Abstract
Salinity changes resulting from storm surge, tides, precipitation, and stormwater run-off are common in coastal wetlands. Soil microbial communities respond quickly to salinity changes, altering the rate of soil organic carbon (SOC) loss and associated biogeochemical processes. This study quantified the impact of salinity-altering pulses on SOC loss, defined as microbial respiration (CO2 flux) at high and low tide, CH4 flux, and dissolved OC (DOC) release, in 3 intertidal wetlands (Jacksonville, FL, USA). Intact soil cores from a freshwater tidal, brackish, and salt marsh were exposed to simulated tides and 3 salinity pulsing events during a 53-day laboratory experiment. Soil and water physio-chemical properties, nutrient release, and microbial indicators were measured. Microbial respiration was the dominate pathway of SOC loss (>97 %). Soil hydraulic conductivity was greater in brackish and salt marshes and was critical to overall soil respiration. High tide CO2 flux was greatest in the freshwater marsh (58 % of SOC loss) and positively correlated with DOC concentration; low tide CO2 flux was greatest in brackish and salt marshes (62 and 70 % of SOC loss, respectively) and correlated with NH4 + and microbial biomass. The freshwater marsh was sensitive to brackish pulses, causing a 112 % increase in respiration, presumably from accelerated sulfate reduction and N-cycling. SOC loss increased in the salt marsh pulsed with freshwater, suggesting freshwater run-off may reduce a salt marsh’s ability to keep-pace with sea level rise. Increased inundation from storm surges could accelerate SOC loss in freshwater marshes, while decreasing SOC loss in brackish and salt marshes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal wetlands occupy an ecological niche along the land’s fridge that is unique in its tidally-influenced hydrology and gradation in surface water salinities. With eustatic sea level rising at a rate of 2.8–3.1 mm y−1, and urban development or coastal morphology impeding the landward transgression of many intertidal wetlands in response to sea level rise (SLR), these ecosystems are being geographically ‘squeezed’ between terrestrial and marine environments (IPCC 2007; Nicholls et al. 1999). As such, the influence of surface water inputs from the two salinity extremes (i.e., freshwater and seawater) are expected to increase. For example, storm surges and extreme tidal events are predicted to increase in frequency and elevation, causing more extensive land inundation (Mousavi et al. 2011; IPCC 2007; Michener et al. 1997). Freshwater pulses to coastal wetlands resulting from large precipitation events, stormwater run-off, or point source discharges from surrounding urban areas may also increase in some regions (Mulholland et al. 1997).
Coastal wetlands are considered a significant sink for global carbon (C) and can accumulate approximately 30–100 kg organic C m−2 in the same time period an adjacent upland accumulates 5–10 kg organic C m−2 (Coultas 1996). Total global C burial in salt marshes is estimated to be 218 ± 24 g m−2 y−1, more than 40 times higher than the average terrestrial forest (McLeod et al. 2011). This large C sequestration and storage capacity is a function of high primary production, slow decomposition, and the ability to trap and bury significant amounts of allochthonus C from terrestrial run-off and tidal deposition (Armentano and Menges 1986). Soil organic C (SOC) is a C storage reservoir in coastal wetlands controlled by the balance between C inputs (e.g., biomass production and deposition) and C losses (e.g., mineralization and export) that can be influenced by salinity. Variations in SOC inputs are manifested as distinct vegetation patterns observed in coastal wetlands that are driven in large part by salinity tolerance; these patterns include decreases in plant productivity (McKee and Mendelssohn 1989), species richness (Tuxen et al. 2011) and diversity (Wieski et al. 2010) with increasing salinity, as well as differences in belowground biomass allocation (Neubauer et al. 2005) and species composition (Smith 2009; Williams et al. 1999), all of which orient along salinity gradients. The effect of salinity on SOC loss is less clear. Comparing decomposition rates along existing coastal salinity gradients has produced varying results, with some studies indicating the highest decomposition rates occur in the most saline wetlands (Craft 2007), others indicating the highest rates in freshwater tidal wetlands (Quintino et al. 2009), and still others suggesting no direct relationship between salinity and decomposition rate (Mendelssohn et al. 1999). The numerous confounding variables affecting in situ decomposition rates suggests that isolating the effect salinity on SOC loss may be better discerned by changes in microbial community structure and activity. Soil microbes, with their quick turnover rate and large surface-to-volume ratio, exhibit a high sensitivity to salinity that is demonstrated by variations in the rate of SOC mineralization under different seawater concentrations (Chambers et al. 2011).
A pulse of higher salinity surface water in wetland soils introduces two main biogeochemical drivers that influence SOC cycling: an ionic effect caused by increased ionic strength and a sulfate effect caused by the abundant SO4 2− in seawater. Higher ionic strength can cause the rapid displacement of cations (e.g., NH4 +, Fe2+ and Al3+) from the soil exchange complex by the plentiful Na2+, Mg2+, Ca2+ and K+ in seawater, subsequently releasing these elements into the soil porewater (Weston et al. 2006; Portnoy and Giblin 1997). The efflux of NH4 + and Fe2+ from the cation exchange complex typically occurs within 1–3 days of seawater addition and persists for ~16 days (Weston et al. 2006) with the potential to enhance nutrient supply to soil microbes and promote redox reactions during this timeframe. Physiologically, high ionic strength can induce osmotic stress in microorganisms, interfering with cellular functions and reproduction, or even causing cell lysis and the divergence of microbial community composition along salinity gradients (Ikenaga et al. 2010; Van Ryckegem and Verneken 2005). Some ions present in seawater are known to disrupt specific processes or microbial guilds in the soil. For example, chloride is the most abundant anion in seawater and can inhibit both nitrification and denitrification in soils and organic matter (Seo et al. 2008; Hale and Groffman 2006; Roseberg et al. 1986). One study found the addition of Cl− to organic matter never previously exposed to the ion caused a significant reduction in denitrification enzyme activity, but the effect did not occur in systems with a history of high Cl− concentrations (Hale and Groffman 2006). This suggests some degree of adaptation or microbial community shifts after prolonged Cl− exposure. Sulfide ions often accumulate in saline environments as a byproduct of sulfate reduction and can have deleterious effects on both vegetation (King et al. 1982) and nitrifying bacteria (Joye and Hollibaugh 1995). Understanding the effects of Cl− and HS− on N cycling is critical to understanding C biogeochemistry because N and C cycling are often tightly coupled (White and Reddy 2003) and can account for significant C mineralization in low salinity wetland soils (Craft et al. 2009; Gribsholt et al. 2005). There is still much debate in the literature regarding the effects of salinity on microbial indicators such as microbial biomass C (Gennari et al. 2007; Rasul et al. 2006; Muhammad et al. 2006) and enzyme synthesis (Wu et al. 2008; Tripathi et al. 2007), with most of the previous studies being conducted in soils exposed to evaporative salinity, rather than seawater, and none having compared results along coastal salinity gradients. For this reason, we quantified two key enzymes involved in C mineralization (ß-glucosidase and dehydrogenase) in the present study.
Arguably the most significant change in SOC cycling in low-salinity wetlands exposed to saltwater intrusion is the increased availability of sulfate (SO4 2−), which functions as an alternative electron acceptor during anaerobic respiration (Capone and Kiene 1988). The introduction of seawater to a freshwater wetland soil in the laboratory increased CO2 flux rate 20–32 % in direct proportion to the amount of SO4 2− added under anaerobic conditions, but the effect was short-term as other resource limitations (e.g., labile C, N or P) begin to constrain the soil respiration rate over time (Chamber et al. Chambers et al. 2011). A similar study showed that the addition of 10 ppt seawater to a freshwater sediment caused sulfate reduction to become the dominate pathway for C mineralization in just 12 days, and can be responsible for up to 95 % of C loss after 35 days (Weston et al. 2006). However, high SO4 2− concentrations also allow sulfate-reducing bacteria to outcompete methanogens with their higher energy yield, dramatically reducing CH4 production in saline wetlands (Capone and Kiene 1988). Adding approximately 10–14 ppt seawater to a freshwater soil can cause the near-complete suppression of methanogenesis in just 1 week (Chambers et al. 2011; Edmonds et al. 2009). A recent meta-analysis of in situ methanogenesis in coastal marshes revealed the lowest CH4 flux rates when salinities were >18 ppt (1.1 ± 2 g m−2 y−1), and the highest rates in freshwater (<0.5 ppt) and oligohaline (0.5–5 ppt) marshes (41.9 ± 76 and 150 ± 221 g m−2 y−1, respectively) (Poffenbarger et al. 2011). Research suggests that freshwater wetland soils exposed to low salinities (<10 ppt) may have the highest overall rate of SOC mineralization due to the combined effects of accelerated sulfate reduction and the maintenance of high in situ rates of methanogenesis (Chambers et al. 2011).
Pulses of lower salinity surface water from heavy precipitation events or stormwater run-off could also alter soil biogeochemistry and the rate of SOC cycling in brackish and saline wetlands. Freshwater reduces the ionic strength and concentration of SO4 2− in the soil porewater, initiating a shift in microbial community composition and biogeochemical pathways that is comparable to the effects of increasing salinity. To our knowledge, no one has directly measured the impact of lower-salinity pulsing events on SOC loss in saline coastal wetland soils, but there is ample evidence that C mineralization pathways tend to differ along wetland salinity gradients. In freshwater marshes, CO2 flux is often dominated by nitrate and iron reduction (Craft et al. 2009; Neubauer et al. 2005) and CH4 flux rates are higher than in saline marshes (Poffenbarger et al. 2011). Increasing the input of freshwater may also assist in flushing some of the deleterious compounds (e.g., Cl− and HS−) that accumulate in saline soils (Jolly et al. 2008), subsequently providing more favorable conditions for microbial respiration.
Inundation, whether the result of a storm surge or the daily tidal cycle, are also important aspects of coastal wetland biogeochemistry because they control the balance between aerobic and anaerobic respiration. The acceleration of the CO2 flux following a drop in the water table is well established in the inland wetland literature (e.g., Blodau and Moore 2003; Wright and Reddy 2001; Freeman et al. 1993) and can cause the C mineralization rate to be as much as 50 times faster during a draw-down period, compared to flooded conditions (Clymo 1983). In intertidal wetlands, as tides recede or the water table drops, CO2 flux has also been shown to increase (Krauss and Whitbeck 2012) possibly due to a combination of greater oxygen availability and relief from the ionic stress caused by saltwater inundation. Few studies have investigated the effects of tidal cycles on SOC loss in coastal systems and none have looked at differences across salinity gradients (Gribsholt and Kristensen 2003; Neubauer et al. 2000).
Many of the responses to salinity changes described above are relatively rapid and short-lived. Therefore, we hypothesized that abrupt, dynamic changes in salinity will have a greater impact on the rate SOC loss in intertidal wetlands than more gradual changes (e.g. eustatic SLR). To test this hypothesis, replicate intact soil cores collected from three intertidal wetlands (freshwater tidal, brackish, and salt marsh) were exposed to pulses of salinity-altering surface water under laboratory conditions. Throughout the salinity manipulations, SOC loss was quantified through soil respiration, methanogenesis, and DOC release measurements collected during simulated high tide (inundated soil) and low tide (exposed soil) conditions. Based on the type of wetland where the soils were collected, the salinity of the surface water pulse, and knowledge gained from previous studies, it was predicted that freshwater soils pulsed with brackish salinity water and salt marsh soils pulsed with freshwater would exhibit the greatest change in the rate of SOC loss (Table 1).
Methods
Study area
Thirty-six intact soil cores (12 from each of 3 sites) were collected along the natural salinity gradient of intertidal wetlands in the City of Jacksonville, Florida, USA. The sites were chosen based on accessibility and ambient surface water salinity. Soil cores were collected from the intertidal marsh platform adjacent to tributaries of the St. John’s River. The freshwater tidal site was located along Cedar Creek (30°26′48.5″N, 81°40′17.1″W), the brackish site along Broward River (30°26′22.4″N, 81°37′33.1″W), and the salt marsh site along Pablo Creek (30°18′29.9″N, 81°25′9.8″W). Emergent marsh vegetation typical of a sub-tropical estuarine wetland dominated each site. The freshwater wetland had the highest species diversity with Sagittaria lancifolia, Zizaniopsis miliacea, and Alternanthera philoxeroides being the dominant species. Juncus roemerianus and Spartina patens dominated the brackish marsh, and the salt marsh was a monoculture of Spartina alterniflora. The freshwater marsh soil was classified as Maurepas muck and the salt and brackish marsh soils were classified as Tisonia mucky peat (USDA 1978). All three sites were subject to diurnal micro-tidal fluctuations averaging 0.7–1.0 m in range (NOAA 2011).
Experimental design
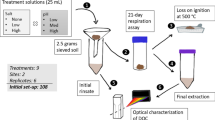
The experimental design consisted of a 3 × 3 × 3 mixed model treatment design. Twelve intact soil cores were collected in each of the three wetland types (freshwater, brackish, and salt marsh) in 40 cm long × 10 cm diameter polyvinyl chloride (PVC) tubes on April 15, 2011. All 12 soil cores from each site were collected within a 6 m2 plot to minimize heterogeneity between cores and using care to minimize soil compaction. Aboveground vegetation was removed by clipping to the soil surface; the cores were capped on top and bottom, and then transported back to the laboratory. At the time of soil collection, 400-L of surface water from the adjacent tidal creek was also collected, field-filtered through a 1-micron filter bag, and transported back to the lab. During sampling, all three sites were near low tide, with the freshwater and salt marsh sites on the ebb tide and the brackish site on the rising tide.
Once at the laboratory, the 12 field-replicate soil cores from each site were randomly assigned to one of 4 conditions (freshwater, brackish, or saltwater salinity-altering pulses, or immediate analysis). Standard window screen mesh was affixed to the bottom of the cores and a 1-cm diameter drain hole was drilled exactly 10 cm above the soil surface (to maintain a 10-cm deep water column). The 10 cm deep water column was chosen to mimic the average tidal range of the St. John’s River estuarine marshes where the soils were collected (NOAA 2011). The bottom of each core was plugged and flooded with ambient surface water (i.e., collected from the same site location) and allowed to acclimate for 1 week. The acclimation period was intended to provide time for any labile C released during the shearing of root structures at core collection to be assimilated. Following acclimation, bottom plugs were removed, a leachate collection container was placed under each core, and surface water was allowed to drain through the soil profile and mesh screen for the first 24 h dry-down period. After the first dry-down, the 3 cores from each site selected for immediate analysis were destructively sampled.
Following acclimation and initial dry-down, the remaining 9 cores from each site were subjected to 3 cycles of 3–5-day salinity-altering pulses, punctuated by 2 ambient surface water periods, each lasting ~12 days. Between each cycle of surface water addition, all cores were unplugged, allowed to drain for 24 h, and leachate was collected. Once the 53 day manipulation experiment was complete, the remaining 27 cores were destructively sampled. The goal of this design was to investigate both the short-term and cumulative impacts of pulsing events in each wetland type. Data from a hurricane storm surge in the Gulf of Mexico indicated that estuarine surface water salinities peak quickly to a maximum of ~25 ppt, and then receded slowly over the course of approximately 4 days (Li et al. 2009). Due to the abrupt, temporary, and dynamic nature of most saltwater (or freshwater) pulses that occur in coastal wetlands, the design included a return to ambient conditions following each salinity pulsing event. This allowed for the identification of any possible legacy impacts from the surge on the rate of SOC loss after natural conditions resumed. Hydroperiod was designated as high tide during periods of surface water flooding (10 cm water column), and low tide during the dry-down periods. Soil cores were stored in the dark at 25 °C and the top remained open to the atmosphere throughout the laboratory experiment.
Soil and water properties
All soil cores were sectioned into 3 depth segments (0–5, 5–10, and 10–20 cm), stored at 4 °C, and analyzed within 30 days. Soil property analysis included % moisture, bulk density, % organic matter (OM), total C, total N, and C fiber analysis (% fines (<0.025 mm), cellulose + hemicellulose, and lignin content). Moisture content and bulk density were determined after drying a subsample at 70 °C until constant weight. Percent OM was estimated by mass loss on ignition (LOI) where dry soils were combusted at 550 °C for 5-h and final weight was subtracted from initial weight. Total C and N content were determined using a Costech Model 4010 Elemental Analyzer (Costech 121 Analytical Industries, Inc., Valencia, CA). Dissolved OC was measured on a TOC Analyzer (Shimadzu Scientific Instrument TOC 5050A, Columbia, MD) following EPA method 415.1, which included filtering the water sample through a 0.45 μm membrane filter and acidifying the sample with H2SO4 until analysis (USEPA 1993). The fiber analysis was performed using a modified Ankom fiber fractionation method after Roberts and Rowland (1998). Fines (<0.025 mm) were defined as soil particles released from a 0.025 mm mesh bag placed in DI water, agitated, and rinsed. During sequential analysis, cellulose + hemicellulose was the fraction solubilized in 24 N H2SO4, lignin content was the fraction combusted in a muffle furnace at 550 °C, and inorganic ash >0.025 mm was the material remaining following combustion.
During the laboratory experiment the surface water in each core was regularly monitored for salinity, conductivity, pH, dissolved oxygen (DO), and temperature using a hand-held YSI model 85 (YSI Inc., Yellow Springs, OH). Twenty-mL surface water was collected from each core 2–3 times during each event/condition to monitor SO4 2− concentration and rate of loss. Sulfate samples were un-acidified, manually diluted, and analyzed on a Dionex DX 600 Ion Chromatograph (Thermo Scientific, Sunnyvale, CA) using standard method 4110B (Standard Methods 1997).
The surface water collected from each wetland type was analyzed for ammonium–N (NH4 +–N) and soluble reactive P (SRP) on an AQ-2 Automated Discrete Analyzer (Seal Analytical, Mequon, WI) using EPA Methods 104-A Rev. 3, and 118-A Rev. 2, respectively (USEPA 1993). Depending on salinity, the sample matrix used was either de-ionized (DI) water or 13 ppt artificial seawater (Neomarine Reef Salt mix, Brightwell Aquatics, Elysburg, PA), with the 26 ppt salinity samples diluted by half. For total Kjeldahl nitrogen (TKN) quantification, 10 mL of surface water was digested in glass tubes with a TKN salt catalyst and 0.5 mL of concentrated H2SO4. Samples were digested for 2 h at 160 °C, and then at 360 °C for 30 min. Tubes were cooled, 10-mL DI water added, vortexed, and the concentration was determined calorimetrically using a Technicon Autoanalyzer II (Seal Analytical, Mequon, WI), EPA Method 351.2 (USEPA 1993).
Soil organic carbon loss
The rate of SOC loss was estimated by measuring the major pathways of organic C loss- CO2 production (soil respiration, during both low and high tide), CH4 production (methanogenesis), and DOC release. Soil respiration was determined using a portable infrared gas analyzer (Li-Cor 8100, Lincoln, NB) that was factory calibrated and equipped with a 10-cm diameter chamber. The cores were plugged/sealed and CO2 flux was measured (1-min) a total of 16 times during the 53 day study, including a minimum of one sampling during each dry-down, salinity-altering pulse, and ambient surface water condition.
Methane was determined using soil slurry microcosms created at the conclusion of the study after Chambers et al. (2011). Approximately 5-g (wet weight) soil sub-samples from each treatment condition (0–5 cm) were prepared in triplicate and added to four 60-mL glass serum bottles. Bottles were capped with butyl stoppers and aluminum crimp-caps, evacuated to −75 kpa, and flushed with O2-free N2 gas for 1-min to create anaerobic conditions. Eight mL of 0.5 ppt, 13 ppt, or 26 ppt seawater was added to create a slurry (mimicking a high tide condition) with duplicates every third. All incubations were maintained at a slight over-pressure and stored in the dark at 30 °C. Headspace was extracted and measured on a gas chromatograph (Shimadzu Scientific Instruments GC 8A, Columbia, MD) fitted with a flame ionization detector (FID) on days 3, 7, 12, and 17. Potential methanogenesis was calculated as CH4–C production per g dry soil, per day.
Dissolved OC release was quantified in the leachate collected during the soil core dry-down and represented total DOC loss. Studies indicate that porewater seepage during ebb tide in salt marshes occurs primarily through the face of tidal creek banks (Gardner 2005). Therefore, we attempted to simulate a receding tide when surface water is drawn through the soil profile and then released to tidal creeks. Leachate was collected in 125-mL nalgene bottles, acidified, and stored at 4 °C until analyzed. All DOC samples were filtered through a 0.45 μm membrane filter and analyzed using a TOC Analyzer (Shimadzu Scientific Instrument TOC 5050A, Columbia, MD) following EPA method 415.1 (USEPA 1993).
Nutrient release
Nutrient release was determined by analyzing the leachate water collected during the dry-down periods for a total of 5 samplings during the study. Ammonium–N, SRP, and TKN were analyzed as described above for surface water properties. Organic-N was calculated as the difference between TKN and NH4 +–N.
Microbial indicators
Microbial biomass C (MBC) was determined by fumigation-extraction after Vance et al. (1987) and White and Reddy (2001). Duplicate 5-g (wet weight) samples were prepared in 25-mL centrifuge tubes. One set was fumigated with chloroform for 24 h and the other set served as the non-fumigated control. Following the chloroform treatment, both fumigates and non-fumigates were extracted with 25 mL of 0.5 M K2SO4, agitated for 30 min on a circulating shaker, and centrifuged at 5,000 rpm for 10 min. The supernatant was vacuum-filtered through a Whatman # 42 filter paper and stored at 4 °C until analyzed for total organic carbon (Shimadzu Scientific Instrument TOC 5050A, Columbia, MD). An extraction efficiency coefficient of kEC = 0.37 was applied to all samples (Sparling et al. 1990). Total labile organic C (TLOC) was defined as the TOC for the fumigated samples, labile organic C (LOC) was defined as the TOC for the non-fumigated samples, and MBC was defined as the difference between TLOC and LOC. The metabolic quotient (qCO2) was calculated as the rate of soil respiration (mg CO2–C kg soil−1 d−1) divided by MBC (mg MBC kg soil−1).
Beta-glucosidase enzyme activity was measured fluorometrically as described by Marx et al. (2001). Soil samples were homogenized, diluted by 100 with autoclaved distilled de-ionized (DDI) water, and sonicated for 10 s. Replicate soil slurry samples (150 μL) were added to each column of a 96 well plate. The top 4 rows were incubated with 100 μL (200 μM final concentration) of fluorescently labeled substrate (methyl umbelliferone (MUF)-glucoside) for 4 h. After 4 h, labeled MUF-glucoside was added to the bottom 4 rows and 10 μL of 0.1 M NaOH was added to all wells. Formation of the fluorescent product MUF was measured at excitation/emission wavelength of 360/460 on a Synergy HT Multi-Mode Microplate Reader (BioTek, Winooski, VT). Quenching curves were prepared for each of the three wetland soils and coefficients were applied to the final values, expressed as mg MUF kg dry soil−1 h−1.
Dehydrogenase enzymes activity was determined using the TTC (triphenyltetrazoliym chloride) method developed by Thalmann (1968) and modified by Alef (1995). Five-g wet weight soil was added to a 60 mL amber glass vial along with 5 mL TTC solution. Slurries were incubated in the dark for 24 h, 40 mL acetone was added, the solutions were filtered through a Whatmann #2 filter paper, and the optical density of the solution was measured at 546 nm wavelength on a Spectrophotometer (Shimadzu Scientific Instruments UV-160, Columbia, MD). Dehydrogenase activities were expressed as mg TPF kg dry soil−1 h−1.
Data analysis
Statistical analysis was performed using SAS 9.1 software (SAS Institute Inc., Cary, NC). All data sets were first tested to determine if the assumptions of homogeneity and normality were met using the Brown and Forsythe’s Test and Shapiro–Wilk Test, respectively. Where these assumptions were not met, the raw data was log transformed and further statistical analysis was conducted using the dataset that fulfilled the assumptions. A three-way repeated measures ANOVA model (α = 0.05) was used to determine the interaction between soil respiration rate, treatment condition, and time. A two-way repeated measures ANOVA model was used to determine significantly different means for complete data sets collected at common times for each soil core where time (t) was significant (p < 0.05, according to a treatment specific two-way ANOVA). This was determined to be true for DOC release and nutrient release. When t was deemed non-significant (p > 0.05; two-way ANOVA), the multivariate response was reduced to a univariate response and a two-way ANOVA model was used to determine significantly different means (Davis 2002). This method was determined to be appropriate for CO2 flux. Non-repeated variables (soil and water properties, CH4 flux, and microbial indicators) were measured using one and two-way ANOVA models (α = 0.05). Pearson’s Product correlations were performed to determine correlations between SOC loss, soil and water properties, microbial indicators, and nutrient release. A Chi Square test of independence was used to test if the percent of SOC lost to each pathway (respiration, methanogenesis, and DOC release) depends upon the treatment condition applied, and a Chi Square of Goodness of Fit was used to test if the percent of SOC lost to each pathway deviated significantly from that of the control condition (all at α = 0.05). One intact core (a freshwater marsh soil pulsed with salt (26 ppt) water) was completely removed from the analysis as an outlier (>2.5 times the standard deviation). A simple theoretical model was developed to illustrate the effects of SLR on SOC loss using the results for the partitioning of C loss via the 4 pathways (high tide CO2, low tide CO2, CH4, and DOC release) in each of the 3 wetland types. In this model, SLR is defined as an increase in time of high tide and decreased time of low tide. The linear model assumes current conditions are 50 % high tide and 50 % low tide and models an increase to 100 % high tide and 0 % low tide, with no real-time SLR scenario specified.
Results
Soil and water properties
The brackish marsh soil had a higher bulk density (p < 0.01) than the freshwater and salt marsh soils; bulk density did not vary with depth in any of the soils (Table 2). Soil organic matter content showed an inverse relationship with bulk density, with consistently higher OM in the freshwater and salt marsh soils (41–52 %) compared to the brackish marsh soil (29–34 %). Similar to % OM, total C and total N were higher in the freshwater and salt marsh soils compared to the brackish marsh soil (p < 0.05) and did not change with depth. The ratios of soil total C:N were 16.7 ± 1.4, 18.0 ± 1.7, and 17.9 ± 1.6 for the freshwater, brackish, and salt marsh soils, respectively. The C:N did not differ significantly with wetland type or depth. Carbon fiber analysis revealed the freshwater marsh soil had fewer fines (<0.025 mm; p < 0.01) than the brackish and salt marsh soils (Fig. 1). Both the freshwater and salt marsh soils had higher lignin content (p < 0.05) than the brackish soil and the brackish soil had higher inorganic ash content (>0.025 mm; p < 0.05) than the salt marsh. Cellulose + hemicelluloses content did not differ with wetland type.
Surface water salinities were 0.56 ± 0.07, 13.5 ± 0.8, and 26.5 ± 1.8 ppt for the freshwater, brackish, and salt marsh surface water, respectively. Sulfate concentration and specific conductivity increased with salinity (Table 3). Surface water pH was similar for all sites (averaging 7.7), and dissolved oxygen also did not vary between sites (ranging from 6.4 to 6.9 mg L−1; data not shown). Ammonium concentration was higher (p < 0.05) in the freshwater, compared to the brackish water, whereas TKN and DOC did not different between wetland types. SRP concentration was greater (p < 0.01) in the freshwater compared to both the brackish and salt marsh site water.
Soil organic carbon loss
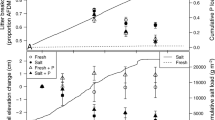
Soil respiration was strongly affected by inundation level, with CO2 flux being greater at low tide (following a 24-h dry-down), compared to high tide (10-cm water column), in the brackish and salt marsh soils (Fig. 2). Using only the soil cores treated with the control condition, the average rate of CO2 flux during high tide was greater (p < 0.001) in the freshwater marsh soil (1,033 ± 347 mg CO2–C m−2 d−1) than the salt marsh soil (500 ± 160 mg CO2–C m−2 d−1) and was negatively correlated with salinity (p < 0.05; Table 4). The rate of high tide CO2 flux was also positively correlated with DOC release, methanogenesis, qCO2, SRP, and Organic-N. During low tide in the control condition soil cores, average CO2 fluxes were 2,450 ± 680 mg CO2–C m−2 d−1 in the brackish marsh soil, 2,052 ± 1,031 mg CO2–C m−2 d−1 in the salt marsh soil, and 1,578 ± 649 mg CO2–C m−2 d−1 in the freshwater marsh soil (Fig. 2). Soil respiration rate at low tide in the control condition cores was not correlated with any of the soil, water, or microbial indicators of interest. Comparing average high tide and low tide CO2 flux in each wetland type revealed significant differences in the magnitude of the tidal effect on soil respiration. Mean soil respiration rates were 53 % greater at low tide than at high tide in the freshwater marsh soil, 230 % greater at low tide than high tide in the brackish marsh soil, and 310 % greater at low tide than high tide in the salt marsh soil.
Effect of tidal cycle (low tide = post 24-h dry-down; high tide = 10 cm water column) on CO2 flux rate according to wetland type. Each bar represents mean flux rate of the control condition (n = 9); error bars represent standard error. Different letters represent significantly different means (p < 0.05) based on a one-way ANOVA
Time was not a significant factor in soil respiration rate over the course of the experiment (p = 0.25; three-way repeated measures ANOVA). Therefore, CO2 fluxes from the three salinity-altering pulses were treated as replicates. Focusing on the effects of the salinity pulsing manipulation, results indicate that all of the freshwater marsh soil cores had higher (p < 0.01) rates of CO2 flux during high tide (1,056 ± 420 mg CO2–C m−2 d−1) than all of the other soil cores (Fig. 3a). Soil respiration rates in the brackish marsh soil were negatively affected by the pulse of fresh (0.5 ppt) water, decreasing the average rate of CO2 flux from 426 ± 85 mg CO2–C m−2 d−1 in the control, to 286 ± 5 mg CO2–C m−2 d−1 following the pulse (p < 0.05; Fig. 3a). Meanwhile, the pulse of salt (26 ppt) water had no impact on the brackish marsh soil CO2 flux during high tide. In the salt marsh soil, respiration rates during high tide were not affected by the addition of either fresh (0.5 ppt) or brackish (13 ppt) water. Overall, the rates of CO2 flux during the high tide salinity pulsing events were positively correlated with DOC release, CH4 flux, and SRP (all p < 0.01; Table 4).
Effect of salinity-altering pulse during: a high tide and b low tide, on CO2 flux rate according to wetland type and surface water salinity. Horizontal bars represent mean (solid line) and standard error (dashed lines) of the control condition. Each bar represents mean flux rate (n = 18); error bars represent standard error; NS = not significantly different from the control condition; significantly different means (p < 0.05) represented by percentages and based on a two-way ANOVA
Following each pulsing event, the soils demonstrated a legacy effect from the surface water addition, resulting in significantly different rates of CO2 flux during the subsequent low tide under certain treatment conditions (Fig. 3b). The freshwater marsh soil receiving a pulse of brackish (13 ppt) water had a CO2 flux of 2,587 ± 1,230 mg CO2–C m−2 d−1, more than double the rate in the control treatment during the same period (1,221 ± 426 mg CO2–C m−2 d−1; p < 0.001; Fig. 3b). In contrast, the freshwater marsh soil pulsed with salt (26 ppt) water showed no legacy effect during low tide. In the brackish marsh soil, respiration rate during low tide was unaffected by either the freshwater or saltwater pulses, with an overall average flux rate of 1,645 ± 291 mg CO2–C m−2 d−1. The salt marsh soil pulsed with fresh (0.5 ppt) water had a significant legacy effect, increasing CO2 flux from 1,578 ± 313 mg CO2–C m−2 d−1 in the control treatment, to 2,775 ± 710 mg CO2–C m−2 d−1 following the pulse (p < 0.05). The pulse of brackish (13 ppt) water had no legacy effect on soil respiration in the salt marsh soil. The concentrations of NH4 + and MBC were positively correlated with CO2 flux during the low tide that followed the salinity pulsing events (p < 0.05; Table 4).
Between each salinity pulsing event, all soil cores were flooded with their original ambient site water for ~12 days to mimic a return to baseline, or natural field conditions. During this ambient water phase, respiration rates for each salinity pulsing treatment condition were not different than the control for that wetland type during both high tide and low tide measurements, suggesting a return to baseline conditions between each pulsing event (data not shown).
The rate of methane production was an order of magnitude higher (p < 0.001) in the freshwater marsh control treatment than in all other treatments (114 ± 9 mg CH4–C m−2 d−1; Fig. 4). The addition of brackish (13 ppt) and salt (26 ppt) water to the freshwater marsh soil decreased methanogenesis by 98 and 97 %, respectively. In the brackish marsh soil, CH4 flux ranged from below detection to 2.2 mg CH4–C m−2 d−1, with no affect of surface water salinity changes. The salt marsh soil exposed to fresh (0.5 ppt) water had a CH4 flux of 8.0 ± 6.4 mg CH4–C m−2 d−1, marginally different (p = 0.08) from the control condition (0.12 ± 0.13 mg CH4–C m−2 d−1). Overall, CH4 flux was positively correlated with DOC release, qCO2, and SRP (Table 4). Methane flux had high within-treatment variability and should be interpreted as a measurement of potential methanogenesis, rather than an approximation of in situ methanogenesis, since the soil slurry incubations modified the physical soil structure.
Effect of salinity-altering pulse (high tide) on CH4–C flux rate according to wetland soil type and water salinity. Horizontal bars represent mean (solid line) and standard error (dashed lines) of the control condition. Each bar represents mean flux rate (n = 3); error bars represent standard error; NS = not significantly different from the control condition; significantly different means (p < 0.05) represented by percentages and based on a two-way ANOVA
DOC release from the control condition soil cores decreased from freshwater, to brackish, to salt marsh wetland types (p < 0.001; Table 5). DOC release was inversely correlated with salinity and directly correlated with CH4 flux, qCO2, SRP, and organic-N (Table 4). The salinity pulsing events did have a significant impact of the rate of DOC release in both the freshwater and the salt marsh soils. The pulse of brackish (13 ppt) water in the freshwater marsh soil caused a decrease (p < 0.01) in DOC release, from 18.3 ± 2.8 mg L−1 in the control, to 10.8 ± 2.2 mg L−1 following the pulse. Additionally, the pulse of fresh (0.5 ppt) water in the salt marsh soil caused an increase (p < 0.01) in DOC release, from 7.6 ± 0.7 mg L−1 in the control, to 11.4 ± 0.9 mg L−1 following the pulse. With the exception of the pulsing effects, the rate of DOC release was constant over time. Overall, DOC release following salinity pulsing events was positively correlated with CH4 flux, qCO2 and SRP, and negatively correlated with NH4 + and MBC (Table 4).
More than 97 % of SOC loss in this study occurred through soil respiration, with the exception of the freshwater marsh control, which lost 94 % of SOC to respiration. The average total mass of SOC lost in the freshwater marsh control treatment was greater than in the brackish marsh salt (26 ppt) water treatment (p < 0.01), the salt marsh control (26 ppt) treatment (p < 0.01), and the salt marsh brackish (13 ppt) water treatment (p = 0.05; Fig. 5). Since the total mass of SOC loss reflected the amount of time each measurement or condition occurred during the experimental manipulation (i.e., cores were exposed to high tide conditions a greater proportion of the time than low tide conditions), a time-corrected average % SOC loss to each pathway was also calculated for the control condition to demonstrate the implications of this data under a natural tidal cycle (Fig. 6). Based on mass C per unit time, the freshwater marsh control lost the largest % of total SOC through high tide CO2 flux (55 %), while the brackish and salt marsh controls lost the largest % SOC through low tide CO2 flux (62 and 70 %, respectively). The freshwater marsh soil pulsed with brackish (13 ppt) water was the only treatment to deviate significantly from its’ respective control in the % SOC lost to each of the 4 pathways. This treatment more closely resembled the SOC loss partitioning of the brackish marsh control (54 % loss to low tide CO2 flux, 46 % to high tide CO2 flux, and <1 % to methanogenesis) than the freshwater marsh control.
Mean percent of SOC lost by different pathways (controlling for duration of measurement) according to soil type. Charts represent control condition (n = 3); the freshwater marsh soil treated with brackish (13 ppt) ‘pulses’ was the only treatment deviating significantly from its’ respective control, according to a Chi square goodness of fit (p < 0.05)
Nutrient release
Among the soil cores receiving the control condition, the salt marsh soil released more NH4 + than the freshwater and the brackish marsh soils (p < 0.01; Table 5). The salinity pulsing events had a significant effect on the freshwater marsh soil, increasing the concentration of NH4 + release by more than an order of magnitude when a pulse of brackish (13 ppt) water was added, from 0.02 ± 0.19 mg L−1 in the control, to 0.57 ± 0.79 mg L−1 following the pulse (p < 0.05). TKN release showed no difference between wetland type, salinity-altering pulse, or time, averaging 1.2 ± 0.3 mg L−1. Organic-N release differed significantly between wetland types and was greater in the freshwater and brackish marsh soils than the salt marsh soil (p < 0.05). Salinity pulsing events and time had no effect on organic-N release. SRP release for the control condition was greater for the freshwater and brackish marsh soils than from the salt marsh soil, even when controlling for differences in the concentration in the surface water (p < 0.01).
Microbial indicators
The concentration of MBC in the salt marsh soil control was more than twice that of the freshwater and brackish marsh soil controls (p < 0.001; Table 6). The salinity-altering pulse of brackish water to the salt marsh soil caused an increase in MBC (p < 0.01) from 2,105 ± 159 mg kg−1 in the control, to 2,974 ± 365 mg kg−1 following the 13 ppt pulse. Time was not a significant factor for MBC and the amount of MBC was similar between the baseline cores (sampled on day 10) and the final cores (sampled on day 53). MBC was positively correlated with soil OM content, TLOC, LOC, dehydrogenase activity, and NH4 + concentration (p < 0.01; data not shown). TLOC among the control condition cores was highest in the salt marsh soils (p < 0.001). Similar to MBC, TLOC increased in the salt marsh soil following the pulse of brackish (13 ppt) water, from 2,345 ± 389 mg kg−1 in the control, to 3,236 ± 389 mg kg−1 following the pulse (p < 0.001). LOC did not differ between wetland types, nor was it affected by the salinity pulsing events. LOC was positively correlated with ß-glucosidase activity (p < 0.05; data not shown). The metabolic quotient (qCO2) was lower in the salt marsh soil compared to the freshwater marsh soil (p < 0.05) and was unaffected by the salinity pulsing events. The qCO2 was positively correlated with high tide CO2 flux and DOC release in the control condition soils, and positively correlated with CH4 flux and DOC release following the salinity pulsing events (Table 4).
Beta-glucosidase activity was lower in the brackish marsh soil compared to the freshwater marsh soil (p < 0.05; Table 6). The salinity pulsing events did not affect ß-glucosidase activity, but activity was positively correlated with dehydrogenase activity (p < 0.05), and organic matter content (p < 0.001). Dehydrogenase activity showed a similar pattern to ß-glucosidase activity, but differences between wetland types were not significant. Dehydrogenase activity was positively correlated with organic matter content and MBC (p < 0.05).
Discussion
Soil and water properties
The brackish marsh soil had higher bulk density, lower OM, total C, and total N content, and a large contribution of fines and inorganic ash, all of which is indicative of a system with greater tidal influence and higher inorganic sediment deposition. Although these characteristics are more commonly found in salt marshes (Craft 2007; DeLaune et al. 2002; Odum 1988), unique sedimentation patterns and/or the location of the estuary’s turbidity maximum in relation to our sampling sites may have contributed to the brackish marsh site being the most inorganic of the three wetland types (Wieski et al. 2010; Nyman et al. 1990). Despite the many physical and chemical similarities of the freshwater and salt marsh soils (e.g., bulk density, OM, C, and N content), the composition and classification of these soils varied greatly. The freshwater marsh soil consisted of highly decomposed herbaceous histisols (USDA 1978). Saprist soils such as these represent ~80 % of North American wetland soils and have one of the lowest soil hydraulic conductivities, acting similar to a sponge in its’ ability to retain water against gravitational pull (Bridgham et al. 2006; Boelter 1965). In contrast, the salt marsh soil was composed of less decomposed hemist soils with higher clay and silt content (USDA 1978). These distinctions become important when discussing the impact of tidal cycle on CO2 flux (see below).
Soil organic carbon loss
Despite extensive knowledge about the influence of inundation and water table on soil respiration rates in non-coastal wetlands, very few studies have investigated the impact of tides on coastal wetland CO2 flux. Low tide CO2 flux rates were higher than high tide rates in the brackish and salt marsh soils, as would be expected from increased soil oxygenation and redox potential following the drop in water level (D’Angelo and Reddy D’Angelo and Reddy 1999). However, there was no significant difference in CO2 flux from high tide to low tide in the freshwater marsh soil (Fig. 2). As a result, the magnitude of the tidal effect for the freshwater marsh was small, averaging only a 53 % increase in mean CO2 flux between high and low tides. This tidal effect is similar to the 50 % increase at low tide found by Neubauer et al. (2000) in a temperate tidal freshwater marsh, but is substantially less than the mean tidal effects observed for the brackish marsh (230 % increase at low tide) and salt marsh (310 % increase at low tide) in this study. Differences in the magnitude of the tidal effect along the salinity gradient is likely attributable to differences in the hydraulic conductivity of the soils. The brackish marsh soil drained the quickest during the simulated ebb tide (<2 h), followed by the salt marsh soil (2–6 h), and the freshwater marsh soil (up to 24 h); a pattern that mirrors the low tide CO2 flux rates. Soils with high water retention could allow anaerobic soil conditions to persist during low tide, and subsequently decrease the rate and efficiency of microbial respiration (Freeman et al. 1993). Additionally, salt can function as both a flocculating agent, causing hydrophilic colloids to aggregate, and as a dispersing agent, causing hydrophobic colloids to repel one another (Gregory 1989). Therefore, high concentrations of Na2+ in the brackish and salt marsh soils could increase both the size of soil aggregates and the quantity of macropore spaces in the soil profile, allowing water to drain quickly and the soil to become aerobic faster during low tide (Brady and Weil 2004). Knowledge of the general patterns in soil hydraulic conductivity along coastal wetland salinity gradients could improve the ability to predict how changes in inundation patterns affect C storage in these systems.
The effect of the pulsing events on SOC loss were overall most pronounced in the freshwater tidal marsh soil pulsed with both brackish (13 ppt) and salt (26 ppt) water, and the salt marsh soil pulsed with fresh (<0.5 ppt) water (Table 7). When the freshwater marsh soil was pulsed with brackish water, high tide CO2 flux was unaffected, but a 112 % increase in CO2 flux was observed during the subsequent low tide. This increase could be partially explained by an influx of SO4 2− to support sulfate reduction (Weston et al. 2006), but our data also show a strong correlation between the acceleration of low tide CO2 flux and a tripling in the concentration of NH4 + release. This correlation suggests a short-term disruption of N cycling following pulses of seawater, a concept supported by previous literature on chloride-induced inhibition of nitrification and denitrification (Seo et al. 2008; Roseberg et al. 1986), especially in systems not previously exposed to Cl− (Hale and Groffman 2006). Chloride concentrations as low as 80 mg L−1 caused the complete inhibition of denitrification enzyme activity in stream organic matter (Hale and Groffman 2006), and 13 ppt seawater contains approximately 7,000 mg Cl− L−1 (Kester et al. 1967). Hydrogen sulfide accumulation of 60–100 μM HS− in estuarine sediments has also been shown to suppress nitrification by 50–100 % (Joye and Hollibaugh 1995). Saltwater can further disrupt N-cycling by releasing NH4 + ions into solution through ionic exchange (Baldwin et al. 2006; Weston et al. 2006; Portnoy and Giblin 1997). Therefore, we hypothesize that during the high tide13 ppt pulse there was an increase in NH4 + availability, a precursor to coupled nitrification–denitrification, but nitrifiers were not able to utilize the additional inorganic N until the stress caused by Cl− and HS− were alleviated during the low tide dry-down. The pulse of saltwater (26 ppt) to the freshwater marsh soil had no effect on soil respiration during high tide or the subsequent low tide. Although the increase in SO4 2− occurred, the increase in NH4 + release seen with the 13 ppt pulse was not seen following the saltwater pulse. The reason for this is unclear, but it could be that the salinity was so high the freshwater marsh microbial community was under too much osmotic stress to respond to the increased SO4 2− availability or rebound from the inhibitory salinity effects on N-cycling bacteria. Overall, the freshwater marsh soil pulsed with brackish water was the only treatment in the entire study that showed a significant deviation from the control condition in the partitioning of SOC loss via the 4 pathways measured.
The pulse of fresh (0.5 ppt) water to the salt marsh soil did not affect the rate of soil respiration during high tide, but did cause an average of a 76 % increase in CO2 flux during the subsequent low tide. Past research has found that increasing tidal flushing can enhance productivity in salt marshes through decreased osmotic stress and the removal of deleterious compounds such as sulfide (King et al. 1982; King and Wiebe 1980). It follows that a flush of freshwater could accelerate microbial respiration in a salt marsh soil, as observed in this study. The only effect on CO2 flux during high tide was observed in the brackish marsh soil pulsed with fresh (0.5 ppt) water, which caused a 33 % decline in soil respiration rate relative to the control. Sulfate reduction is the dominate pathway for soil respiration in brackish marshes and SO4 2− tends to have a short residence time in the soil profile, being quickly utilized by microbes (Weston et al. 2006; DeLaune et al. 1983). The abrupt decline in SO4 2− availability when freshwater was added would have diminished the rate of sulfate reduction and required non-sulfate reducing microbes to activate quickly to maintain the same overall rate of soil respiration. The brackish marsh soil had lower microbial biomass than the salt marsh soil (Table 6) and microbial biomass and microbial diversity tends to be directly correlated (Cordova-Kreylos et al. 2006). Therefore, the low biomass of the brackish marsh soil may have prevented the microbial community from adapting quickly to the decrease in SO4 2− availability, whereas the salt marsh soil (with high microbial biomass) was unaffected. The absence of a low tide legacy effect from the freshwater pulse in the brackish marsh soil supports the earlier assertion that low tide respiration in the brackish marsh soil is dominated by aerobic microbes due to the high hydraulic conductivity of the soil, making SO4 2− availability superfluous during low tide.
Methanogenesis was an order of magnitude greater in the freshwater marsh control compared to all other treatments, as would be expected from pervious literature indicating a higher energy yield for sulfate reducers, compared to methanogens (D’Angelo and Reddy D’Angelo and Reddy 1999). However, the contribution of CH4 flux to overall SOC loss was still minimal in the freshwater marsh soil, representing only ~3 % of the total SOC loss (Fig. 6). In-situ rates of methanogenesis in freshwater tidal marshes in another study represented about 6 % of C loss; our rates may be underestimated because 95 % of CH4 is normally released through macrophytes, which were not included in the present study (Neubauer et al. 2000). Methanogenesis in the freshwater marsh soil was near-completely suppressed (97–98 %) following the addition of brackish and saltwater. Past laboratory studies have indicated that salinity concentrations of 14 and 35 ppt reduced CH4 flux by 79 and 94 %, respectively (Chambers et al. 2011). The addition of freshwater to the salt marsh soil caused a slight increase in methanogenesis. Although marginally significant, this finding may still have important global implications since CH4 has a radiative forcing 25 times greater than CO2 (IPCC 2007). These results suggests the discharge of precipitation run-off from urban development directly into salt marshes can accelerate both the production of CO2 during low tide and the release of CH4 to the atmosphere.
Dissolved OC release decreased in the freshwater marsh soil pulsed with brackish (13 ppt) water. In other laboratory studies, the addition of artificial seawater (no C source) to a freshwater wetland soil caused no change in DOC release (Weston et al. 2011). Since the ambient concentration of DOC in the brackish water was less than the freshwater (Table 3), the decrease in release may have been due to DOC absorption to the peat matrix, or the utilization of DOC as a substrate for microbial respiration (Freeman et al. 1997). An increase in the concentration of DOC release was observed following the freshwater pulse in the salt marsh soils, but may also simply be a result of higher DOC concentration in the freshwater marsh surface water. Past studies have shown dramatic increases in DOC release following the flooding of dry soils (Blodau and Moore 2003) and there has been speculation about the impact saltwater intrusion may pose on DOC release (Henman and Poulter 2008). Based on our findings, DOC release appears to be controlled by the mass balance between the concentration of DOC in the pulsed water relative to the ambient concentration in the soil porewater, rather than any short-term ionic displacement from the soil matrix.
Nutrient release
Water nutrient concentrations showed no clear correlation with salinity, nor were they strongly impacted by the salinity pulsing events. The only exception was NH4 +, which increased 3-fold in the freshwater marsh leachate following the addition of brackish (13 ppt) water. As mentioned, this could be the result of ionic displacement and/or the inhibition of nitrification by Cl− and/or HS−, which have been shown to cause an accumulation of NH4 + in the porewater (Azam and Ifzal 2006; Roseberg et al. 1986). The addition of 10 ppt artificial seawater to a freshwater sediment displaced 70 % of NH4 + from the soil exchange complex in an intact core study (Weston et al. 2011). Similarly, a 50-fold increase in porewater NH4 + concentration was observed in a microcosm study where non-saline wetland soils were flushed with 30 ppt seawater (Portnoy and Giblin 1997). A previous investigation of coastal wetland soils suggested PO4 2− (SRP) can be displaced by SO4 2− due to their similar chemical structure, but we found no evidence of this displacement in the current study (Bruland and DeMent 2009).
Microbial indicators
Microbial biomass C is a strong indicator of soil microbial community size that has been largely ignored in the coastal wetland literature. It is believed that the density and diversity of bacteria in freshwater and marine soils are comparable, while differing only in community composition (Capone and Kiene 1988). This study found MBC in the salt marsh soil to be more than double that of the freshwater and brackish marsh soils; TLOC, an indicator of bioavailable C, also showed the same pattern (Table 6). This could result from the combination of a strong marine influence introducing additional bacterial communities to the system and high organic matter content to support the additional marine microorganisms (Ikenaga et al. 2010; Blum et al. 2004). Interestingly, both MBC and TLOC increased even more in the salt marsh soil following the addition of brackish water (Table 7).
High ionic conductivity is known to inhibit enzyme activity in the soil, so this study also investigated the impact of pulsing events on enzyme synthesis (Frankenberger and Bingham 1982). ß-glucosidase, a soil enzyme involved in glucose production, is considered a good indicator of soil quality and general C mineralization rate (Makoi and Ndakidemi 2008). We found ß-glucosidase to be lower in the brackish marsh soil than the freshwater marsh soil, which is in agreement with other indicators of lower soil quality in the brackish marsh soil (e.g., lower organic matter content and higher C:N). No relationship was found between ß-glucosidase and salinity, as was also the case in a study by Jackson and Vallaire (2009). Dehydrogenase is produced during microbial respiration and was of interest due to existing contradictions in the literature. Some research has found that dehydrogenase activity is strongly inhibited by salinity (Saviozzi et al. 2011; Frankenberger and Bingham 1982); others have found a positive relationship between dehydrogenase activity and salinity (Wu et al. 2008); and the current study found no difference in dehydrogenase activity with salinity (Table 6). Although the chemical composition of the salinity treatment does vary in the previously cited literature, all contained Cl− and/or SO4 2−, both of which can inhibit dehydrogenase activity (Dinesh et al. 1995). In the present study, it appears that substrate availability may have exerted a greater constraint on dehydrogenase activity than salinity, as evidence by correlations between activity and indicators of soil quality (e.g., LOC (r = 0.77, p < 0.05) and inorganic ash content (r = –0.73. p < 0.05)).
The qCO2 is an index of the efficiency of the microbial community, such that stressed populations tend to produce more CO2 per individual and have a higher qCO2 (Wong et al. 2008). More saline environments are presumed to be more stressful to microbes and typically have a higher metabolic quotient (Saviozzi et al. 2011; Tripathi et al. 2006), but previous studies have found a decrease in qCO2 with increasing salinity, as we did in the present study (Wong et al. 2008). This could be a consequence of the type of microorganisms present in the soil. For example, fungi are known to produce less CO2 per individual than bacteria and are commonly associated with the decomposition of Spartina spp., the dominant source of litter material in our salt marsh site (Wong et al. 2008; Torzilli et al. 2006). With the exception of MBC and TLOC, no microbial indicators were affected by the salinity pulsing events.
Sea level rise implications
Aside from salinity pulsing effects, this study also identified noteworthy differences in the influence of tidal cycles (duration of inundation) on coastal wetland SOC loss that could have far-reaching repercussions for wetland C storage capacity during eustatic SLR. According to our results, as coastal marshes become more saline, carbon loss during low tide becomes increasingly important (Fig. 6). Using a simple linear model to extrapolate these SOC loss findings to a scenario of increased tidal inundation due to SLR rise suggests total SOC loss from freshwater tidal marsh soils may increase with greater tidal inundation, while SOC loss in brackish and salt marsh soils would decrease (not accounting for accretion). As the percent of time a marsh is inundated under high tide increases from 50 % (assumed present condition) to 100 % (always inundated), the mg C m−2 d−1 lost from the freshwater tidal marsh soil is expected to increase by approximately 4.3 mg for each 1 % increase in inundation time, while C loss in the brackish and salt marsh soils are expected to decrease by ~4.3 and 5.5 mg C m−2 d−1, respectively, for each 1 % increase in inundation time. A decrease in the rate of SOC loss could allow more C to remain stored in the soils of brackish and salt marshes, but it is important to consider that C inputs will likely also be altered by SLR. For example, one study found that tidal inundation (fully or partially submerging marsh vegetation) reduced the rate of C fixation by approximately 46 % compared to non-inundated conditions (Kathilankal et al. 2008). On an ecosystem scale, increased inundation may enhance plant productivity by promoting the deposition of sediments and nutrients (Kirwan and Mudd 2012). Predicting the overall effect of SLR on a wetland’s C balance requires a complete C budget to be calculated (including aboveground and belowground biomass and sediment deposition rates). Possibly the only study that has attempted to establish a complete C budget in a coastal wetland impacted by saltwater intrusion was performed by Neubauer (2011) in a tidal freshwater marsh. This study found that a 10 ppt increase in salinity decreased net ecosystem productivity (NEP) by 55 %, while greater freshwater inundation increased NEP by 75 %, resulting in no overall change when these two parameters (salinity and inundation) where combined.
Experimental design considerations
The use of intact soil cores to measure the response of SOC loss, nutrient release, and microbial indicators affected by salinity pulsing events allowed for greater control of environmental variables such as temperature, water column depth, C inputs via photosynthesis, and soil redox effects caused by primary producers. Although additional research is necessary to determine if all of our findings can be replicated under in situ conditions, a field study in a freshwater tidal marsh documented decreases in CO2 flux with both increased inundation and moderate increases in salinity (Neubauer 2011). Over longer time spans, processes such as soil accretion and deposition will be important in determining if the marsh platform does indeed experience longer periods of inundation and increased saltwater pulsing with SLR, or if deposition and OM accumulation compensate for the increased elevation of ocean. In field studies, a wide variety of responses to SLR have been documented in coastal wetlands (e.g., DeLaune and White 2011, and references therein) with local geomorphic and ecological factors often determining the ultimate fate of a wetland (Fagherazzi et al. 2012; Nicholls et al. 1999).
Conclusions
Coastal wetlands function as large storage reservoirs for global C in the form of SOC. In the contiguous United States, only 25,000 km2 of coastal (estuarine) wetlands sequester over 10 Tg C y−1 (Bridgham et al. 2006). The combined effects of global SLR and coastal urban development are well documented threats to the health and sustainability of coastal wetlands, as well as the SOC stored within them (DeLaune and White 2011; Craft et al. 2009). However, limited attention has been given to the short-term, dynamic fluctuations in salinity that occurs in coastal wetlands as a result of storm surges, extreme tidal events, and urban stormwater discharge. This study found that the impact of pulsing events on the rate of SOC loss along a coastal salinity gradient differs with wetland type and salinity. Freshwater tidal marsh SOC biogeochemistry was highly sensitive to pulses of brackish water; this type of pulsing event could become increasingly common in low relief coastal zones experiencing more extreme tidal events and storm surges with rising sea levels. Brackish water additions caused an increase in low tide CO2 flux and NH4 + release, a decrease in CH4 flux, and a shift in the partitioning of SOC loss via the 4 pathways studied (low and high tide CO2 flux, CH4 flux, and DOC release) in the freshwater marsh soil during a 3–5 day pulsing event. Based on knowledge of biogeochemical mechanisms described in previous literature, it is likely the brackish water pulse accelerated soil respiration by increasing SO4 2− reduction and coupled nitrification–denitrification of NH4 +, while simultaneously causing the near-complete suppression of methanogenesis through competition with sulfate-reducers. The brackish marsh soil was the least sensitive to pulses of higher or lower salinity surface water, possibly because these intermediate systems are already adapted to significant salinity fluctuations. However, decreases in CO2 flux were observed during pulses of freshwater in the brackish soil and were presumed to be the product of reduced SO4 2− availability for sulfate-reducing bacteria. The salt marsh soil responded to freshwater pulses with accelerated SOC loss, which may have been due to the flushing of accumulated Cl− and HS−, a reduction in osmotic stress, and the stimulation of low levels of methanogenesis. The salt marsh soil used in this study also benefited from a large soil microbial community, which could have allowed for quicker adaptation to changing environmental conditions. The increase in low tide CO2 flux in the salt marsh soil following pulses of freshwater has broad implications considering the juxtaposition of urban develop and many salt marshes, particularly where stormwater is discharged directly into adjacent tidal creeks and coastal wetlands. Most salt marshes respond to SLR through a natural feedback loop that promotes vertical marsh accretion (Fagherazzi et al. 2012), but our data indicates high volumes of freshwater urban run-off could diminish the ability of salt marshes to keep pace with SLR due to accelerated SOC loss. All three wetland types recovered quickly from salinity pulsing events, returning to baseline rates of SOC loss and nutrient release when natural salinities were re-established during the 9–12 day ambient site water phase between pulsing events. This demonstrates the rapidity of soil microbial responses to salinity change and the community’s flexibility in adapting to variations in the abundance of electron acceptors.
The ability of intertidal wetland soils to drain quickly during ebb tides proved crucial to the overall rate of CO2 flux in this study. High soil hydraulic conductivity, a function of both organic matter composition and the aggregation/dispersive forces of salts, tends to magnify the difference between high and low tide respiration rates in brackish and saline soils by allowing for the quick conversion from anaerobic to aerobic respiration following the drop in water level. In contrast, the highly decomposed organic soils of the freshwater marsh remained saturated even during low tide, diminishing the impact of tidal fluctuations of CO2 loss. Such variations in soil properties along natural salinity gradients could affect how C storage capacity responds to eustatic SLR, particularly with changes in inundation patterns. Based on our findings, an increase in the duration of inundation due to SLR could decrease the overall rate of SOC loss in brackish and salt marshes, while increasing the rate of SOC loss in freshwater tidal marshes.
References
Alef K (1995) Dehydrogenase activity. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, London, pp 228–230
Armentano TV, Menges ES (1986) Patterns of change in the carbon balance of organic soil- wetlands of the temperate zone. J Ecol 74(3):755–774
Azam F, Ifzal M (2006) Microbial populations immobilizing NH4 +–N and NO3 −–N differ in their sensitivity to sodium chloride salinity in soil. Soil Biol Biochem 38(8):2491–2494
Baldwin DS, Rees GN, Mitchell AM, Watson G, Williams J (2006) The short-term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland. Wetlands 26:455–464
Blodau C, Moore TR (2003) Micro-scale CO2 and CH4 dynamics in a peat soil during a water fluctuation and sulfate pulse. Soil Biol Biochem 35(4):535–547
Blum LK, Roberts MS, Garland JL, Mills AL (2004) Distribution of microbial communities associated with the dominant high marsh plants and sediments of the United States east coast. Microb Ecol 48(3):375–388
Boelter DH (1965) Hydraulic conductivity of peats. Soil Sci 100:227–231
Brady NC, Weil RR (2004) Elements of the nature and properties of soils, 2nd edn. Pearson Prentice Hall, New Jersey
Bridgham SD, Megonigal JP, Keller JK, Bliss NB, Trettin C (2006) The carbon balance of North American wetlands. Wetlands 26(4):889–916
Bruland GL, DeMent G (2009) Phosphorus sorption dynamics of Hawaii’s coastal wetlands. Estuaries Coasts 32(5):844–854
Capone DG, Kiene RP (1988) Comparison of microbial dynamics in marine and fresh-water sediments: contrasts in anaerobic carbon catabolism. Limnol Oceanogr 33(4):725–749
Chambers LG, Reddy KR, Osborne TZ (2011) Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Sci Soc Am J 75:2000–2007
Clymo RS (1983) Peat. Mines: swamp, bog, fen and moor. Gen Stud A:159–224
Cordova-Kreylos AL, Cao YP, Green PG, Hwang HM, Kuivila KM, LaMontagne MG, Van De Werfhorst LC, Holden PA, Scow KM (2006) Diversity, composition, and geographical distribution of microbial communities in California salt marsh Sediments. Appl Environ Microbiol 72:3357–3366
Coultas CL (1996) Soils of the intertidal marshes of Florida’s Gulf Coast. In: Coultas CL, Hesieh Y-P (eds) Ecology and management of tidal marshes. CRC Press, Boca Raton, pp 61–69
Craft C (2007) Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and U.S. tidal marshes. Limnol Oceanogr 52:1220–1230
Craft C, Clough J, Ehman J, Joye S, Park R, Pennings S, Guo HY, Machmuller M (2009) Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front Ecol Environ 7(2):73–78
D’Angelo EM, Reddy KR (1999) Regulators of heterotrophic microbial potentials in wetland soils. Soil Biol Biochem 31(6):815–830
Davis CS (2002) Statistical methods for the analysis of repeated measurements. Springer, New York
DeLaune RD, White JR (2011) Will coastal wetlands continue to sequester carbon in response to an increase in global sea level?: a case study of the rapidly subsiding Mississippi river deltaic plain. Clim Change 110(1–2):297–314
DeLaune RD, Smith CJ, Patrick WH (1983) Methane release from Gulf-coast wetlands. Tellus B 35:8–15
DeLaune RD, Devai I, Crozier CR, Kelle P (2002) Sulfate reduction in Louisiana marsh soils of varying salinities. Commun Soil Sci Plant Anal 33:79–94
Dinesh R, Ramanathan G, Singh H (1995) Influence of chloride and sulfate-ions on soil enzymes. J Agron Crop Sci-Zeitschrift Fur Acker Und Pflanzenbau 175(2):129–133
Edmonds JW, Weston NB, Joye SB, Mou XZ, Moran MA (2009) Microbial community response to seawater amendment in low-salinity tidal sediments. Microb Ecol 58:558–568
Fagherazzi S, Kirwan ML, Mudd SM, Guntenspergen GR, Temmerman S, D’Alpaos A, van de Koppel J, Rybczyk JM, Reyes E, Craft C, Clough J (2012) Numerical models of salt marsh evolution: ecological, geomorphic, and climate factors. Rev Geophys 50:28
Frankenberger WT, Bingham FT (1982) Influence of salinity on soil enzyme-activities. Soil Sci Soc Am J 46:1173–1177
Freeman C, Lock MA, Reynolds B (1993) Fluxes of CO2, CH4 and N2O from a Welsh peatland following simulation of water table draw down- potential feedback to climatic-change. Biogeochemistry 19:51–60
Freeman C, Liska G, Ostle NJ, Lock MA, Hughes S, Reynolds B, Hudson J (1997) Enzymes and biogeochemical cycling in wetlands during a simulated drought. Biogeochemistry 39(2):177–187
Gardner LR (2005) Role of geomorphic and hydraulic parameters in governing pore water seepage from salt marsh sediments. Water Resour Res 41:11
Gennari M, Abbate C, La Porta V, Baglieri A, Cignetti A (2007) Microbial response to Na2SO4 additions in a volcanic soil. Arid Land Res Manag 21:211–227
Gregory J (1989) Fundamentals of flocculation. Crit Rev Environ Control 19(3):185–230
Gribsholt B, Kristensen E (2003) Benthic metabolism and sulfur cycling along an inundation gradient in a tidal Spartina anglica salt marsh. Limnol Oceanogr 48(6):2151–2162
Gribsholt B, Boschker HTS, Struyf E, Andersson M, Tramper A, De Brabandere L, van Damme S, Brion N, Meire P, Dehairs F, Middelburg JJ, Heip CHR (2005) Nitrogen processing in a tidal freshwater marsh: a whole-ecosystem (15)N labeling study. Limnol Oceanogr 50(6):1945–1959
Hale RL, Groffman PM (2006) Chloride effects on nitrogen dynamics in forested and suburban stream debris dams. J Environ Qual 35(6):2425–2432
Henman J, Poulter B (2008) Inundation of freshwater peatlands by sea level rise: Uncertainty and potential carbon cycle feedbacks. J Geophys Res Biogeosci 113(G1):11
Ikenaga M, Guevara R, Dean AL, Pisani C, Boyer JN (2010) Changes in community structure of sediment bacteria along the Florida coastal everglades marsh-mangrove-seagrass salinity gradient. Microb Ecol 59(2):284–295
IPCC (2007) Climate change 2007: a synthesis report. Valencia, Spain
Jackson CR, Vallaire SC (2009) Effects of salinity and nutrients on microbial assemblages in Louisiana wetland sediments. Wetlands 29(1):277–287
Jolly ID, McEwan KL, Holland KL (2008) A review of groundwater-surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology. Ecohydrology 1(1):43–58
Joye SB, Hollibaugh JT (1995) Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270(5236):623–627
Kathilankal JC, Mozdzer TJ, Fuentes JD, D’Odorico P, McGlathery KJ, Zieman JC (2008) Tidal influences on carbon assimilation by a salt marsh. Environ Res Lett 3:6
Kester DR, Duedall IW, Connors DN, Pytkowic RM (1967) Preparation of artificial seawater. Limnol Oceanogr 12:176–178
King GM, Wiebe WJ (1980) Regulation of sulfate concentrations and methanogenesis in salt-marsh soils. Estuar Coast Mar Sci 10:215–223
King GM, Klug MJ, Wiegert RG, Chalmers AG (1982) Relation of soil-water movement and sulfide concentration to Spartina alterniflora production in a Georgia salt marsh. Science 218:61–63
Kirwan ML, Mudd SM (2012) Response of salt-marsh carbon accumulation to climate change. Nature 489(7417):550–554
Krauss KW, Whitbeck JL (2012) Soil greenhouse gas fluxes during wetland forest retreat along the lower Savannah River, Georgia (USA). Wetlands 32(1):73–81
Li CY, Weeks E, Rego JL (2009) In situ measurements of saltwater flux through tidal passes of Lake Pontchartrain estuary by Hurricanes Gustav and Ike in September 2008. Geophys Res Lett 36:5
Makoi J, Ndakidemi PA (2008) Selected soil enzymes: Examples of their potential roles in the ecosystem. Afr J Biotechnol 7(3):181–191
Marx MC, Wood M, Jarvis SC (2001) A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem 33:1633–1640
McKee KL, Mendelssohn IA (1989) Response of a fresh-water marsh plant community to increased salinity and increased water level. Aquat Bot 34(4):301–316
McLeod E, Chmura GL, Bouillon S, Salm R, Bjork M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO(2). Front Ecol Environ 9(10):552–560
Mendelssohn IA, Sorrell BK, Brix H, Schierup HH, Lorenzen B, Maltby E (1999) Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetland in Denmark. Aquat Bot 64(3–4):381–398
Michener WK, Blood ER, Bildstein KL, Brinson MM, Gardner LR (1997) Climate change, hurricanes and tropical storms, and rising sea level in coastal wetlands. Ecol Appl 7:770–801
Mousavi ME, Irish JL, Frey AE, Olivera F, Edge BL (2011) Global warming and hurricanes: the potential impact of hurricane intensification and sea level rise on coastal flooding. Clim Change 104:575–597
Muhammad S, Muller T, Joergensen RG (2006) Decomposition of pea and maize straw in Pakistani soils along a gradient in salinity. Biol Fertil Soils 43:93–101
Mulholland PJ, Best GR, Coutant CC, Hornberger GM, Meyer JL, Robinson PJ, Stenberg JR, Turner RE, VeraHerrera F, Wetzel RG (1997) Effects of climate change on freshwater ecosystems of the south-eastern United States and the Gulf Coast of Mexico. Hydrol Process 11:949–970
Neubauer SC (2011) Ecosystem responses of a tidal freshwater marsh experiencing saltwater intrusion and altered hydrology. Estuar Coasts DIO. doi:10.1007/s12237-011-9455-x
Neubauer SC, Miller WD, Anderson IC (2000) Carbon cycling in a tidal freshwater marsh ecosystem: a carbon gas flux study. Mar Ecol Prog Ser 199:13–30
Neubauer SC, Givler K, Valentine SK, Megonigal JP (2005) Seasonal patterns and plant-mediated controls of subsurface wetland biogeochemistry. Ecology 86(12):3334–3344
Nicholls RJ, Hoozemans FMJ, Marchand M (1999) Increasing flood risk and wetland losses due to global sea-level rise: regional and global analyses. Glob Environ Change-Hum Policy Dimens 9:S69–S87
NOAA (2011) National weather service Jacksonville. FL Weather Forecast Office. http://www.srh.noaa.gov/jax/, Accessed 2 February 2012
Nyman JA, Delaune RD, Patrick WH (1990) Wetland soil formation in the rapidly subsiding Mississippi River deltaic plain- mineral and organic-matter relationships. Estuar Coast Shelf Sci 31:57–69
Odum WE (1988) Comparative ecology of tidal fresh-water and salt marshes. Annu Rev Ecol Syst 19:147–176
Poffenbarger HJ, Needelman BA, Megonigal JP (2011) Salinity influence on methane emissions from tidal marshes. Wetlands 31(5):831–842
Portnoy JW, Giblin AE (1997) Biogeochemical effects of seawater restoration to diked salt marshes. Ecol Appl 7:1054–1063
Quintino V, Sangiorgio F, Ricardo F, Mamede R, Pires A, Freitas R, Rodrigues AM, Basset A (2009) In situ experimental study of reed leaf decomposition along a full salinity gradient. Estuar Coast Shelf Sci 85(3):497–506
Rasul G, Appuhn A, Muller T, Joergensen RG (2006) Salinity-induced changes in the microbial use of sugarcane filter cake added to soil. Appl Soil Ecol 31(1–2):1–10
Roberts J, Rowland A (1998) Cellulose fractionation in decomposition studies using detergent fiber pretreatment methods. Commun Soil Sci Plant Anal 25(11–14):269–277
Roseberg RJ, Christensen NW, Jackson TL (1986) Chloride, soil solution osmotic potential, and soil-pH effects on nitrification. Soil Sci Soc Am J 50(4):941–945
Saviozzi A, Cardelli R, Di Puccio R (2011) Impact of salinity on soil biological activities: a laboratory experiment. Commun Soil Sci Plant Anal 42(3):358–367
Seo DC, Yu K, Delaune RD (2008) Influence of salinity level on sediment denitrification in a Louisiana estuary receiving diverted Mississippi River water. Arch Agron Soil Sci 54:249–257
Smith SM (2009) Multi-decadal changes in salt marshes of Cape Cod, MA: Photographic analyses of vegetation loss, species shifts, and geomorphic change. Northeast Nat 16(2):183–208
Sparling GP, Feltham CW, Reynolds J, West AW, Singleton P (1990) Estimation of soil microbial C by fumigation extraction method- use on soils of high organic matter content and a reassessment of the KEC-factor. Soil Biol Biochem 22(3):301–307
Standard Methods (1997) Standard methods for the examination of water and wastewater, 20th edn. 4110 Determination of Anions by Ion Chromatography with chemical suppression of eluent conductivity
Thalmann A (1968) Zur Methodik der Bestimmung der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch Forsch 21:249–259
Torzilli AP, Sikaroodi M, Chalkley D, Gillevet PM (2006) A comparison of fungal communities from four salt marsh plants using automated ribosomal intergenic spacer analysis (ARISA). Mycologia 98(5):690–698
Tripathi S, Kumari S, Chakraborty A, Gupta A, Chakrabarti K, Bandyapadhyay BK (2006) Microbial biomass and its activities in salt-affected coastal soils. Biol Fertil Soils 42(3):273–277
Tripathi S, Chakraborty A, Chakrabarti K, Bandyopadhyay BK (2007) Enzyme activities and microbial biomass in coastal soils of India. Soil Biol Biochem 39(11):2840–2848
Tuxen K, Schile L, Stralberg D, Siegel S, Parker T, Vasey M, Callaway J, Kelly M (2011) Mapping changes in tidal wetland vegetation composition and pattern across a salinity gradient using high spatial resolution imagery. Wetlands Ecol Manage 19(2):141–157
USDA (1978) Soil survey of city of Jacksonville. Duval County, Florida
USEPA (1993) Methods for the determination of inorganic substances in environmental samples. USEPA 600/R-93/100: method 365.1
Van Ryckegem G, Verbeken A (2005) Fungal diversity and community structure on Phragmites australis (Poaceae) along a salinity gradient in the Scheldt estuary (Belgium). Nova Hedwigia 80:173–197
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707
Weston NB, Dixon RE, Joye SB (2006) Ramifications of increased salinity in tidal freshwater sediments: Geochemistry and microbial pathways of organic matter mineralization. J Geophys Res Biogeosci 111:14
Weston NB, Vile MA, Neubauer SC, Velinsky DJ (2011) Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102(1–3):135–151
White JR, Reddy KR (2001) Influence of selected inorganic electron acceptors on organic nitrogen mineralization in everglades soils. Soil Sci Soc Am J 65(3):941–948
White JR, Reddy KR (2003) Nitrification and denitrification rates of everglades wetland soils along a phosphorus-impacted gradient. J Environ Qual 32(6):2436–2443
Wieski K, Guo HY, Craft CB, Pennings SC (2010) Ecosystem functions of tidal fresh, brackish, and salt marshes on the Georgia coast. Estuaries Coasts 33:161–169
Williams K, Ewel KC, Stumpf RP, Putz FE, Workman TW (1999) Sea-level rise and coastal forest retreat on the west coast of Florida, USA. Ecology 80(6):2045–2063
Wong VNL, Dalal RC, Greene RSB (2008) Salinity and sodicity effects on respiration and microbial biomass of soil. Biol Fertil Soils 44(7):943–953
Wright AL, Reddy KR (2001) Heterotrophic microbial activity in northern Everglades wetland soils. Soil Sci Soc Am J 65(6):1856–1864
Wu Y, Tam NFY, Wong MH (2008) Effects of salinity on treatment of municipal wastewater by constructed mangrove wetland microcosms. Mar Pollut Bull 57:727–734
Acknowledgments
This research was supported in part by the Florida Sea Grant Nutrient Dynamics Fellowship, with funds from Scotts Miracle-Gro, Inc. The views expressed are those of the authors and do not necessarily reflect the view of these organizations. The authors would like to thank Matt Norton and Gavin Wilson for field and laboratory assistance during this project and Dr. Kanika Inglett for assistance with the soil enzyme analysis. The helpful comments of two anonymous reviewers improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chambers, L.G., Osborne, T.Z. & Reddy, K.R. Effect of salinity-altering pulsing events on soil organic carbon loss along an intertidal wetland gradient: a laboratory experiment. Biogeochemistry 115, 363–383 (2013). https://doi.org/10.1007/s10533-013-9841-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9841-5