/10.1016/j.scitotenv.2020.142934
Velásquez L, Dussan J (2019) Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J Hazard Mater 167:713–716. https://doi.org/10.1016/j.jhazmat.2009.01.044
Xiao L, Guan D, Chen Y et al (2019) Distribution and availability of heavy metals in soils near electroplating factories. Environ Sci Pollut Res Int 26(22):22596–22610. https://doi.org/10.1007/s11356-019-04706-0
Xu H, Xie Z, Guo J, Men Q (2021) Morphological changes and bioaccumulation in response to cadmium exposure in Morchella spongiola, a fungus with potential for detoxification. Can J Microbiol 67(11):789–798. https://doi.org/10.1139/cjm-2020-0571
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci 11:359. https://doi.org/10.3389/fpls.2020.00359
Zango UU, Yadav M, Sharma V, Sharma JK, Panwar S, Dan S, Sharma AK (2020) Microbial bioremediation of heavy metals: emerging trends and recent advances. Res J Biotech 15(1):164–178
Zhang C, Wu X, Wu Y, Li J, An H, Zhang T (2021) Enhancement of dicarboximide fungicide degradation by two bacterial cocultures of Providencia stuartii JD and Brevundimonas naejangsanensis J3. J Hazard Mater 403:123888. https://doi.org/10.1016/j.jhazmat.2020.123888
Zhang T, Zhang H (2022) Microbial consortia are needed to degrade soil pollutants. Microorganisms 10(2):261. https://doi.org/10.3390/10020261
Zhang H (2014) Biosorption of heavy metals from aqueous solutions using keratin biomaterials. Dissertation, Universitat Autònoma de Barcelona (UAB), Barcelona.
Acknowledgements
This study was sponsored by Fundamental Research Grant Scheme (FRGS) [(KPT Reference Code: FRGS/1/2019/STG05/UPM/02/11) (Vote Number: 5540228) (Project Code: 01-01-19-2103FR)] awarded by the Ministry of Higher Education, Malaysia (MOHE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflicts of interest, and all the authors are interested to publish the manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Maryam Shabani.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alhammadi, E., Halimoon, N., Johari, W.L.W. et al. Potentially applicable bioremediation mechanisms for metal-tolerant bacteria from industrial waste electroplating. Int. J. Environ. Sci. Technol. 21, 4817–4836 (2024). https://doi.org/10.1007/s13762-023-05313-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05313-w