Abstract
This study assessed the effects of different selenium (Se) supplementation levels on oxidative stress, cytokines, and immunotoxicity in chicken thymus. A total of 180 laying hens (1 day old; Mianyang, China) were randomly divided into 4 groups (n = 45). The chickens were maintained either on a basic diet (control group) containing 0.2 mg/kg Se, a low-supplemented diet containing 5 mg/kg Se, a medium-supplemented diet containing 10 mg/kg Se, or a high-supplemented diet containing 15 mg/kg Se for 15, 30, and 45 days, respectively. Over the entire experimental period, serum and thymus samples were collected and used for the detection of the experimental index. The results indicated that the antioxidative enzyme activities and messenger RNA (mRNA) levels of antioxidative enzymes, IFN-γ and IL-2 in the thymus, and the content of IFN-γ and IL-2 in the serum of excessive-Se-treated chickens at all time points (except for the 5 mg/kg Se supplement group at 15 days) were significantly decreased (P < 0.05) compared to the corresponding control groups. Interestingly, a significantly increase (P < 0.05) in the content of IFN-γ was observed in the serum and thymus in the 5 mg/kg Se supplement group at 15 and 30 days compared to the corresponding control groups. In histopathological examination, the thymus tissue from excessive-Se-treated chickens revealed different degrees of cortex drop, incrassation of the medulla, and degeneration of the reticular cells. These results suggested that the excessive Se could result in a decrease in immunity, an increase in oxidative damage, and a series of clinical pathology changes, such as cortex drop, incrassation of the medulla, and degeneration of the reticular cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential trace element for a wide range of species, including birds, and insufficient intake of Se in humans has been proven to be linked to “Keshan disease” and “White muscle disease” [1, 2]. Numerous studies have shown that Se plays an important role in chemoprevention [3, 4], neurobiology [5], aging [6], immune functions [7, 8], muscle metabolism [9], reproduction [10], redox reactions [11], and many other aspects of health [12, 13]. Many studies also demonstrated that adequate Se prevents cancers in animals and humans [14, 15]. However, Se is toxic at levels slightly above homeostatic requirement [16]. Excessive Se intake can result in adverse health problems, including such symptoms as loss of hair and nails, skin lesions, nervous system disorders, increased risk of adverse type-2 diabetes and cardiovascular disease, and even paralysis and death [2, 17, 18].
Se is important for many cellular processes because it is a component of several selenoproteins and selenoenzymes with essential biological functions [13, 19, 20]. Oxidative stress is defined as a disruption of the pro-antioxidant balance that leads to potential damage. The main antioxidative enzymes for detoxification of reactive oxygen species (ROSs) in all organisms are superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). An essential component of GSH-Px is Se [21]. The Se-deficiency-induced oxidative stress and antioxidant protection of selenoproteins such as GSH-Px are perceived to be involved in the pathogenesis of Se-deficiency-related diseases [22, 23]. Environmental contaminants, including excessive Se, can cause oxidative stress that leads to generation of free radicals and alterations in antioxidants or free oxygen radical scavenging enzyme systems [24]. Lipid peroxidation (LPO) has been suggested as one of the molecular mechanisms involved in Se-exposure-induced toxicity [25].
Histological changes in animal tissues provide a rapid method for detection of the toxicity effects of environmental contaminants in various organisms, because of the relationship between pollutant concentrations and animal tissue lesions [26, 27]. However, a lack of experimental findings exists for the histopathological impact of Se-exposure on animal tissues, including birds. In chickens, the thymus has important functions in implementation of immune defense and maintenance of a stable internal environment. Toxicopathic thymus lesions in bird species are sensitive signs of immune organ damage and have been used as biomarkers of chemicals in environmental risk assessments [28, 29].
Cytokines are major modulators of immune responses to infection, and therefore represent natural sources of immunostimulation that could be used as an adjuvant in vaccines. Interleukin 2 (IL-2) and interferon-γ (IFN-γ) are produced by T-helper-1 cells and are critical components in immune responses such as delayed hypersensitivity, macrophage activation, and enhanced non-specific immunity to parasites [30, 31]. Several studies on the messenger RNA (mRNA) levels of IL-2 and IFN-γ have been reported in chickens treated with environmental contaminants [32].
Previous studies on Se have focused primarily on Se deficiency in birds, but studies on the toxic effects of excessive Se in chickens are scarce in birds. Consequently, this study aimed to evaluate the effect of excessive Se on the oxidative status, histopathology, and cytokines of chicken thymus in vitro.
Materials and Methods
Animals and Experimental Design
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Southwest University of Science and Technology. A total of 180 laying hens (1 day old; Mianyang, China) were randomly divided into 4 groups (n = 45). Each group was separated into five pens (nine chickens per pen). The chickens were maintained either on a basic diet (control group) containing 0.2 mg/kg Se or a low-supplement diet containing 5 mg/kg Se, a medium-supplement diet containing 10 mg/kg Se, and a high-supplement diet containing 15 mg/kg Se for 15, 30, and 45 days, respectively. Over the entire experimental period, experimental animals were given free access to feed and water. On days 15, 30, and 45, blood and thymus tissue were collected from chickens in each group after sodium pentobarbital administration. The prepared serum was stored at −80 °C. The tissues were divided into three portions: one portion was retained for protein and antioxidant enzyme analysis, the second was fixed in Bouin’s solution for histological examination and cell apoptosis analysis, and the third was stored at −80 °C for RNA isolation.
Measurement of IL-2 and IFN-γ in Serum
The levels IL-2 and IFN-γ in the serum were measured according to the method of Chen et al. [33].
Measurement of Antioxidative Enzymes
Thymus samples were homogenized on ice in physiological saline and centrifuged at 700×g to collect supernatants for biochemical assays. Formation of MDA was determined as an indicator of LPO using the thiobarbituric acid assay [34] (MDA detection kit A003, Nanjing Jiancheng Bioengineering Institute). GSH-Px (GSH-Px assay kit A001-3, Nanjing Jiancheng Bioengineering Institute), CAT (CAT assay kit A007-1, Nanjing Jiancheng Bioengineering Institute), and SOD (SOD assay kit A003, Nanjing Jiancheng Bioengineering Institute) were determined using the method of Yao [35–37], according to the kit protocol. Protein concentrations of samples were measured using the Bradford method [38].
Histology Analysis
For histological examination, tissues were fixed in Bouin’s solution, dehydrated with a graded series of ethanol, cleared in xylene, and embedded in paraffin. Sections with 5–6-mm thickness were prepared from paraffin blocks using a Reichert microtome and subsequently stained with hematoxylin and eosin. Histopathological changes were examined under a Leica DME 100 light microscope.
Quantitative Real-Time PCR Analysis
The primers for real-time amplification of the CuZn-SOD, Mn-SOD, CAT, GSH-Px, IL-2, IFN-γ, and β-actin cDNA were designed using Oligo_6.0 Software (Molecular Biology Insights, Cascade, CO) based on the deposited sequences in GenBank (Table 1). The PCR products were electrophoresed on 2 % agarose gels, extracted, cloned into the pMD18-T vector (Takara, Ohtsu, Japan), and sequenced. BLASTX and BLASTN were used to determine the PCR assay specificity. The reaction specificity of each assay was verified by observing a single peak in the melting curve. The qRT-PCR work was conducted according to the MIQE guidelines.
Total RNA was isolated from the testes of each chicken using Trizol reagent according to the manufacturer’s instructions (Invitrogen). The RNA preparation, qPCR procedure, and relative mRNA abundance qualification processes were the same as previously described [39]. The chicken β-actin gene was used as an internal reference. The magnitude of change in gene expression relative to the controls was determined using the 2−△△Ct method reported by Livak and Schmittgen [40].
Statistical Analyses
Statistical analyses of all data were performed using SPSS for Windows (version 13, SPSS Inc., Chicago, IL). Data were expressed as the mean ± standard deviation, and differences were considered significant if P < 0.05. Tukey’s paired test was used to determine significant differences between different Se supplementation levels at the same time points in the same indicators.
Results
Histopathological Analysis
The general histological examination indicated a low to moderate incidence of thymus tissue damage of excessive-Se-treated chickens. The histopathological changes in the thymus tissue were shown in Fig. 1. Individuals in the control group exhibited intact structures with regular morphology and did not display any histological changes in the thymus tissues. In contrast, the thymus tissues from excessive-Se-treated chickens exhibited structural alterations, and the severity of the alterations increased with Se supplementation level. As shown in Fig. 1, the thymus tissue in excessive-Se-treated chickens revealed different degrees of cortex drop, incrassation of the medulla, and degeneration of the reticular cells.
Analysis of Antioxidative Enzymes in the Thymus
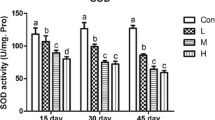
The effects of different Se supplementation levels on antioxidative enzyme activity and MDA content in the thymus tissue at all time points are summarized in Table 2. The activities of GSH-Px, SOD, and CAT in different Se supplement groups were significantly decreased (P < 0.05) compared to the corresponding control group at all time points, except for the 5 mg/kg Se supplement group at 15 days. Interestingly, a significantly increase (P < 0.05) in the activities of GSH-Px, SOD, and CAT was observed in the 5 mg/kg group at 15 days. The activities of GSH-Px, SOD, or CAT between different Se supplement groups showed a significant difference (P < 0.05) at the same time points. In contrast, the MDA content in different Se supplement groups was significantly increased (P < 0.05) compared to the corresponding control group at all time points, except for the 5 mg/kg group at 15 days. The MDA content between different Se supplement groups showed a significant difference (P < 0.05) at the same time points, except for the 10 and 15 mg/kg groups at 15 days.
The mRNA levels of antioxidative enzymes in chicken thymus under different Se supplementation levels are shown in Fig. 2. A significant change (P < 0.05) in the mRNA levels of GSH-Px, CuZn-SOD, Mn-SOD, and CAT between control group and Se supplement groups was observed at all time points, except for GSH-Px in 10 mg/kg group and CAT in the 5 mg/kg group at 30 days. The mRNA levels of GSH-Px, CuZn-SOD, Mn-SOD, and CAT in 5 mg/kg group were significantly increased (P < 0.05) compared to the corresponding control groups at 15 days, and the increases reached maxima. A significant difference (P < 0.05) in the mRNA levels of GSH-Px, CuZn-SOD, Mn-SOD, or CAT between different Se supplement groups at the same time points was observed except for CuZn-SOD in the 5 and 10 mg/kg groups at 45 days.
Effects of different Se supplementation level on the mRNA level of antioxidative enzymes of chicken thymus. Each value represents the mean ± SD of five individuals. Tukey’s paired test was used to determine significant differences between different Se supplementation levels at the same time points in the same indicators. The different small letters on top of the bars in panels a to d indicate significant differences (P < 0.05) in the mRNA level of antioxidative enzymes between different Se supplementation levels at the same time points. Those groups that share a common letter on top of the bars are not significantly different (P > 0.05)
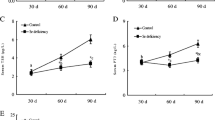
Analysis of IL-2 and IFN-γ Content in the Serum and IL-2 and IFN-γ mRNA Level in the Thymus
The concentrations of IL-2 and IFN-γ in chicken serum and thymus are summarized in Table 3. The contents of IFN-γ in different Se supplement groups in chicken serum and thymus were significantly decreased (P < 0.05) compared to the corresponding control groups at all time points except for the 5 mg/kg Se supplement group at 15 and 30 days. Interestingly, a significantly increase (P < 0.05) in the content of IFN-γ in the serum and thymus was observed in the 5 mg/kg Se supplement group at 15 and 30 days compared to the corresponding control groups. The content of IFN-γ between different Se supplement groups in the serum showed a significant difference (P < 0.05) at the same time points in the serum and thymus, except for the 5 and 10 mg/kg Se supplement groups at 45 days.
The contents of IL-2 in chicken serum and thymus in different Se supplement groups were significantly decreased (P < 0.05) compared to the corresponding control groups at all time points except for the 5 mg/kg Se supplement group at 15 days. In contrast, a significant increase (P < 0.05) in the content of IL-2 was observed in the 5 mg/kg Se supplement group at 15 days compared to the corresponding control group in the serum and thymus. The content of IL-2 between different Se supplement groups in the serum showed a significant difference (P < 0.05) at the same time points in the serum and thymus, except for the 5 and 10 mg/kg Se supplement groups at 30 days.
The effects of different Se supplementation levels on the mRNA levels of IL-2 and IFN-γ in chicken thymus are shown in Fig. 3. The mRNA levels of IL-2 or IFN-γ in different Se supplement groups at all time points were significantly decreased (P < 0.05) compared to the corresponding control groups except for the 5 mg/kg Se supplement group at 15 and 30 days. In contrary, a significantly increase (P < 0.05) in the mRNA level of IL-2 or IFN-γ was observed in the 5 mg/kg Se supplement group at 15 and 30 days compared to the corresponding control group. The mRNA levels of IL-2 or IFN-γ between different Se supplement groups showed a significant difference (P < 0.05) at the same time points.
Effects of different Se supplementation level on the mRNA level of IFN-γ and IL-2 in the thymus tissues of chicken. Each value represents the mean ± SD of five individuals. Tukey’s paired test was used to determine significant differences between different Se supplementation levels at the same time points in the same indicators. Different small letters on the top of the bars in panels a and b indicate significant differences (P < 0.05) in the mRNA level between different Se supplement levels at the same time points. A shared common letter on top of the bars indicated a lack of significant difference (P > 0.05)
Discussion
Se has a notably narrow concentration range from sufficiency to deficiency and toxicity [41], and Se is toxic at concentrations of only three to five times the essential concentration [42]. The toxic effects of excessive Se intake have become a great threat to the health of humans and animals. However, studies are limited on the effect of different Se supplementation levels on the immune organs of chickens. Hence, this study was conducted to evaluate the effect of different Se supplementation levels on the oxidative status, histopathology, cytokines, and immunotoxicity in the thymus of laying hens. To the best of our knowledge, this study was the first to examine the effects of Se supplementation on histopathology, inflammation, and biomarkers of oxidative stress in the thymus of laying hens.
Excess or deficiencies of Se has been implicated in major pathophysiologies in animals and humans due to its major role in redox processes. Studies have shown that Se plays an important role in the antioxidant defense system of animals and humans [35, 36]. Xu et al. reported that the GSH-Px activity and levels of Se and glutathione (GSH) in Se-deficient chickens are seriously reduced by 33.8–96 % (P < 0.001), 24.51–27.84 % (P < 0.001), and 20.70–64.24 % (P < 0.01), respectively [21]. Se supplementation can cause increases in erythrocyte and plasma total antioxidant status (TAS), erythrocyte-reduced GSH and GSH-Px in patients with epilepsy and refractory epilepsy [43]. In our present study, biomarkers of oxidative stress via enzyme activity and mRNA levels in chicken thymus were significantly changed in the 10 and 15 mg/kg Se supplement groups at all time points, which might be due to Se influences on growth of animals via biomarkers of oxidative stress [44]. These results are in agreement with the findings by Hoffmann and Berry [8] and Rayman [7], who reported that Se deficiency impacts immune function. Interestingly, the activities of SOD, CAT, and GSH-Px in the low Se supplementation group at 15 days were higher than those in the corresponding control groups. An important feature of antioxidant enzymes is their inducibility under conditions of oxidative stress, which might be an important adaptation to poisoning-induced stress [26]. The induced antioxidant activity indicated a response against increased ROS production in Se poisoning. These results also revealed that thymus plays an important role in the immune response to concurrently protect immune cells from ROS damage and high Se status might exacerbate oxidative stress. These results are in agreement with the histopathological changes observed in our study. Our histological results showed that the thymus tissue in excessive Se-treated chickens revealed different degrees of cortex drop, incrassation of medulla, and degeneration of the reticular cells. The results were similar to histopathological alterations of chicken thymus under manganese poisoning [28]. The detection of inflammatory cytokines has become one of the indices that could be used to evaluate the state of organism immunity. Certain studies showed that the levels of Se in organisms are closely associated with the function of inflammatory cytokines [45, 46]. Wu et al. reported that the content of serum IL-2β in the Se-poisoned ducks increased slightly in the early stage but showed a notable decrease in the later stage. Similarly, the concentrations of IL-2 and IFN-γ in serum and thymus and the mRNA levels of IL-2 and IFN-γ in the thymus were also observed in excessive-Se-treated chickens in this study, indicating disruption of organism homeostasis by this element. The changes in these inflammatory cytokines in the Se poisoning animals could be due to the excess Se, which caused injury to the immune organ (such as thymus), a decrease in production of T lymphocyte, and a decrease in the IL-2 and IFN-γ produced by T lymphocytes [47]. No reports were found in the literatures on the effects of subchronic Se exposure on IL-2 and IFN-γ in chickens, making the current study, to the best of our knowledge, the first report on inflammatory cytokines caused by excess Se exposure. It is possible that Se toxicity could be a consequence of the disruption of normal metal and trace elements. This disruption could in turn cause problems in metabolic pathways and cascades in the organism, which might subsequently lead to inflammation and, finally, to increased oxidative stress [48].
In conclusion, this study indicated that excessive Se influences the concentrations of IL-2 and IFN-γ in the serum and thymus, mRNA levels of IL-2 and IFN-γ, histopathological changes in the tissues, and changes in the antioxidant status of chicken thymus. Our study suggested that excessive dietary Se could result in a decrease in immunity, an increase in oxidative damage, and a series of clinical pathology changes, such as a cortex drop, incrassation of the medulla, and degeneration of the reticular cells.
References
Shan H, Yan R, Diao J, Lin L, Wang S, Zhang M et al (2015) Involvement of caspases and their upstream regulators in myocardial apoptosis in a rat model of selenium deficiency-induced dilated cardiomyopathy. J Trace Elem Med Biol 31:85–91
Qin H, Zhu J, Liang L, Wang M, Su H (2013) The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ Int 52:66–74
Combs GF Jr, Clark LC, Turnbull BW (2001) An analysis of cancer prevention by selenium. Biofactors 14:153–159
Li JL, Gao R, Li S, Wang JT et al (2010) Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals 23:695–705
Schweizer U, Schomburg L, Savaskan NE (2004) The neurobiology of selenium: lessons from transgenic mice. J Nutr 134:707–710
Martin-Romero FJ, Kryukov GV, Lobanov AV et al (2001) Selenium metabolism in Drosophila: selenoproteins, selenoprotein mRNA expression, fertility, and mortality. J Biol Chem 276:29798–29804
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52:1273–1280
Chariot P, Bignani O (2003) Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve 27:662–668
Kaur P, Bansal MP (2005) Effect of selenium-induced oxidative stress on the cell kinetics in testis and reproductive ability of male mice. Nutrition 21:351–357
Brown KM, Arthur JR (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4:593–599
Mahmoud KZ, Edens FW (2005) Influence of organic selenium on hsp70 response of heat-stressed and enteropathogenic Escherichia coli-challenged broiler chickens (Gallus gallus). Comp Biochem Physiol Part C Toxicol Pharmacol 141:69–75
Behne D, Kyriakopoulos A (2001) Mammalian seleniumcontaining proteins. Annu Rev Nutr 21:453–473
Clark LC, Combs GF Jr, Turnbull BW et al (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 276:1957–1963
Rederstorff M, Krol A, Lescure A (2006) Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol Life Sci 63:52–59
Zhang H, Feng X, Chan H−M, Larssen T (2014) New insights into traditional health risk assess-ments of mercury exposure: implications of selenium. Environ Sci Technol 48:1206–1212
Bajaj M, Eiche E, Neumann T, Winter J, Gallert C (2011) Hazardous concentrations of selenium in soil and groundwater in North-West India. J Hazard Mater 189:640–646
Lemire M, Philibert A, Fillion M, Passos CJ, Guimarães JR, Barbosa F Jr et al (2012) No evidence of selenosis from a selenium-rich diet in the Brazilian Amazon. Environ Int 40:128–136
Stadtman TC (2000) Selenium biochemistry. Mammalian selenoenzymes. Ann NY Acad Sci 899:399–402
Driscoll DM, Copeland PR (2003) Mechanism and regulation of selenoprotein synthesis. Annu Rev Nutr 23:17–40
Xu SW, Yao HD, Zhang J, Zhang ZW, Wang JT, Zhang JL, Jiang ZH (2013) The oxidative damage and disbalance of calcium homeostasis in brain of chicken induced by selenium deficiency. Biol Trace Elem Res 151:225–233
Ghazi Harsini S, Habibiyan M, Moeini MM, Abdolmohammadi AR (2012) Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol Trace Elem Res 148:322–330
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxi-dase. Science 179:588–590
Abbas HHH, Authman MMN (2009) Effects of accumulated selenium on some physiological parameters and oxidative stress indicators in tilapia fish (Oreochromis spp.). Am Eurasian J Agric Environ Sci 5:219–225
Miller LL, Wang F, Palace VP, Hontela A (2007) Effects of acute and subchronic exposures to waterborne selenite on the physiological stress response and oxidative stress indicators in juvenile rainbow trout. Aquat Toxicol 83:263–271
Xing H, Li S, Wang Z, Gao X, Xu S, Wang X (2012) Histopathological changes and antioxidant response in brain and kidney of common carp exposed to atrazine and chlorpyrifos. Chemosphere 88:377–383
Wang X, Xing H, Jiang Y, Wu H, Sun G, Xu Q, Xu S (2013) Accumulation, histopathological effects and response of biochemical markers in the spleens and head kidneys of common carp exposed to atrazine and chlorpyrifos. Food Chem Toxicol 62:148–158
Liu X, Li Z, Tie F, Liu N, Zhang Z, Xu S (2013) Effects of manganese-toxicity on immune-related organs of cocks. Chemosphere 90:2085–2100
Chen K, Shu G, Peng X, Fang J, Cui H, Chen J et al (2013) Protective role of sodium selenite on histopathological lesions, decreased T-cell subsets and increased apoptosis of thymus in broilers intoxicated with aflatoxin B1. Food Chem Toxicol 59:446–454
Wang D, Li X, Xu L, Hu Y, Zhang B, Liu J (2006) Immunologic synergism with IL-2 and effects of cCHMIs on mRNA expression of IL-2 and IFN-γ in chicken peripheral T lymphocyte. Vaccine 24:7109–7114
Shah MAA, Song X, Xu L, Yan R, Li X (2011) Construction of DNA vaccines encoding Eimeria acervulina cSZ-2 with chicken IL-2 and IFN-c and their efficacy against poultry coccidiosis. Res Vet Sci 90:72–77
Ren Z, Wang Y, Deng H, Deng Y, Deng J, Zuo Z et al (2015) Deoxynivalenol-induced cytokines and related genes in concanavalin A-stimulated primary chicken splenic lymphocytes. Toxicol in Vitro 29(2015):558–563
Chen K, Yuan S, Chen J, Peng X, Wang F, Cui H, Fang J (2013) Effects of sodium selenite on the decreased percentage of T cell subsets, contents of serum IL-2 and IFN-cinduced by aflatoxin B1in broilers. Res Vet Sci 95:143–145
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Yao H, Liu W, Zhao W, Fan R, Zhao X, Khoso PA, Zhang Z, Xu S (2014) Different responses of selenoproteins to the altered expression of selenoprotein W in chicken myoblasts. RSC Adv 4:64032–64042
Yao H, Wu Q, Zhang Z, Li S, Wang X, Lei X, Xu S (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta 1830:3112–3120
Yao HD, Wu Q, Zhang ZW, Zhang JL, Li S, Huang JQ, Ren FZ, Xu SW, Wang XL, Lei XG (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of Se-deficient chicks1–3. J Nutr 143:613–619
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Xing H, Li S, Wang X, Gao X, Xu S, Wang X (2013) Effects of atrazine and chlorpyrifos on the mRNA levels of HSP70 and HSC70 in the liver, brain, kidney and gill of common carp (Cyprinus carpio L.). Chemosphere 90:910–916
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Sun M, Liu G, Wu Q (2013) Speciation of organic and inorganic selenium in selenium-enriched rice by graphite furnace atomic absorption spectrometry after cloud point extraction. Food Chem 141:66–71
Tuzen M, Pekiner OZ (2015) Ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction combined with graphite furnace atomic absorption spectrometric for selenium speciation in foods and beverages. Food Chem 188:619–624
Yurekli VA, Naziroglu M (2013) Selenium and topiramate attenuates blood oxidative toxicity in patients with epilepsy: a clinical pilot study. Biol Trace Elem Res 152:180–186
Lemly AD (2014) Teratogenic effects and monetary cost of selenium poisoning of fish in Lake Sutton, North Carolina. Ecotoxicol Environ Saf 104:160–167
Zeng J, Zhou J, Huang K (2009) Effect of selenium on pancreatic proinflammatory cytokines in streptozotocin-induced diabetic mice. J Nutr Biochem 20:530–536
Liu J, Zhao H, Liu Y, Liu Y, Wang X (2012) Effect of two selenium sources on hepatocarcinogenesis and several angiogenic cytokines in diethylnitrosamine-induced hepatocarcinoma rats. J Trace Elem Med Biol 26:255–261
Wu R, Lian X, Tang X, Fu Y, He C (2007) Study on the effects of cytokine level in ducklings with selenium poisoning. Agric Sci China 6:375–380
Hauser-Davis RA, Silva JAN, Rocha RCC, Pierre TS, Ziollib RL, Arruda MAZ (2016) Acute selenium selenite exposure effects on oxidative stress biomarkers and essential metals and trace-elements in the modelorganism zebrafish (Danio rerio). J Trace Elem Med Biol 33:68–72
Acknowledgments
Southwest University of Science and Technology Project (No. 15zx7121) and Mianyang Science and Technology Project (No. 14 N043) supported this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
None of the authors have any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Additional information
Yachao Wang and Li Jiang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Y., Jiang, L., Li, Y. et al. Effect of Different Selenium Supplementation Levels on Oxidative Stress, Cytokines, and Immunotoxicity in Chicken Thymus. Biol Trace Elem Res 172, 488–495 (2016). https://doi.org/10.1007/s12011-015-0598-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0598-7