Abstract
Two unicellular cyanobacteria Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942 showed contrasting responses to chromate stress with EC50 of 12 ± 2 and 150 ± 15 μM potassium dichromate respectively. There was no depletion of chromate in growth medium in both the cases. Using labeled chromate, very low accumulation (<1 nmol/108 cells) was observed in Synechocystis after incubation for 24 h in light. No accumulation of chromate could be observed in Synechococcus under these conditions. Chromate oxyanion is known to enter the cells using sulfate uptake channels. Therefore, inhibition of sulfate uptake caused by chromate was monitored using 35S labeled sulfate. IC50 values of chromate for 35sulfate uptake were higher in Synechococcus as compared to Synechocystis. The results suggested that the sulfate transporters in Synechococcus have lower affinity to chromate than those from Synechocystis possibly due to differences in affinity of sulfate receptors for chromate. Bioinformatic analyses revealed presence of sulfate and chromate transporters with considerable similarity; however, minor differences in these may play a role in their differential response to chromate. In both cases the IC50 values decreased when sulfate concentration was reduced in the medium indicating competitive inhibition of sulfate uptake by chromate. Interestingly, Synechococcus showed stimulation of growth at concentrations of chromate less than 100 μM, which affected its cell size without disturbing the ultrastructure and thylakoid organization. In Synechocystis, growth with 12 μM potassium dichromate damaged the ultrastructure and thylakoid organization with slight elongation of the cells. The results suggested that Synechococcus possesses efficient strategies to prevent entry and to remove chromate from the cell as compared to Synechocystis. This is the first time a differential response of Synechococcus 7942 and Synechocystis 6803 to chromate is reported. The contrasting characteristics observed in the two cyanobacteria will be useful in understanding the basis of resistance or susceptibility to chromate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium is a member of transition metals and exhibits various oxidation states from +2 to +6, of which +3 and +6 are predominant in chromium compounds. Hexavalent chromium (Cr6+) is highly soluble and hence toxic; it usually exists as oxyanions such as chromate (CrO4 2−) and dichromate (Cr2O7 2−) whereas the trivalent form (Cr3+) is less soluble, less toxic and is found in the form of oxides, hydroxides or sulfates (Cheung and Gu 2007). The hexavalent form is released in the environment as aqueous waste from leather, paints, electroplating and other industries. Chromate is highly mobile and hence available, resulting in biological toxicity mainly due to oxidative damage to biomolecules (Cervantes et al. 2001). Microorganisms have developed different strategies to thrive in the presence of chromate in the aquatic environment. One of these strategies deals with reduction of chromate to the less toxic chromium(III) by chromate reductase identified in diverse bacterial species (Cervantes et al. 2001). Another strategy in prokaryotes uses chromate efflux system using a plasmid encoded gene, chrA. ChrA belongs to a small family of proteins (CHR), which occur in bacteria and archaea and represents a novel kind of prokaryotic proton motive force driven chromate transporters. Several members of CHR superfamily have been shown to confer resistance to chromate (Nies et al. 1998; Alvarez et al. 1999; Rami’rez-Diaz et al. 2008).
Among photoautotrophs, cyanobacteria and algae isolated from chromate contaminated sites or some mutants are able to tolerate chromate to varied extent (Garnham and Green 1995; Khattar et al. 2004; Anjana et al. 2007; Yewalkar et al. 2007; Kiran et al. 2007, 2008; Ozturk et al. 2009). Some of these adsorb chromate on the surface or reduce chromate to chromium(III). Recently an investigation into mechanism of chromate resistance in Synechococcus elongatus showed that it possesses a homologue of chromate transporter gene srpC on pANL plasmid which harbors genes of sulfur metabolism (Aguilar-Barajas et al. 2012). Although overexpression of srpC conferred chromate resistance to E. coli by reducing chromate uptake, it did not complement E. coli cysA sulfate uptake mutant, suggesting that srpC is not sulfate transporter. Since chromate oxyanion is structurally related to sulfate, chromate actively crosses biological membranes by means of the sulfate uptake pathway (Rami’rez-Diaz et al. 2008; Aguilar-Barajas et al. 2011). This results in sulfate deficiency as well as in creating oxidative stress in cells. Thus a complex relation is known to exist between sulfate and chromate transporters vis a vis the effect of chromate in prokaryotes.
Cyanobacteria are some of the oldest organisms living on the earth and have evolved mechanisms to combat various environmental stresses. The comparative study on the relative responses of different cyanobacteria to variety of stresses is an interesting field of research. The standard cultures of two taxonomically related cyanobacteria Synechococcus PCC 7942 and Synechocystis sp. PCC 6803 are known to differ in their responses to salt, light and oxidative stress (Fulda et al. 1999; Stork et al. 2005). We observed these two standard strains also showed contrasting tolerance to chromate, Synechococcus being more tolerant than Synechocystis. In this paper we report differential responses of these two cyanobacteria to chromate with respect to its effect on growth, ultrastructure, chromate and sulfate uptake. The bioinformatic analysis of chromate and sulfate transporters is also presented.
Materials and methods
Analysis of chromate toxicity
The two strains of unicellular cyanobacteria, S. elongatus PCC 7942 and Synechocystis sp. PCC 6803 (referred to as Synechococcus and Synechocystis respectively hereafter) were inoculated in 50 ml of BG-11 medium (Rippka et al. 1979) and grown at 30 °C under continuous white light of intensity 21 W m−2. To study the effect of chromate on growth, ~108 cells of log phase culture were inoculated in BG-11 medium containing K2Cr2O7 ranging from 0 to 200 μM and growth was monitored periodically as cell density and expressed as number of cell ml−1 using Neubauer’s hemacytometer in the Zeiss Axio imager digital microscope. Growth was monitored as OD730 up to 30 days. EC50 value represents the concentration of potassium dichromate at which number of cells ml−1 was 50 % as compared to control.
Determination of 51chromate accumulation
Chromate uptake was determined by using 51chromium labeled chromate as a tracer. Log phase cultures of Synechococcus and Synechocystis were washed and incubated (108 cells ml−1) in light (21 W m−2) for 24 h at room temperature in fresh BG-11 medium containing 10 or 100 μM K2Cr2O7 with 20 μCi 51Cr labeled sodium chromate (0.24 μCi nmol−1). Cells were washed with BG-11 medium followed by 1 mM EDTA, resuspended in 90 % methanol and mixed with 175 μl of Perkin-Elmer’s Hidex aqualight cocktail. 51Chromate in the cells was estimated by counting β emission by using liquid scintillation counter.
Determination of IC50 of chromate for 35sulfate uptake
Inhibitory concentration (IC50) of chromate for sulfate uptake was determined by monitoring uptake of 35S labeled sodium sulfate. Log phase cultures of Synechococcus and Synechocystis were washed and suspended in fresh BG-11 medium (108 cells ml−1) containing 30 or 300 μM sulfate along with 40 μCi 35sodium sulfate (28.6 μCi μmol−1) and increasing concentration of chromate (0–10 mM) for 2 h in light at room temperature. Cell pellets were washed with BG-11 medium followed by 1 mM EDTA. The washed cells were resuspended in 90 % methanol and mixed with 175 μl of Perkin-Elmer’s Hidex aqualight cocktail. 35Sulfate in the cells was measured by liquid scintillation counting.
Transmission electron microscopy
Synechococcus and Synechocystis cells were grown with different concentrations of potassium dichromate for 9 days as indicated in figures. The cells were harvested and washed with sodium phosphate buffer (100 mM, pH 7.4), fixed with 0.5 % glutaraldehyde—2 % paraformaldehyde for 2 h at room temperature followed by washing with water. Serial dehydration was carried out using 35, 50, 75 and 100 % ethanol for 30 min each. Ethanol was removed by incubation with propylene oxide for 3 h followed by further incubation with 3:1, 1:1, 1:3 (v/v propylene oxide:araldite) for 2 h each. The samples were infiltrated with Araldite for 16 h and embedded in it by incubation at 60 °C for 72 h. Thin (70 nm) sections were contrasted for 15 min with 10 % uranyl acetate in 50 % methanol followed by staining with lead citrate (Reynolds 1963) for 2 min. The sections were viewed under Libra 120 keV transmission electron microscope.
Sequence comparison of sulfate and chromate transporters
Amino acid sequences of sulfate and chromate transporters from Synechococcus and Synechocystis were downloaded from cyanobase (http://genome.kazusa.or.jp/cyanobase) and compared with similar well characterized genes from other bacteria. Alignment and analyses of proteins were performed using BioEdit v7.0.8. Conserved domains were obtained using conserved domain database (CDD) available at http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml with CDDv2.3.CDD) and simple modular architecture research tool (SMART) available at http://smart.embl-heidelberg.de.
Results
Differential effect of chromate on growth of Synechococcus and Synechocystis
When log phase culture of Synechococcus and Synechocystis were inoculated in 50 ml of BG-11 medium, the exponential growth phase started after 4 days and lasted for about 30 days in untreated Synechococcus where as in case of untreated Synechocystis the log phase lasted for 15 days after which slow decline in growth was observed under similar growth conditions. The two organisms showed comparable growth rates in absence of chromate with doubling time in the range of 40–50 h till 15 days period indicating that both were growing normally under the conditions used for their growth. Growth was monitored as OD730 and also by measuring number of cells ml−1. Synechococcus cultures showed over twice the number of cells at equivalent OD730 as compared to Synechocystis culture suggesting difference in scattering properties of the cells.
To measure the effect of chromate on cell survival and growth, ~108 cells were inoculated in BG-11 medium containing different concentrations of chromate and growth was monitored by cell count.
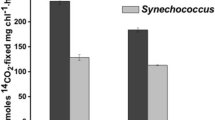
In case of Synechocystis survival was reduced in the presence of potassium dichromate at concentration as low as 10–20 μM. On the contrary in case of Synechococcus, cells could grow even in presence of 200 μM potassium dichromate (Fig. 1). In Synechococcus inclusion of potassium dichromate up to 100 μM in growth medium stimulated growth; highest stimulation was observed with 70–80 μM potassium dichromate. Concentration of potassium dichromate required to inhibit growth completely in Synechococcus was 300 μM. In case of Synechocystis, survival declined with increasing concentration of chromate and was completely inhibited in presence of 30 μM potassium dichromate. Since the growth rates of Synechocystis and Synechococcus were comparable under the experimental conditions, the growth inhibition seen in Synechocystis in presence of chromate was due to chromate toxicity rather than difference in growth characteristics between the two under normal conditions.
Tolerance of Synechococcus and Synechocystis to potassium dichromate. BG11 medium containing increasing concentration of potassium dichromate was inoculated with log phase cultures of Synechococcus and Synechocystis containing ~108 cells ml−1. Growth was monitored as number of cells ml−1. EC50 for potassium dichromate was calculated after growth for 9 days. The points represent average of four experiments and bars represent SE
Since both cultures showed exponential growth from 4 to 15 days, the concentration of potassium dichromate at which number of cells ml−1 was 50 % as compared to control was monitored after 9 days of inoculation and was used to compare the tolerance of Synechococcus and Synechocystis to chromium stress. The 9 days EC50 of potassium dichromate was 12 ± 2 μM for Synechocystis and 150 ± 15 μM for Synechococcus (Fig. 1). The EC50 values increased with time after inoculation as well as with increase in the size of inoculum (Table 1 on line resource; Fig. 1 on line resource).
Differential accumulation of chromate by Synechococcus and Synechocystis
Synechococcus and Synechocystis were grown in the presence of increasing concentration of potassium dichromate. Cell free medium from each culture was obtained and chromate concentration in the medium was determined using di phenyl carbazide (DPC) reduction assay as described by Urone (1955). There was no change in chromate concentration in the supernatant of the growth medium indicating that chromate was not absorbed or reduced by both the cyanobacteria (data not shown). Chromate accumulation was also monitored using radiolabeled chromate as a tracer. In Synechocystis after 24 h of incubation in light, the chromate accumulation up to 0.04–0.05 nmol 108 cells−1 was observed when extracellular concentration of chromate was 10 μM. This increased to 0.4 nmol 108 cells−1 when chromate concentration in the medium was increased to 100 μM (Fig. 2). In contrast, there was no accumulation of chromate in Synechococcus cells under these conditions (Fig. 2). In Synechocystis there was no accumulation of chromate when the cells were boiled in the medium or when they were incubated in dark (data not shown).
Uptake of 51chromate by Synechococcus and Synechocystis in light. Log phase cultures of Synechococcus and Synechocystis (108 cells ml−1) were incubated in BG11 medium containing 10 or 100 μM of potassium dichromate with 51Cr as tracer. The intracellular 51Cr was measured after incubation for 24 h in light at room temperature
Differential effect of chromate on uptake of sulfate by Synechococcus and Synechocystis
Sulfate uptake using 35S labeled sodium sulfate in the presence of varying concentrations of chromate was monitored in Synechococcus (Fig. 3a, b) and Synechocystis (Fig. 3c, d) incubated in medium containing 30 and 300 μM sodium sulfate. Chromate was found to decrease the uptake of sulfate in Synechococcus and Synechocystis. When concentration of sulfate was increased from 30 to 300 μM the concentration of chromate required for IC50 was increased from 30.35 μM to 1.7 mM in case of Synechococcus. In Synechocystis when concentration of sulfate was increased from 30 to 300 μM the concentration of chromate required for IC50 was increased from 8.6 to 99 μM.
Effect of chromate on uptake of 35S labeled sulfate by Synechococcus and Synechocystis. Log phase cultures of Synechococcus and Synechocystis (108 cells ml−1) were incubated in BG11 medium containing 30 or 300 μM sodium sulfate with 35S labeled sodium sulfate as tracer and different concentrations of potassium dichromate as mentioned in figure. Uptake of 35sulfate was monitored after 2 h. The average of four estimations is shown. Bars represent SE. a Synechococcus incubated in medium containing 30 μM sodium sulfate. b Synechococcus incubated in medium containing 300 μM sodium sulfate. c Synechocystis incubated in medium containing 30 μM sodium sulfate and d Synechocystis incubated in medium containing 300 μM sodium sulfate
Differential effect of chromate on ultrastructure of Synechococcus and Synechocystis
Ultrastructural changes in Synechococcus and Synechocystis grown with EC50 concentration of potassium dichromate were observed by transmission electron microscopy. The length of cells in case of Synechococcus was reduced whereas there was slight elongation of cells in case of Synechocystis while the cell width was not affected. In Synechococcus the cells grown with chromate did not show extensive damage to ultrastructure (Fig. 4a, b, c). The cell wall, thylakoids and carboxysomes were not affected by the presence of dichromate in medium. However, growth in the presence of chromate resulted in reduction of length to breadth (L/B) ratio (Fig. 4f). In Synechocystis growth in the presence of chromate resulted in distortions in thylakoid membranes and damage to cell wall in addition to slight elongation of regular spherical shape with increase in L/B ratio (Fig. 4d, e, f).
Ultrastructure of Synechococcus and Synechocystis cells grown with or without potassium dichromate. Log phase cultures of Synechococcus and Synechocystis containing 108 cells ml−1 were inoculated in BG11 medium containing potassium dichromate as mentioned below. Cells were harvested after 9 days of growth and were processed for transmission electron microscopy as described in “Materials and methods” section. a Synechococcus control. b Synechococcus grown with 75 μM of potassium dichromate c Synechococcus grown with 150 μM of potassium dichromate. Bars represent 500 nm. d Synechocystis control. e Synechocystis grown with 12 μM of potassium dichromate. Bars represent 200 nm. f Length to breadth ratio of Synechococcus and Synechocystis grown with above mentioned chromate concentrations in the growth medium. The values represent mean ratio obtained from electron micrographs of 15–20 cells and bars represent SE. Th thylakoids, Cb carboxysomes, Cw cell wall
Bioinformatic analysis of sulfate and chromate transporters in Synechococcus elongatus PCC 7942 and Synechocystis sp. PCC 6803
To investigate if the differences in the IC50 of chromate for sulfate uptake in the two organisms could be attributed to the differences in primary structure of the sulfate and chromate transporters, a comparative bioinformatic analysis of amino acid sequences of these proteins from Synechococcus and Synechocystis was carried out (Tables 1, 2); (Figs. 2, 3, 4, 5, 6 on line resource). The analysis included SulT permease constituting the permease, membrane proteins and ATPase; and chromate transporter ChrA. Their sequences were also compared with the sequences of well characterized sulfate and chromate uptake related proteins from other prokaryotic organisms.
Sulfate-thiosulfate transporters
Sulfate-thiosulfate (SulT) permease complex from some bacteria is well characterized and typically consists of sulfate or thiosulfate binding protein Sbp or CysP, and the proteins of ABC transporter viz CysA, ATPase, CysW and CysT. These were used as reference to identify differences among the corresponding proteins in the two organisms under study. Ten and nine genes for sulfate transporters are identified in Synechococcus and Synechocystis respectively in cyanobase (Tables 1, 2). The sulfate binding proteins (SbpA) in Synechococcus and Synechocystis have ~58.9 % identity. SbpA from these organisms showed 99 conserved amino acid residues with SbpA from Salmonella typhimurium (Fig. 2 on line resource). Cys A, the ATPase subunit of the SulT permease of Synechococcus and Synechocystis have ~63 % identity (Tables 1, 2). Figure 3 (on line resource) shows that there were 148 conserved amino acid residues among them and the CysA from Pseudomonas syringae. Bacterial CysT and CysW constitute the transport channel of the SulT permease typically with six transmembrane helices which are also present in CysT and CysW from Synechocystis. Synechococcus CysT and CysW show ~46–64 % identities with homologs from Synechocystis. All of these have six transmembrane helices except Synpcc7942_1687 (Cys T) which has seven transmembrane helices. Synechocystis and Synechococcus CysT and CysW showed 70 and 96 conserved amino acid residues with CysT and CysW from E. coli. (Figs. 4, 5 on line resource).
In addition to the conserved SulT components, the genomes of Synechococcus PCC 7942 and Synechocystis PCC 6803 show presence of some putative sulfate transporters, thiosulfate binding protein, low and high affinity sulfate transporter and sulfate permease (Tables 1, 2). Some of these have transmembrane domains suggesting a possible membrane location for them.
Chromate transporters
Chromate resistance in prokaryotes has been attributed to ChrA which is a chemiosmotic pump responsible for chromate efflux using proton motive pump (Alvarez et al. 1999). Two ORFs have been annotated as chromate transporters in Synechococcus and Synechocystis, one on plasmid and other on chromosome (Tables 1, 2). These showed ~29–40 % identity, and presence of two CHR domains containing homologous halves with membrane spanning regions which is a feature of long chain CHR family proteins in bacteria. The cyanobacterial chromate transporters showed 37 conserved amino acid residues when compared with chromate transporter from Pseudomonas aeruginosa (Fig. 6 on line resource).
Discussion
Chromate toxicity in aquatic organisms is mainly examined by exposing cultures to different concentrations of chromate for a given time, followed by investigations on physiological parameters. We compared two non-nitrogen fixing unicellular aquatic cyanobacteria belonging to order Chroococcales for their sensitivity to chromate when included in their growth media. Comparative analysis of chromate tolerance in S. elongatus PCC 7942 and Synechocystis PCC 6803 revealed that the former showed ~ 12 times higher tolerance to chromate than the latter, with EC50 values of 150 ± 15 and 12 ± 2 μM respectively. The EC50 values were dependent on the inoculum size in both cases indicating stoichiometric relation between chromate receptors per cell and number of chromate ions available in the growth medium. Thompson et al. (2002) have compared Synechococcus PCC 7942 and Nostoc PCC 7120 for resistance to chromate under high and low density conditions and have shown that there is general decrease in toxicity in dense cultures. Interestingly, stimulation in growth at less than 100 μM potassium dichromate was observed in Synechococcus. Lesser extent of ultrastructural damage in Synechococcus supports this argument.
In bacteria, two mechanisms of chromate tolerance are known: reduction of Cr(VI)–Cr(III) and efflux of chromate ions from cytoplasm (Rami’rez-Diaz et al. 2008). DPC assays showed that these two cyanobacteria were not chromate reducers. Only nanomolar concentration of chromate accumulation was detected in Synechocystis using 51chromate and accumulation was not observed if cells were killed or incubated in dark indicating involvement of an active process in uptake of chromate (data not shown). Although in Synechococcus, no chromate accumulation was observed under these conditions, there was stimulation of growth along with reduction of cell length when chromate concentration in medium was less than 100 μM. This entailed that Synechococcus was able to sense the presence of low concentration of chromate in the growth medium. These observations indicated that in Synechococcus there are efficient mechanisms for sensing and efflux of chromate. In contrast, Synechocystis cells showed deterioration of ultrastructure, reduction in growth and elongation of cells with 12 μM chromate in medium. Recently effects of chromate on cell morphology are reported in case of Rhodobacter sphaeroides by Italiano et al. (2012).
It is known that chromate ion enters the cells using sulfate transporters present in cell membrane as chromate ion is nearly identical in size shape and charge as sulfate ion (Riedel 1985). IC50 values of chromate in both Synechococcus and Synechocystis were dependent on sulfate concentration in medium indicating that sulfate uptake was competitively inhibited by chromate in both the cases. The inhibition of sulfate uptake by chromate suggested an interaction of chromate ions with sulfate uptake mechanism. However, the IC50 value of chromate was much higher in Synechococcus as compared to Synechocystis showing that Synechocystis has higher affinity for chromate as compared to Synechococcus. Sequence analysis showed that both the cyanobacteria have similar type of sulfate uptake channels and also contain similar genes related to efflux of chromate having typical bi-domain structure of LCHR. The differences in IC50 value of chromate suggested that the sequence variation in the non-conserved regions of sulfate uptake systems in both these organisms may be contributing to higher affinity of the sulfate uptake system for chromate in Synechocystis 6803 as compared to Synechococcus 7942. Synechococcus elongatus PCC 7942 possesses a plasmid (pANL) that contains a gene (srpC/chrA) conferring chromate resistance. The higher chromate susceptibility of Synechocystis cannot be attributed to absence of pANL as the sequence analysis shows that it has chrA homologues on its chromosome. Whether the location of chrA is responsible for the differential tolerance remains to be explored.
IC50 of chromate for sulfate uptake was higher in Synechococcus as compared to Synechocystis indicating that differential affinity of sulfate transporters for chromate may be contributing to the chromate tolerance in Synechococcus 7942 as compared to Synechocystis 6803. While IC50 for chromate at 10-fold increase in sulfate needed ~50-fold increase in chromate in case of Synechococcus, in case of Synechocystis with similar increase in sulfate, IC50 was attained by only ~12-fold increase in chromate concentration. Thus in Synechococcus the sulfate limitation by chromate would be avoided by the its sulfate transporters which have lower affinity to chromate as well as by the efficient chromate efflux systems resulting in higher EC50 for chromate. Although bioinformatic comparison of chromate and sulfate transporters revealed identity to a varying extent, the differences in their primary sequences could account for the difference in IC50 of chromate. Thus in addition to differences mentioned above, the different putative sulfate permease in these two organisms may play a role in their distinct chromate response. Chromate resistance of Synechococcus would give it growth advantage in chromate contaminated sites over Synechocystis.
Thus the multifarious interaction of sulfate/chromate transporters could be the basis of contrasting response of Synechococcus and Synechocystis to chromate.
In case of Synechococcus there was stimulation of growth at concentrations of chromate lower than 100 μM. It may be due to hormetic response characterized by low dose stimulation and high dose inhibition resulting in typical inverted U type dose response. Although biochemical mechanism of hormesis is not well understood it is possible that at low doses, the stressor activates repair processes which can repair the damage caused by chromate as well as other accumulated damages. Even though no chromate accumulation was seen in Synechococcus at low concentration, the effect on morphology and ultrastructure indicated presence of sensing mechanism and response. Another possibility may be that the reduced sulphate uptake due to chromate and subsequent adjustment of metabolic rate in Synechococcus has effect on growth. Also at low concentration, chromate may interact with growth regulators, a possibility which needs to be addressed in future.
Bioinformatic analysis of sulfate and chromate transporters of Synechococcus and Synechocystis shows the complexity of these transporters. Chromate resistance is a manifestation of numerous biochemical processes governed by different genes and their homologues. The complexity in composition of sulfate and chromate transporters and subsequent metabolic adjustments to sulfate deficiency in these two strains may be the cause of differential response of these two cyanobacteria. On this back ground, exploring the molecular basis of the contrasting chromate resistance in these two organisms and also to comprehend the phenomenon of stimulation of growth and induction of morphological changes in Synechococcus at low concentration of chromate is a challenge in future.
Summary
The standard strains of S. elongatus PCC 7942 and Synechocystis sp. PCC 6803 showed significant differences in the EC50 to chromate. There was difference in uptake of chromate; the resistant Synechococcus prevented entry by virtue of lower affinity of its sulfate transporters to chromate (IC50 was higher). Synechococcus probably also removed chromate from the cells more efficiently as no chromium was detected in the cells. The resistant type sensed chromate at low concentration and possibly brought about changes in metabolism which resulted in stimulation of growth in terms of cell numbers. Bioinformatic analysis showed that chromate transporters are present in both the organisms; however the location of the responsible genes is different. Also, there is 29–40 % identity in the chromate transporter genes. Whether and how much, the differences in location and sequence variations contribute to the functioning of chromate transporter needs to be seen. The components of sulphate transporters from the two organisms also revealed identity in the range of ~40–60 % indicating that there is adequate scope for variation which can account for the observed differences. Although the cell length in the resistant type was reduced, the ultrastructure of the cell was protected in presence of chromate. The results indicate that the large difference in response to chromate by the two organisms was due to a multi-component process. Although the comparison is between two genera, the large difference between the two makes it a suitable system for investigations on the resistance contributing factors and their interplay.
Abbreviations
- EC50 of chromate:
-
Concentration of potassium dichromate at which number of cells ml−1 was 50 % as compared to control
- IC50 of chromate:
-
Concentration of chromate required for 50 % reduction in uptake of sulfate as compared to control
- OD:
-
Optical density
References
Aguilar-Barajas E, Dı′az-Pe′rez C, Ramirez-Diaz M, Riveros-Rosas H, Cervantes C (2011) Bacterial transport of sulfate, molybdate, and related oxyanions. Biometals 24:687–707
Aguilar-Barajas E, Jeronimo-Rodriguez P, Ramirez-Diaz MI, Rensing C, Cervantes C (2012) The ChrA homologue from a sulfur-regulated gene cluster in cyanobacterial plasmid pANL confers chromate resistance. World J Microbiol Biotechnol 28:865–869
Alvarez AH, Moreno-Sanchez R, Cervantes C (1999) Chromate efflux by means of the ChrA chromate resistance protein from Pseudomonas aeruginosa. J Bacteriol 181:7398–7400
Anjana K, Kaushik A, Kiran B, Nisha R (2007) Biosorption of Cr(VI) by immobilized biomass of two indigenous strains of cyanobacteria isolated from metal contaminated soil. J Hazard Mater 148:383–386
Cervantes C, Compos-Garcia J, Devars S, Gutie’rrez-Corona F, Loza-Tavera H, Torres-Guzman JC, Moreno-Sanchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
Cheung KH, Gu JD (2007) Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeter Biodegrad 59:8–15
Fulda S, Huckauf J, Schoor A, Hagemann M (1999) Analysis of stress responses in the cyanobacterial strains Synechococcus sp. PCC 7942, Synechocystis sp. PCC 6803, and Synechococcus sp. PCC 7418: osmolyte accumulation and stress protein synthesis. J Plant Physiol 154:240–249
Garnham GW, Green M (1995) Chromate(VI) uptake and interactions with cyanobacteria. J Ind Microbiol 14:247–251
Italiano F, Rinalducci S, Agostiano A, Zolla L, Leo DF, Ceci LR, Trotta M (2012) Changes in morphology, cell wall composition and soluble proteome in Rhodobacter sphaeroides cells exposed to chromate. Biometals. doi:10.1007/s10534-012-9561-7
Khattar JIS, Sarma TA, Sharma A (2004) Effect of Cr6+ stress on photosynthetic pigments and certain physiological processes in the cyanobacterium Anacystis nidulans and its chromium resistant strain. J Microbiol Biotechnol 14:1211–1216
Kiran B, Kaushik A, Kaushik CP (2007) Response surface methodological approach for optimizing removal of Cr(VI) from aqueous solution using immobilized cyanobacterium. Chem Eng J 126:147–153
Kiran B, Rani N, Kaushik A (2008) Chromium(VI) tolerance in two halotolerant strains of Nostoc. J Environ Biol 29:155–158
Nies DH, Koch S, Wachi S, Pietzsch N, Saier MH (1998) CHR, a novel family of prokaryotic proton motive force-driven transporters probably containing chromate/sulfate antiporters. J Bacteriol 180:5799–5802
Ozturk S, Aslim B, Suludere Z (2009) Evaluation of chromium(VI) removal behavior by two isolates of Synechocystis sp. in terms of exopolysaccharide (EPS) production and monomer composition. Bioresource Technol 100:5588–5593
Rami’rez-Diaz MI, Diaz-Perez C, Vargas E, Riveros-Rosa H, Compos-Garcia J, Cervantes C (2008) Mechanisms of bacterial resistance to chromium compounds. Biometals 21:321–332
Reynolds ES (1963) The use of lead citrate at high pH as electron-opaque stain for electron microscopy. J Cell Biol 17:208–212
Riedel GF (1985) The relationship between chromium(VI) uptake, sulfate uptake and chromium (VI) toxicity in the estuarine diatom Thalassiosira pseudonana. Aquatic Toxicol 7:191–204
Rippka R, Deruelles J, Waterbury J, Herdman M, Stanier R (1979) Generic assignments strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Stork T, Michel KP, Pistorius EK, Dietz KJ (2005) Bioinformatic analysis of the genomes of the cyanobacteria Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942 for the presence of peroxiredoxins and their transcript regulation under stress. J Exp Bot 56:3193–3206
Thompson SL, Manning FCR, McColl SM (2002) Comparison of the toxicity of chromium(III) and chromium(VI) to cyanobacteria. Bull Environ Contam Toxicol 69:286–293
Urone PF (1955) Stabiltiy of colorimetric reagent for chromium, s-diphenylcarbazide, in various solvents. Anal Chem 27:1354–1355
Yewalkar SN, Dhumal KN, Sainis JK (2007) Chromium(VI)-reducing Chlorella spp. isolated from disposal sites of paper-pulp and electroplating industry. J Appl Phycol 19:459–465
Acknowledgments
The authors thank Dr. A. Ballal and Dr. R. Agarwal MBD, BARC, Mumbai for critical reading of the manuscript. We also thank Drs. S.F. D’Souza and J.S. Melo for useful suggestions. We thank Ms. N. Jadhav for sample preparation for Electron Microscopy. JKS acknowledges Department of Atomic Energy, India for Raja Ramanna Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, A., Bhagwat, S.G. & Sainis, J.K. Synechococcus elongatus PCC 7942 is more tolerant to chromate as compared to Synechocystis sp. PCC 6803. Biometals 26, 309–319 (2013). https://doi.org/10.1007/s10534-013-9614-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9614-6