Abstract
Humans have dramatically increased the deposition and availability of nutrients, such as nitrogen (N), worldwide. Soil organic matter (SOM) is a significant global reservoir of carbon (C); however, the effects of N enrichment on this large, heterogeneous C stock are unclear. Nitrogen has variable effects on the biological, chemical, and physical factors that determine SOM pool mean residence time; consequently, we predicted that N enrichment would have distinct effects on SOM pools, including the pool that is readily available for microbial decomposition, as well as the pools that have been stabilized against microbial decomposition via aggregate occlusion and mineral association. We addressed this gap in knowledge by measuring the effects of N addition on different SOM pools at five grassland experiments in the US Central Great Plains that participate in the Nutrient Network and have been fertilized for three or five years. Overall, N addition decreased microbial respiration of unoccluded OM by as much as 29 % relative to control plots, and consequently, decreased C loss from this pool. Furthermore, N addition tended to increase soil aggregation and C occlusion in large macro-aggregates. These results suggest that N addition will increase C sequestration by slowing the decomposition of SOM, as well as stabilizing SOM against microbial decomposition in aggregate-occluded pools. However, the effects of N on all pools studied varied among sites, possibly due to site variation in soil texture. Consequently, increased sequestration of soil C in response to N enrichment may not be universal across grasslands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the late 1800s, anthropogenic creation of reactive nitrogen (N) has increased 10-fold (Galloway et al. 2004, 2008), due to fossil fuel combustion, cultivation of N-fixing crops, and fertilizer production, causing a concomitant rise in atmospheric N deposition. Perturbation of the global N cycle influences global carbon (C) stocks as well. The addition of N can significantly increase net primary production, and total C aboveground stocks (Gough et al. 2000; LeBauer and Treseder 2008; Lee et al. 2010). Soil organic matter (SOM)—a globally significant C reservoir—contains two to five times as much C than aboveground biomass, and two to four times as much C than is present in the atmosphere (Ciais et al. 2013). Consequently, either positive or negative changes to soil C sequestration, in response to N addition, could significantly alter atmospheric carbon dioxide (CO2) concentrations, and thus have implications for future global climate. However, the influence of added N on soil C remains uncertain.
The effects of N addition on soil C storage differ across studies. Specifically, N can increase (Frey et al. 2014), decrease (Waldrop et al. 2004), or not change (Zeglin et al. 2007) the total SOM C stock (for meta-analyses of N addition studies see Liu and Greaver 2010 and Lu et al. 2011). However, the variable effects of N addition on total soil C are not surprising. The total SOM stock is comprised of multiple pools with mean residence times (MRTs) that vary from days to centuries (Von Lützow et al. 2006). Three key factors play a role in determining the variation in pool MRT: recalcitrance of SOM, physical protection of SOM in soil aggregates, and associations between soil minerals and SOM (Dungait et al. 2012; Cotrufo et al. 2013). Nitrogen addition can directly affect each of these key factors; consequently, the effects of N enrichment on each of these pools may differ. Unfortunately, few studies have examined the effects of N addition on multiple SOM pools with distinct MRTs and the results of these studies have not been concordant. Researchers report no change (Reid et al. 2012), increased (Neff et al. 2002), and decreased (Torn et al. 2005) decomposition of the unprotected SOM pool (rapidly cycling C with short MRT) and no change (Kaye et al. 2002), increased (Bradford et al. 2008), and decreased (Hagedorn et al. 2003) decomposition of aggregate-occluded or mineral-associated SOM pools (slowly cycling C with long MRT) in response to N addition. Current knowledge, which is riddled with inconsistent patterns and little mechanistic understanding, is still insufficient to predict future soil C sequestration potential in response to N enrichment.
What are the possible effects of N addition on SOM pools with distinct MRTs? Microbial decomposition of physically unoccluded, available SOM is influenced by SOM biochemistry, environmental conditions, and microbial physiology (Schimel and Schaeffer 2012). Consequently, the effects of N addition on the chemistry of the decomposition substrates and/or soil microbes (e.g., enzyme activity, physiology, community composition) will determine how these rapidly cycling C pools (MRT days to years) will respond to N addition (Janssens et al. 2010). Since leaf litter decomposition studies measure the decomposition of unoccluded (e.g., physically accessible) organic matter, results from litter N addition studies can offer insight into how unoccluded soil organic matter pools may respond to N. For example, Berg and Matzner (1997) and Hobbie et al. (2012) have found that N addition increased the decomposition rate of the litter fraction comprising more labile substrates that decomposes first (the “fast” decomposing litter fraction), but decreased the decomposition rate of the remaining litter comprising more chemically complex substrates (the “slow” decomposing litter fraction). The contrasting effects of N on “fast” versus “slow” litter fractions are likely due to differing effects of N on the decomposition of the substrates that make up each litter fraction. Specifically, previous litter studies have shown that the addition of N alleviated N limitation of microbes seeking C in relatively labile OM substrates, such as polysaccharides, which would lead to faster decomposition of the “fast” decomposing, labile litter fraction (Berg and Staaf 1980; Talbot and Treseder 2011). By contrast, N addition can inhibit lignin-degrading enzyme activity, leading to slower decomposition of the more chemically complex substrates that dominate the “slow” decomposing litter fraction (Carreiro et al. 2000). These effects could also occur in the unoccluded soil organic matter pool, which is also heterogeneous in substrate composition. Consequently, we expect divergent effects of N addition on the decomposition of unoccluded SOM: N addition may increase the decomposition of unoccluded labile SOM, and decrease the decomposition of unoccluded chemically complex SOM.
By contrast, in pools comprised of slowly cycling C (MRT years to centuries), SOM is stabilized against microbial decomposition via physical protection (occlusion) within soil aggregates (Jastrow et al. 1996). Along with soil particles, biological components such as roots, fungal hyphae, and microbial biomass and necromass, all contribute to aggregate formation (Oades and Waters 1991; Jastrow et al. 1998; Six et al. 2004; Wilson et al. 2009; King 2011; Gupta and Germida 2015). Nitrogen addition can decrease plant investment in belowground nutrient acquisition (such as root biomass and mycorrhizae), leading to less belowground biomass (Feng et al. 2010; Janssens et al. 2010; Li et al. 2015). Consequently, we expect aggregate-binding “agents” and aggregate-occluded SOM to decrease in response to N addition.
Finally, the very slowest cycling C pools (MRT centuries to millennia) are stabilized against microbial decomposition via adsorption onto soil mineral surfaces (Torn et al. 1997). Nitrogen addition can lead to acidification and increased solubility of polyvalent, hydrolyzing cations, such as Al3+ and Fe3+ in soils (Bouwman et al. 2002; Mueller et al. 2012). In turn, these pH-mediated effects can increase mineral surface reactivity and the abundance of exchangeable cations, respectively, resulting in increased SOM binding to mineral surfaces (Oades 1988; Baldock and Skjemstad 2000). Consequently, we expect that N addition will increase mineral-associated SOM through changes to mineral surface reactivity.
The objective of this study was to determine the effects of N enrichment on the decomposition of multiple SOM pools using empirical measurements of key SOM pools and fluxes. Specifically, we examined the effects of N enrichment on: (1) rapidly cycling C in the unoccluded SOM pools, (2) slowly cycling C in the aggregate-occluded SOM pools, and (3) very slowly cycling C in the mineral-associated SOM pools. Understanding the effects of N addition on soil C cycling is particularly important in grassland ecosystems as they comprise a significant proportion of the earth’s land area (~24 %) and soil C (~28 %) and contain more soil C per unit area than the global average (Watson et al. 2000). Consequently, this study focused on examining the effects of N addition at multiple grasslands sites.
We tested the following hypotheses for the effects of N addition on distinct SOM pools:
Hypothesis 1 (H1)
Nitrogen addition will increase the decomposition (and decrease the amount) of relatively labile unoccluded SOM by alleviating microbial nutrient limitation, and decrease the decomposition (and increase the amount) of more chemically complex unoccluded SOM by inhibiting its decomposition.
Hypothesis 2 (H2)
Nitrogen addition will decrease the size of the aggregate-occluded SOM pool through decreased root and mycorrhizal biomass and associated soil aggregation.
Hypothesis 3 (H3)
Nitrogen addition will increase the size of the mineral-associated SOM pool through pH-mediated increases to mineral surface reactivity.
Methods
Study sites
Soils were sampled from nutrient addition plots at five participatory sites of the Nutrient Network (www.nutnet.org; Table 1). The Nutrient Network is a collaborative, global network of experiments established to investigate the effects of multiple nutrient additions, including N, on ecosystem processes in grasslands. Participatory sites are located across the globe and follow standard protocols for sampling and analysis (Borer et al. 2014). We focused on the nutrient addition plots at five Nutrient Network sites located in the U.S. Central Great Plains region (Table 1): Cedar Creek Ecosystem Science Reserve (East Bethel, Minnesota; 45.40°, −93.20°), Cedar Point Biological Station (Ogallala, Nebraska; 41.2°, −101.63°); Chichaqua Bottoms Greenbelt (Maxwell, Iowa; 41.79°, −93.39°); Konza Prairie Biological Station (Manhattan, Kansas; 39.07°, −95.58°); and Shortgrass Steppe (Nunn, Colorado; 40.82°, −104.77°). At each of these sites, nitrogen (N), phosphorus (P), and potassium plus micronutrients (K), have been added in a full factorial manner since 2008 (Colorado, Kansas, Minnesota, Nebraska) or 2010 (Iowa). N, P, and K were applied annually (10 g m−2 year−1); a micronutrient mix, including B, Cu, Fe, Mg, Mn, Mo, S, and Zn, was applied once at the start of the experiment in the K plots only. The treatments are replicated across three blocks in Colorado, Kansas, and Nebraska, across five blocks in Minnesota, and across six blocks in Iowa. More details on the experimental set-up and nutrient sources are available in Borer et al. (2014).
Our focus was on the effects of N on SOM decomposition and stabilization. To maximize the statistical power for detecting N effects, we sampled the full factorial of nutrient treatments for most analyses. For the more labor-intensive analyses, we analyzed the control and N addition plots only (see Appendix 1 for a summary of sampled plots included in each analysis).
Soil sampling
Soil samples were collected in July and August of 2012. Three cores (5 cm diameter and 10 cm deep) were sampled from each plot and composited across the full factorial of nutrient treatments; a fourth core was sampled from the control and N addition treatments for root analyses. Samples were kept on ice or in the refrigerator for a maximum of 6 days until processed in the lab. A subsample of composite soil from each plot was sieved to 2 mm for chemical and biological analysis and 8 mm for soil aggregate isolation. Fresh, 2 mm-sieved soil was used to measure gravimetric soil moisture, microbial respiration, microbial biomass and net N mineralization (details below). Air-dried, 2 mm-sieved soil was used to measure total soil % C and % N by combustion (Costech ESC 4010 Elemental Analyzer, Valencia, California, USA), soil pH (1:1 soil:water slurry method), and particulate organic matter (POM) C and N via density flotation (method detailed below). Additionally, soil texture was measured on air-dried, 2 mm-sieved soil from the control plots using the hydrometer method and sodium hexametaphosphate as the dispersing agent (Ashworth et al. 2001). Soil sieved to 8 mm from the control and N addition plots was air-dried and used to measure water-stable soil aggregates (details below).
Analyses: decomposition of unoccluded SOM
We evaluated the effects of nutrient addition on microbial decomposition of unoccluded SOM by measuring microbial respiration during a long-term laboratory incubation. A subsample of fresh, 2 mm sieved soil from each plot was placed in a 120 ml specimen cup and soil moisture was adjusted to 70 % field capacity. Field capacity was calculated separately for each site by pulling 20 kPa pressure on saturated soil. Microbial respiration rate (mg C g soil−1 day−1) was determined at least 17 times during the 380-day laboratory incubation. For each respiration rate measurement, the specimen cups were placed inside 1 L Mason jars and sealed for either 24- or 48-hour intervals. The CO2 concentration in the airtight jars was measured at the beginning and end of each interval using an infrared gas analyzer (LICOR 7000). When not being measured, specimen cups were covered with gas-permeable, low-density polyethylene film. Throughout the incubation, soil samples were maintained at 70 % field capacity and kept at 20 °C in the dark. We calculated cumulative C respired (mg C g soil−1) during the incubation by averaging the respiration rate between adjacent measurement dates and multiplying by the interval between them, then summing the amount of C respired in between each rate measurement. Additionally, because we were interested in the effects of N on unoccluded SOM substrates that decay at different rates, we evaluated our respiration rate data against both one- and two-pool decay models (see “Statistical analyses” section below).
Since microbes drive the turnover of unoccluded SOM, we also assessed the effects of N addition on microbial biomass C and N at the start of the respiration incubation using chloroform fumigation extraction (Brookes et al. 1985). Briefly, replicate fresh, 2 mm-sieved soil samples were extracted with 0.5 M K2SO4 prior to and following chloroform fumigation under vacuum for 5 days. Following filtration, extracts were analyzed for total organic C and total N (Shimadzu TOC-V, Shimadzu Corporation, Kyoto, Japan). Soil microbial biomass C (MC) and N (MN) were calculated as: MC = EC/k EC and MN = EN/k EN , where EC is the difference between extractable C in the fumigated and unfumigated samples, EN is the difference between extractable N in the fumigated and unfumigated samples, k EC is the C extraction efficiency coefficient, and k EN is the N extraction efficiency coefficient. We used the standard extraction efficiency coefficients of 0.45 (k EC ) and 0.54 (k EN ) from the literature (Brookes et al. 1985; Beck et al. 1997).
Analyses: aggregate-occluded and mineral-associated SOM
We measured the effects of nutrient addition on SOM stabilization in aggregate and mineral fractions using wet sieving fractionation, which isolated water stable soil aggregates (Six et al. 2000; Bach et al. 2010). Briefly, air-dried, 8 mm-sieved soil subsamples from the control and N addition treatments only were wet sieved with a 2 mm sieve for 2 min each to isolate large macro-aggregates (>2000 μm). Soil that passed through the sieve was wet-sieved with a 250 μm sieve to isolate small macro-aggregates (2000–250 μm). Finally, the remaining material was wet-sieved with a 53 μm sieve to isolate micro-aggregates (250–53 μm) and mineral-associated SOM (<53 μm). During wet sieving, floating organic matter was removed so we could test for N effects on C that was occluded within each aggregate fraction. The isolated fractions were dried at 105 °C for 12 h, followed by 60 °C for 48 h. Fractions were weighed and analyzed for C and N concentration (Costech ESC 4010 Elemental Analyzer, Valencia, California, USA) and used to determine percentage of whole soil total C and N contributed by each fraction. We did not perform sand-corrections on the aggregate fractions (Elliott et al. 1991) because our primary goal was to evaluate the distribution of C and N among aggregate fractions at several grassland sites, not a direct comparison of C and N content within specific fractions across sites. The large macro-aggregate, small macro-aggregate, and micro-aggregate fractions were used to evaluate H2 (aggregate-occluded SOM), while the smallest size fraction informed H3 (mineral-associated SOM).
In order to assess mechanisms of aggregation, we supplemented our aggregate fraction data with root biomass and mycorrhizal colonization measurements. Directly following collection, the additional intact core sampled from the control and N treatment plots was washed in wire mesh tubes (0.28 mm mesh) in a rotating elutriator (Wiles et al. 1996) until all soil was removed (~3 h). Remaining material was suspended in water and roots were captured with fine sieves and hand-picking. Root crowns were not considered root biomass and removed. Once free of soil, roots were dried at 65 °C overnight and weighed to calculate dry root biomass per unit area. Colonization of root tissue by arbuscular mycorrhizal fungi was determined by the point intercept method. Roots were removed from soil cores by washing gently with water over a 53 µm sieve. Cleaned roots were stained with Trypan Blue and stored in a 1:1:1 (vol) solution of glycerin:lactic acid:water at 4 °C. Roots were spread in a petri dish marked with 13 mm square grid and examined at ×40 magnification to determine presence of fungal structures (hyphae and/or vesicles) at each root-grid line intersection. One hundred intersects were counted for every sample to determine the proportion of root tissue colonized, and each sample was counted twice to ensure reproducible results. Seven root samples were not prepared for mycorrhizal analysis and, consequently, are not included in the statistical analyses.
Analyses: additional soil measurements
We also measured the effects of nutrient addition on POM chemistry and microbial net N mineralization rate. These two soil properties can change in response to N addition due to the effects of N addition on plant substrate chemistry. POM was measured following gentle agitation and separation in a dense liquid (method modified after Sollins et al. 2006). Briefly, 10 ml of 1.8 g L−1 NaI was added to 10 g of air-dried, 2 mm sieved soil. Samples were gently shaken for 30 min to disperse weakly bound soil aggregates and centrifuged (2400 rpm for 30 min). We separated floating light fraction material (POM) and rinsed and analyzed the fraction for % C and % N (Costech ESC 4010 Elemental Analyzer, Valencia, California, USA). Four samples were contaminated during the procedure and excluded from the statistical analyses.
To measure net N mineralization, we performed a 28-day laboratory incubation. Fresh, 2 mm sieved soil samples were extracted immediately with 2 M KCl. Duplicate samples were adjusted to 70 % field capacity, incubated in the laboratory at 20 °C in the dark for 28 days, and then extracted with 2 M KCl. Extracts were analyzed for inorganic N (ammonium and nitrate) using colorimetric methods (Hood-Nowotny et al. 2010). We calculated net N mineralization (mg N g soil−1 day−1) by subtracting the inorganic N concentration of the original soil extract from the inorganic N concentration of the incubated soil extract and dividing by the number of incubation days.
Statistical analyses
We were interested in whether N had distinct effects on more labile versus chemically complex unoccluded SOM (H1). Consequently, we evaluated whether microbial respiration data should be modeled with two pools that cycle at different rates, or a simple one-pool model (single decomposition rate). We evaluated the fit of the daily respiration rate measurements to two decomposition models: a one-pool decay model, C rate (t) = k (C t e −kt ); and a two-pool decay model, C rate (t) = k f (C f \(e^{{ - k_{f} t}}\)) + k s [(C t –C f ) \(e^{{ - k_{s} t}}\)]. In both models, C rate (t) is the daily respiration rate (mg C g soil−1 day−1) at time t, t is time (days), and C t is total soil C (mg C g soil−1). In the one-pool model, k is the decomposition rate (day−1) of the total soil C pool (C t ). In the two-pool model, k f and k s are the decomposition rates (day−1) of the “fast”- and “slow”-decomposing soil C pools, respectively. The MRT (day) of the fast and slow pools are k −1 f and k −1 s , respectively. The slow pool is defined as the difference between the total soil C pool (C t ; mg C g soil−1) and the fast soil C pool (C f ; mg C g soil−1).
We used maximum-likelihood estimation (MLE) to fit both decomposition models and calculate the decay rate and pool size parameters (bbmle package in R). The Akaike Information Criterion (AIC) was used as a measure of model fit. Since the two-pool model fit the data best (see Results: Decomposition models and parameters below), the parameter estimates from the two-pool model were used in subsequent analyses. We tested for parameter estimate equifinality—or model results where multiple combinations of parameters produce equally good model fits (Beven 2006)—by randomly generating 50,000 parameter combinations for each sample and fitting all parameter combinations to the one- and two-pool models. We evaluated model goodness-of-fit (R2) by comparing the predicted respiration rates from these random parameter combinations against the actual respiration rate data of each soil sample. This test showed no evidence in support of parameter estimate equifinality using MLE (see Appendix 2 for further details on equifinality evaluation methods and results).
We evaluated the effects of N on the unoccluded SOM pool (H1) by testing for the effects of site and nutrient addition (N × P × K) on the two-pool model parameter estimates (k f , k s , C f , and C s ) and cumulative C respired using ANOVA. We evaluated N effects on the aggregate-occluded and mineral-associated SOM pools (H2 and H3) by testing the effects of site and N on the proportion of total C contained in each of the aggregate fractions isolated via wet sieving. We also tested for the effects of site and nutrient addition (N × P × K) on total soil C, N and C:N ratio; microbial biomass C, N, and C:N ratio; POM C, N, and C:N ratio; soil pH; and net N mineralization; as well as the effects of site and N on root biomass and mycorrhizal colonization using ANOVA. Response variables were natural log transformed when needed to meet normality assumptions (tested using the Shapiro-Wilke normality test). Additionally, we assessed the regression assumptions of normality and homogeneity of variance by plotting the residual values versus fitted values and quantile–quantile residual plots of each model.
For all responses, we examined alternate model structures that included covariates (e.g., soil, climate, or plant variables) instead of site identity as main effects along with nutrient treatments (data not shown). None of the available covariates provided as much or more explanatory power than site. Furthermore, the covariate models did not describe the site by nutrient response interactions when they occurred. Consequently, for all models we included site as a fixed effect to account for known (e.g., climatic, pedologic, and plant community) differences among the study sites. Block was included as a random effect (nlme package in R).
For each model, we tested for site by nutrient interactions, and included them in the final model structure when significant. When there was a significant interaction, we used the lsmeans package in R to perform post hoc comparisons; p-values were Bonferroni corrected for multiple comparisons. Both marginal and conditional R2—or the variance explained by fixed effects only versus both fixed and random effects—were calculated for each model using the MuMIn package in R (Nakagawa and Schielzeth 2013). All analyses were performed in R (R version 3.0.1; R Foundation for Statistical Computing 2013).
For the samples collected from the full nutrient factorial (N × P × K plots), the N treatment dominated the observed responses (see Results below) and the +NP, +NK, and +NPK plots enhanced the N response. Consequently, we report significant interactions with P and K below when they occurred, but we focus on the effects of N.
Results
Unoccluded SOM response to N enrichment (H1)
We evaluated our long-term microbial respiration incubation against two decomposition models. Based on AIC, the two-pool decomposition model was the best fit for 85.6 % of the samples (137/160) and the one- and two-pool models were indistinguishable (difference in AIC <3) for 14.4 % of the samples (23/160). The one-pool model was never the best fit. Model fit for the two-pool model ranged from 0.54 to 0.99 R2, with a mean R2 value of 0.85 and median R2 of 0.88. The effects of N addition on the two-pool parameter estimates are reported here.
On average, decomposition rates of the “fast” decomposing soil C pool (k f ) increased with N addition (ambient N average = 0.1008 day−1; added N average = 0.1173 day−1) and the fast pool MRT decreased with N addition (ambient N average = 9.92 days; added N average = 8.53 days), although the direction and magnitude of response varied by site (site by N interaction: p = 0.001; Fig. 1a; see Appendix 3, Table 1 for decomposition parameter ANOVA tables). Post-hoc tests revealed that N addition significantly increased k f at the most northern and sandiest soil sites (Iowa, p = 0.0001; Minnesota, p = 0.03), had no significant effect on k f in Kansas and Nebraska, and significantly decreased k f in Colorado (p = 0.007). In response to N addition, there was a marginally significant decrease in the size of the fast pool (C f ) on average (ambient N average = 0.30 mg C g soil−1; added N average = 0.23 mg C g soil−1; N effect: p = 0.07; Fig. 1b).
Effect of nitrogen addition on decay rate a and size b of the “fast” decomposing C pool, decay rate c and size d of the “slow” decomposing C pool, and cumulative C respired e measured with a long-term microbial respiration incubation. All panels show mean plus/minus one standard error. Stars indicate significance from post hoc pairwise comparisons: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. Treatment codes are the same across all panels. Treatment codes: ambient N (open circles) include all plots where N was not added (control, +P, +K, +PK plots); added N (closed squares) include all N addition plots (+N, +NP, +NK, +NPK). See Appendix 1, Table 2 for sample numbers
In contrast to the fast pool (k f ), the decomposition rate of the “slow” decomposing soil C pool (k s ) decreased with N addition on average (ambient N average = 2.95 × 10−4 day−1; added N average = 2.63 × 10−4 day−1) and the slow pool MRT increased with N addition on average (ambient N average = 9.30 years; added N average = 10.42 years). Responses to N addition varied among the sites, having either negative or neutral effects (site by N interaction: p = 0.03; Fig. 1c). Nitrogen significantly decreased k s in Nebraska (p = 0.05), Kansas (p = 0.03) and Colorado (p = 0.003), but had no effect at the two sandiest sites (Iowa and Minnesota). The size of the slow pool (C s ) did not change significantly (N effect, p > 0.1; Fig. 1d).
Nitrogen addition either significantly decreased or did not change cumulative C respired (site by N interaction: p = 0.02; Fig. 1e). Post-hoc tests showed that N significantly decreased cumulative respiration in Nebraska by 23 % (p = 0.002) and Kansas by 29 % (p = 0.0001), but had no effect at the other three sites. The trend toward decreasing cumulative respired C in response to N addition at Nebraska and Kansas was the same whether evaluated on a per g soil or per g microbial C basis (i.e., metabolic quotient, qCO 2 ; data not shown). Microbial biomass C concentration (per mass soil and per mass soil C) did not change in response to N addition (p > 0.1; see Table 2 for microbial biomass data and Appendix 3, Table 3 for microbial biomass ANOVA tables).
Aggregate-occluded and mineral-associated soil fraction responses to N enrichment (H2 and H3)
At the four coarse-textured sites (Colorado, Iowa, Minnesota, Nebraska), at least half of the total C content was associated with the middle aggregate size classes: small macro-aggregates (2000–250 μm) and micro-aggregates (250–53 μm; Fig. 2). In contrast, at the site with the greatest soil clay content (Kansas), more than 50 % of total soil C was in the large macro-aggregate fraction (>2000 μm). On average, N addition increased the proportion of C in large macro-aggregates (ambient N average: 0.16 g macro-aggregate C g soil C−1; added N average: 0.18 g macro-aggregate C g soil C−1), but the difference was only marginally significant (N effect: p = 0.07). None of the other aggregate fractions or the mineral-associated fraction had statistically significant N effects (p > 0.1; see Appendix 3, Table 2 for aggregate fraction ANOVA tables).
Effects of nitrogen addition on soil C concentration (as % of total soil C) across soil aggregate size classes: large macro-aggregates (>2000 μm), small macro-aggregates (2000–250 μm), micro-aggregates (250–53 μm), and mineral-associated C (<53 μm). Figure shows mean plus/minus one standard error. Treatment codes: ambient N (white) = control plots; added N (gray) = +N plots. Sites are ordered from high to low clay content. See Appendix 1, Table 2 for sample numbers
To assess mechanisms that could lead to the N responses in the aggregate-occluded and mineral-associated fractions, we also evaluated root and mycorrhizal abundance (Table 3), as well as soil pH (Table 2). N addition had no significant effect on root biomass (p > 0.1; see Appendix 3, Table 4 for root variable ANOVA tables). However, N addition caused a marginally significant increase in average percent root colonization by mycorrhizae (ambient N average: 50.96 %; added N average: 59.44 %; N effect: p = 0.052), as well as a statistically significant increase in the absolute root biomass colonized by mycorrhizae (ambient N average: 4.72 mg cm−3; added N average: 4.92 mg cm−3; N effect: p = 0.05). Finally, average soil pH decreased with N addition (ambient N average: 6.0; added N average: 5.8), especially in the presence of either P or K (N by P interaction: p = 0.02; N by K interaction: p = 0.04; see Appendix 3, Table 3 for pH ANOVA table).
Additional soil variables
In addition to evaluating multiple SOM pools, we measured variables that may influence site responses to N addition (Table 2). POM C concentration increased significantly in response to N, but only in P addition plots (N by P interaction: p = 0.008; ambient N/added P = 3.20 mg POM C g soil−1, added N/added P = 4.48 mg POM C g soil−1). Particulate organic matter N concentration increased significantly with N addition (N main effect: p = 0.001; ambient N average: 0.32 mg N g soil−1; added N average: 0.38 mg N g soil−1). The POM C:N ratio also declined significantly with N, but only under ambient P (N by P interaction: p = 0.009; ambient N/ambient P = 11.51, added N/ambient P = 9.73). Nitrogen addition significantly increased net N mineralization rates at three sites (site by N interaction: p < 0.0001): Iowa (p = 0.004), Minnesota (p < 0.0001), and Kansas (p < 0.0001). Finally, there was no statistically significant effect of N on total soil C concentration, but on average, N addition increased total soil N concentration by 6 % (N effect: p = 0.04) and decreased the soil C:N ratio by 3 % (N effect: p = 0.0008) relative to control plots (see Appendix 3, Table 3 for ancillary soil variable ANOVA tables).
Discussion
Overall, we confirmed that N enrichment affects SOM pools with variable MRTs differentially, but our pool-specific hypotheses were only partially supported. In the unoccluded SOM pools, our results supported our hypothesis: on average, N enrichment increased the decomposition rate of the most quickly decomposing unoccluded pool (“fast” decomposing soil C pool) and decreased the decomposition rate of the more slowly decomposing unoccluded pool (“slow” decomposing soil C pool) (average fast pool MRT decreased from 9.92 to 8.53 days, average slow pool MRT increased from 9.30 to 10.42 years with N addition). Additionally, on average cumulative C respired decreased with N addition. By contrast, in the aggregate-occluded and mineral-associated SOM pools, data did not support our hypotheses: the proportion of C occluded within the largest aggregate fraction (>2000 μm) actually increased slightly with N addition and there were no effects of N addition on the mineral-associated soil fraction. Despite some agreement with our hypotheses, the effects of N on multiple SOM pools were not consistent across the five grassland sites studied. Our results suggest that, while C sequestration could increase in response to N, the effect is not universal across grasslands.
Nitrogen enrichment changed the decomposition rates of unoccluded SOM
We found partial support for our first hypothesis (H1): in line with our prediction, N enrichment increased the decomposition rate (k f ) and decreased the pool size (C f ) of the “fast” decomposing soil C in the unoccluded SOM pool at the more northern, sandy soil sites (Iowa and Minnesota). Additionally, the “slow” decomposing soil C in the unoccluded pool had a slower decomposition rate (k s ) under N enrichment at the more southern sites that did not exhibit fast pool responses (Colorado, Kansas, and Nebraska). Furthermore, N enrichment decreased cumulative C respired in Kansas and Nebraska. Overall, these divergent effects of N on more quickly versus more slowly cycling soil C are in accordance with patterns observed in leaf litter decomposition (Berg and Matzner 1997; Hobbie et al. 2012) and predicted from models of microbial activity following N addition (Ågren et al. 2001; Schimel and Weintraub 2003; Moorhead and Sinsabaugh 2006). A number of non-exclusive mechanisms have been proposed to explain increased decomposition of rapidly cycling C and decreased decomposition slowly cycling C in response to N addition. Next we evaluate these mechanisms in light of explaining our pool- and site-specific responses.
In response to N enrichment, the decomposition of the “fast” decomposing C in the unoccluded pool could increase due to increasing N concentration of plant tissue inputs to this pool. Substrates with a low C:N ratio will decompose faster than substrates with higher C:N ratio, at least in the early stages of decomposition (Cornwell et al. 2008). Studies in grasslands have shown that litter N content increases with increasing soil N availability (Wedin and Tilman 1996). Nitrogen addition can also alter plant input chemistry by increasing the abundance of species with high N content tissue (Henry et al. 2004). Although we did not measure plant input chemistry, under N enrichment we observed increased POM N concentration, decreased POM C:N ratio, and increased N mineralization rates, all of which suggest that the N content of substrate inputs increased with N addition. Interestingly, N addition significantly increased N mineralization rates at three sites, two of which also had significantly higher decay rates in the “fast” decomposing soil C pool.
Additionally, N addition could increase the decay rate of the “fast” decomposing C in the unoccluded pool through N effects on the microbial community. Previous research has shown that the production of hydrolytic microbial enzymes, including cellulases that degrade labile organic matter, are stimulated by N addition (Carreiro et al. 2000; Sinsabaugh et al. 2002; Talbot and Treseder 2011; Hobbie et al. 2012) and lead to increased decomposition (Sinsabaugh and Moorhead 1994). Examination of microbial enzyme production, as well as the site-level factors that affect their production (e.g., the availability of non-N nutrient enzyme co-factors), would be needed to assess the relevance of this hypothesis in light of the site by N interaction we found in the response of rapidly cycling unoccluded C.
On the other hand, N addition may decrease the decomposition of the “slow” decomposing C in the unoccluded pool through N effects on microbial growth efficiency. Models (Schimel and Weintraub 2003) and empirical studies (Thiet et al. 2006) have shown N addition can cause more C to be allocated to microbial growth (assuming N-limited growth) instead of lost via respiration and extracellular enzymes, leading to increased microbial efficiency and decreased respiration. We did find that N decreased cumulative C respired per unit microbial biomass C (i.e., the metabolic quotient, qCO 2 ), and that this decline was significant at two of the three sites where N also decreased the decay rate of the slow pool. This result is consistent with a recent meta-analysis which found a significant negative relationship between microbial respiration per unit microbial biomass and litter N concentration (Spohn 2015). Although we only evaluated the microbial biomass C pool at the beginning of the laboratory incubation, this finding does suggest that changing microbial allocation to growth versus respiration may be occurring at some sites.
Additionally, N effects on microbial enzyme activity could explain the decrease in decay rates of the “slow” decomposing C pool with N addition. The production of oxidative enzymes – which decompose more complex C substrates such as lignin—can be inhibited by N addition (Fog 1988), Previous research in forest ecosystems has demonstrated that N addition leads to decreased oxidative enzyme activity and increased soil C sequestration (Waldrop et al. 2004). However, there is some evidence to suggest that oxidative enzymes are not significantly inhibited by N addition in grasslands (Keeler et al. 2008; Sinsabaugh 2010), where plant lignin content is relatively low compared to forests. Alternately, lignin may be degraded as a mechanism of N acquisition, or “N mining.” Consequently, N enrichment could suppress N mining and, consequently, decrease lignin (and slow pool) degradation (Craine et al. 2007). However, it is unclear to what extent N mining occurs in ecosystems (Spohn 2015).
Overall, we have scant evidence to explain the site by N interactions we observed for the divergent N responses of unoccluded SOM pools with “fast” versus “slow” decomposing C among our sites. Although the sites that exhibited positive N effects on fast pool decay are the sandiest, the climate and vegetation characteristics of these sites also differ in comparison with the other sites studied (Table 1). Further research on site differences, along with measurements of the candidate mechanisms described above, would inform site-specific responses.
Aggregate-occluded SOM increased slightly with N enrichment
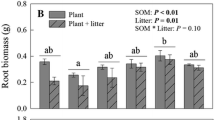
Contrary to our expectation that N addition would decrease C occluded within soil aggregates (H2), we found a trend of increasing proportion of C in the largest aggregate fraction (>2000 μm). The effect of N on macro-aggregate C was, however, consistent with the root responses we observed: we found that root biomass also increased with N addition on average (but not significantly). Although theory predicts that plant proportional allocation belowground should decrease as belowground resources (such as N) increase, previous studies have observed greater absolute root biomass under N addition even if the relative root:shoot ratio decreases (e.g., Fornara and Tilman 2012). It is possible soil texture interacts with N addition to evoke this response; within sites with coarse-textured soil (Minnesota, Iowa, Nebraska, and Colorado), root biomass increased slightly with N addition and at three of these sites (Minnesota, Iowa, and Nebraska), macro-aggregate C increased (although neither response was significant). In contrast, within the most fine-textured soil (Kansas), root biomass was 25 % lower in N addition plots than control (Table 3). In light of the role of roots in aggregate formation (Oades and Waters 1991; Jastrow et al. 1998), the root biomass response to N enrichment, particularly in the coarse-textured sites, may have contributed to increased formation of macro-aggregates and occlusion of C. Future studies replicated across soil texture types are required to support and understand this potential interaction.
Alternately, N enrichment could have increased C content of the large macro-aggregate fraction via another aggregate-formation agent, such as fungal biomass (Guggenberger et al. 1999; Six et al. 2006; Strickland and Rousk 2010). Although we did not measure fungal biomass in this study, we did find that arbuscular mycorrhizal colonization of roots (both absolute mass abundance and percent colonized) increased with N addition. This was surprising, since a meta-analysis by Treseder (2004) found that colonization of both ecto- and arbuscular mycorrizal fungi on roots tends to decrease in response to N addition, although this was a weak relationship when data were limited to colonization rates only. Furthermore, a recent meta-analysis by (Li et al. 2015), reported that the inhibitory effects of N on mycorrhizal colonization was strongest in forests, and insignificant in grasslands. Increased arbusucular mycorrhizal fungi colonization of roots may lead to increased large macro-aggregate C concentration through physical enmeshment of soil particle with organic matter and/or direct inputs of hyphae and exudates, such as glomalin (Rillig 2004), into aggregates.
The large macro-aggregate fraction is relatively dynamic (Yang and Wander 1998; Plante and McGill 2002) and C occluded in large macro-aggregates is not stabilized against microbial decomposition. However, this fraction has the potential to influence C sequestration on the centennial time scale and beyond via interactions with smaller aggregate fractions. Large macro-aggregates can facilitate aggregation (and stabilization) of smaller occluded fractions that cycle much more slowly (Oades 1984; Six et al. 2004). Consequently, even small increases to this aggregate fraction could lead to greater aggregate and mineral occlusion of SOM in the future, along with greater C sequestration.
Mineral-associated SOM was not changed with N enrichment
We expected N enrichment to increase mineral surface-associated C (H3), however, we observed no effect of N enrichment on mineral-associated C despite a 3 % decrease in pH in N addition plots relative to control plots. Lack of N effects on the mineral-associated pool could have been caused by several factors. First, four of the five study sites had very coarse-textured soil with little silt and clay (Kansas average % silt and clay particles = 68.1 %; all other sites ranged from 9.9 to 28.7 % silt and clay); therefore, potential for OM to associate with mineral surfaces in these soils is very small and detecting treatment differences in this already very small pool may be difficult. Second, the mineral-associated fraction (<53 μm) isolated during wet sieving, is itself a heterogeneous mixture of clay micro-structures and silt-sized particles, which may not uniformly associate with C. Furthermore, the site with the highest percentage clay (Kansas) had the largest amount of C occluded in macro-aggregates (and other smaller fractions therein). If there were N enrichment effects on the mineral-associated fraction at this site, they may have been undetectable as they were occluded within larger aggregates. The duration of N addition (5 years in Colorado, Kansas, Minnesota, and Nebraska; 3 years in Iowa) may not have been sufficient to detect N effects in this fraction, despite observable decreases in soil pH. For example, Gillespie et al. (2014), found that N enrichment altered the chemistry of SOM in the fine (<5 μm) fraction following 17 years of N addition. Further investigation into the effects of N enrichment on mineral-associated OM (and the timescale of this effect) is warranted.
Conclusion
Our findings—specifically, the decrease in decomposition rate of “slow” pool unoccluded SOM, decreased cumulative C respired, and trend toward increased aggregation after only 3–5 years of nutrient addition—suggest that N enrichment will lead to increased sequestration of soil C in grassland soils, although unexplained site-to-site variation indicates that this effect may not be universal. Our decomposition results are consistent with findings from forest systems where soil C has increased under N enrichment due to suppressed microbial decomposition (e.g., Zak et al. 2008; Frey et al. 2014). In contrast to previous studies, however, we also examined mechanisms of stabilization that could sequester C beyond the decadal scale (e.g., via aggregate occlusion and mineral association): in addition to slowing microbial decomposition, N addition may increase soil C storage at grassland sites through increased C occlusion in large macro-aggregates. This could eventually lead to C accumulation in the mineral-associated SOM fractions (MRT centuries to millennia) due to the cascading effects of increased aggregation on smaller aggregate fractions. Finally, site-specific factors will influence how N affects the decomposition rates and sizes of multiple SOM pools. Unsurprisingly, but importantly, we observed significant site-by-site variation in response to N addition in both the unoccluded and occluded SOM pools we measured. Soil texture appeared to play a significant role in these interactions; however, we caution against over-interpreting this effect since texture co-varied strongly with climatic and biotic variables among our study sites. In the future, additional multi-study sites that investigate the effects of N while varying a key state factor that influences SOM stabilization (e.g., soil parent material) will inform our understanding of soil C sequestration across ecosystems.
References
Ågren GI, Bosatta E, Magill AH (2001) Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128:94–98. doi:10.1007/s004420100646
Ashworth J, Keyes D, Kirk R, Lessard R (2001) Standard procedure in the hydrometer method for particle size analysis. Commun Soil Sci Plant Anal 32:633–642. doi:10.1081/CSS-100103897
Bach EM, Baer SG, Meyer CK, Six J (2010) Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol Biochem 42:2182–2191. doi:10.1016/j.soilbio.2010.08.014
Baldock JA, Skjemstad JO (2000) Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org Geochem 31:697–710
Beck T, Joergensen RG, Kandeler E et al (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biochem 29:1023–1032. doi:10.1016/S0038-0717(97)00030-8
Berg B, Matzner E (1997) Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ Rev 5:1–25. doi:10.1139/a96-017
Berg B, Staaf H (1980) Decomposition rate and chemical changes of Scots pine needle litter. II. Influence of chemical composition. Ecol Bull 32:373–390
Beven K (2006) A manifesto for the equifinality thesis. J Hydrol 320:18–36. doi:10.1016/j.jhydrol.2005.07.007
Borer ET, Harpole WS, Adler PB et al (2014) Finding generality in ecology: a model for globally distributed experiments. Methods Ecol Evol 5:65–73. doi:10.1111/2041-210X.12125
Bouwman A, Van Vuuren D, Derwent R, Posch M (2002) A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut 141:349–382. doi:10.1023/A:1021398008726
Bradford MA, Fierer N, Jackson RB et al (2008) Nonlinear root-derived carbon sequestration across a gradient of nitrogen and phosphorous deposition in experimental mesocosms. Glob Change Biol 14:1113–1124. doi:10.1111/j.1365-2486.2008.01564.x
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. doi:10.1016/0038-0717(85)90144-0
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Ciais P, Sabine C, Bala G et al (2013) Carbon and other biogeochemical cycles. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Cornwell WK, Cornelissen JH, Amatangelo K et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Cotrufo MF, Wallenstein MD, Boot CM et al (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995. doi:10.1111/gcb.12113
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113. doi:10.1890/06-1847.1
Dentener FJ (2006) Global maps of atmospheric nitrogen deposition, 1860, 1993, and 2050. Data set. Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge. http://daac.ornl.gov/
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Change Biol 18:1781–1796. doi:10.1111/j.1365-2486.2012.02665.x
Elliott ET, Palm CA, Reuss DE, Monz CA (1991) Organic matter contained in soil aggregates from a tropical chronosequence: correction for sand and light fraction. Agric Ecosyst Environ 34:443–451. doi:10.1016/0167-8809(91)90127-J
Feng X, Simpson AJ, Schlesinger WH, Simpson MJ (2010) Altered microbial community structure and organic matter composition under elevated CO2 and N fertilization in the duke forest. Glob Change Biol 16:2104–2116. doi:10.1111/j.1365-2486.2009.02080.x
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Fornara DA, Tilman D (2012) Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition. Ecology 93:2030–2036
Frey SD, Ollinger S, Nadelhoffer K et al (2014) Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121:305–316. doi:10.1007/s10533-014-0004-0
Galloway JN, Dentener FJ, Capone DG et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Galloway JN, Townsend AR, Erisman JW et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892. doi:10.1126/science.1136674
Gillespie AW, Diochon A, Ma BL et al (2014) Nitrogen input quality changes the biochemical composition of soil organic matter stabilized in the fine fraction: a long-term study. Biogeochemistry 117:337–350. doi:10.1007/s10533-013-9871-z
Gough L, Osenberg CW, Gross KL, Collins SL (2000) Fertilization effects on species density and primary productivity in herbaceous plant communities. Oikos 89:428–439. doi:10.1034/j.1600-0706.2000.890302.x
Guggenberger G, Frey SD, Six J et al (1999) Bacterial and fungal cell-wall residues in conventional and no-tillage agroecosystems. Soil Sci Soc Am J 63:1188. doi:10.2136/sssaj1999.6351188x
Gupta VVSR, Germida JJ (2015) Soil aggregation: influence on microbial biomass and implications for biological processes. Soil Biol Biochem 80:A3–A9. doi:10.1016/j.soilbio.2014.09.002
Hagedorn F, Spinnler D, Siegwolf R (2003) Increased N deposition retards mineralization of old soil organic matter. Soil Biol Biochem 35:1683–1692. doi:10.1016/j.soilbio.2003.08.015
Henry HAL, Cleland EE, Field CB, Vitousek PM (2004) Interactive effects of elevated CO2, N deposition and climate change on plant litter quality in a California annual grassland. Oecologia 142:465–473. doi:10.1007/s00442-004-1713-1
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. doi:10.1002/joc.1276
Hobbie SE, Eddy WC, Buyarski CR et al (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr. doi:10.1890/11-1600.1
Hood-Nowotny R, Umana NH-N, Inselbacher E et al (2010) Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Soil Sci Soc Am J 74:1018–1027
Janssens IA, Dieleman W, Luyssaert S et al (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322. doi:10.1038/ngeo844
Jastrow JD, Miller RM, Boutton TW (1996) Carbon dynamics of aggregate-associated organic matter estimated by carbon-13 natural abundance. Soil Sci Soc Am J 60:801–807. doi:10.2136/sssaj1996.03615995006000030017x
Jastrow J, Miller R, Lussenhop J (1998) Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie. Soil Biol Biochem 30:905–916
Kaye J, Barrett J, Burke I (2002) Stable nitrogen and carbon pools in grassland soils of variable texture and carbon content. Ecosystems 5:461–471. doi:10.1007/s10021-002-0142-4
Keeler BL, Hobbie SE, Kellogg LE (2008) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15. doi:10.1007/s10021-008-9199-z
King GM (2011) Enhancing soil carbon storage for carbon remediation: potential contributions and constraints by microbes. Trends Microbiol 19:75–84. doi:10.1016/j.tim.2010.11.006
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379. doi:10.1890/06-2057.1
Lee M, Manning P, Rist J et al (2010) A global comparison of grassland biomass responses to CO2 and nitrogen enrichment. Philos Trans R Soc B 365:2047–2056
Li W, Jin C, Guan D et al (2015) The effects of simulated nitrogen deposition on plant root traits: a meta-analysis. Soil Biol Biochem 82:112–118. doi:10.1016/j.soilbio.2015.01.001
Liu L, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828. doi:10.1111/j.1461-0248.2010.01482.x
Lu M, Zhou X, Luo Y et al (2011) Minor stimulation of soil carbon storage by nitrogen addition: a meta-analysis. Agric Ecosyst Environ 140:234–244. doi:10.1016/j.agee.2010.12.010
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174
Mueller KE, Eissenstat DM, Hobbie SE et al (2012) Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry 111:601–614. doi:10.1007/s10533-011-9695-7
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Neff JC, Townsend AR, Gleixner G et al (2002) Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 419:915–917
Oades JM (1984) Soil organic matter and structural stability: mechanisms and implications for management. Plant Soil 76:319–337
Oades JM (1988) The retention of organic matter in soils. Biogeochemistry 5:35–70
Oades J, Waters A (1991) Aggregate hierarchy in soils. Soil Res 29:815–828
Plante AF, McGill WB (2002) Soil aggregate dynamics and the retention of organic matter in laboratory-incubated soil with differing simulated tillage frequencies. Soil Tillage Res 66:79–92
Reid J, Adair E, Hobbie S, Reich P (2012) Biodiversity, nitrogen deposition, and CO2 affect grassland soil carbon cycling but not storage. Ecosystems 15:580–590. doi:10.1007/s10021-012-9532-4
Rillig MC (2004) Arbuscular mycorrhizae, glomalin, and soil aggregation. Can J Soil Sci 84:355–363. doi:10.4141/S04-003
Schimel JP, Schaeffer SM (2012) Microbial control over carbon cycling in soil. Front Microbiol. doi:10.3389/fmicb.2012.00348
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404. doi:10.1016/j.soilbio.2009.10.014
Sinsabaugh RL, Moorhead DL (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311. doi:10.1016/0038-0717(94)90211-9
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24
Six J, Paustian K, Elliott ET, Combrink C (2000) Soil structure and organic matter I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci Soc Am J 64:681–689
Six J, Bossuyt H, Degryze S, Denef K (2004) A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res 79:7–31
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555. doi:10.2136/sssaj2004.0347
Sollins P, Swanston C, Kleber M et al (2006) Organic C and N stabilization in a forest soil: evidence from sequential density fractionation. Soil Biol Biochem 38:3313–3324
Spohn M (2015) Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences 12:817–823. doi:10.5194/bg-12-817-2015
Strickland MS, Rousk J (2010) Considering fungal:bacterial dominance in soils—methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395. doi:10.1016/j.soilbio.2010.05.007
Talbot JM, Treseder KK (2011) Interactions among lignin, cellulose, and nitrogen drive litter chemistry–decay relationships. Ecology 93:345–354. doi:10.1890/11-0843.1
Thiet RK, Frey SD, Six J (2006) Do growth yield efficiencies differ between soil microbial communities differing in fungal:bacterial ratios? Reality check and methodological issues. Soil Biol Biochem 38:837–844. doi:10.1016/j.soilbio.2005.07.010
Torn MS, Trumbore SE, Chadwick OA et al (1997) Mineral control of soil organic carbon storage and turnover. Nature 389:170–173. doi:10.1038/38260
Torn MS, Vitousek PM, Trumbore SE (2005) The influence of nutrient availability on soil organic matter turnover estimated by incubations and radiocarbon modeling. Ecosystems 8:352–372. doi:10.1007/s10021-004-0259-8
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Von Lützow M, Kögel-Knabner I, Ekschmitt K et al (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions–a review. Eur J Soil Sci 57:426–445
Waldrop MP, Zak DR, Sinsabaugh RL et al (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14:1172–1177
Watson RT, Noble IR, Bolin B et al (2000) Land use, land-use change, and forestry. Special Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Wedin DA, Tilman D (1996) Influence of nitrogen loading and species composition on the carbon balance of grasslands. Science 274:1720
Wiles LJ, Barlin DH, Schweizer EE, et al (1996) A new soil sampler and elutriator for collecting and extracting weed seeds from soil. Weed Technol 10:35–41
Wilson GW, Rice CW, Rillig MC et al (2009) Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett 12:452–461
Yang X-M, Wander MM (1998) Temporal changes in dry aggregate size and stability: tillage and crop effects on a silty loam Mollisol in Illinois. Soil Tillage Res 49:173–183
Zak DR, Holmes WE, Burton AJ et al (2008) Simulated atmospheric NO3-deposition increases soil organic matter by slowing decomposition. Ecol Appl 18:2016–2027
Zeglin LH, Stursova M, Sinsabaugh RL, Collins SL (2007) Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 154:349–359. doi:10.1007/s00442-007-0836-6
Acknowledgments
We thank Nutrient Network collaborators for establishing and maintaining the sites included in this study, including Elizabeth Borer, Adam Kay, and Eric Seabloom at Cedar Creek; Jean Knops at Cedar Point; Lori Biederman, Stan Harpole, Lauren Sullivan, and Ryan Williams at Chichaqua Bottoms; Kim LaPierre at Konza; and Dana Blumenthal, Cynthia Brown, and Julia Klein at Shortgrass Steppe. Numerous individuals at the University of Minnesota and Iowa State University assisted us in the lab and field, including: Daniel Ackerman, Rick Beckel, Chris Buyarski, Katie Kemmitt, Joey Krenz, Eric Lind, Elyssa McFarland, Queenster Nartey, Jennifer Pederson, Kristen Peterson, Missy Rudeen, Kelsey Thurow, and Michael Wells. Eric Lind also provided valuable assistance with data analysis and Nutrient Network database management. This work was supported by the Cedar Creek Long Term Ecological Research Program (NSF DEB-1234162), a National Science Foundation Graduate Research Fellowship to CER (Grant No. 00039202), and the Dayton Fund of the Bell Museum of Natural History. The Nutrient Network has been supported by funding to Elizabeth Borer and Eric Seabloom from the National Science Foundation Research Coordination Network and the Long Term Ecological Research programs, as well as the University of Minnesota’s Institute on the Environment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: E. Matzner.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Riggs, C.E., Hobbie, S.E., Bach, E.M. et al. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 125, 203–219 (2015). https://doi.org/10.1007/s10533-015-0123-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0123-2