Abstract
Soil extracellular enzymes mediate organic matter turnover and nutrient cycling yet remain little studied in one of Earth’s most rapidly changing, productive biomes: tropical forests. Using a long-term leaf litter and throughfall manipulation, we explored relationships between organic matter (OM) inputs, soil chemical properties and enzyme activities in a lowland tropical forest. We assayed six hydrolytic soil enzymes responsible for liberating carbon (C), nitrogen (N) and phosphorus (P), calculated enzyme activities and ratios in control plots versus treatments, and related these to soil biogeochemical variables. While leaf litter addition and removal tended to increase and decrease enzyme activities per gram soil, respectively, shifts in enzyme allocation patterns implied changes in relative nutrient constraints with altered OM inputs. Enzyme activity ratios in control plots suggested strong belowground P constraints; this was exacerbated when litter inputs were curtailed. Conversely, with double litter inputs, increased enzymatic investment in N acquisition indicated elevated N demand. Across all treatments, total soil C correlated more strongly with enzyme activities than soluble C fluxes, and enzyme ratios were sensitive to resource stoichiometry (soil C:N) and N availability (net N mineralization). Despite high annual precipitation in this site (MAP ~5 m), soil moisture positively correlated with five of six enzymes. Our results suggest resource availability regulates tropical soil enzyme activities, soil moisture plays an additional role even in very wet forests, and relative investment in C, N and P degrading enzymes in tropical soils will often be distinct from higher latitude ecosystems yet is sensitive to OM inputs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil extracellular enzymes play a critical role in organic matter decomposition, regulating both carbon (C) storage and the supply of essential nutrients to below- and above-ground communities (e.g. Aber and Melillo 1980; Berg 2000; Burns and Dick 2002). A better understanding of the feedbacks between soil physico-chemical conditions and enzyme activities can help improve our understanding of steady-state biogeochemical dynamics as well as how soil biogeochemical cycles may respond to current and future global changes (Sinsabaugh et al. 2009; Allison et al. 2010; Cusack et al. 2011). Theoretical and empirical work to date suggests soil enzyme production is coupled to local resource availability. Economic, “optimal allocation” models of enzyme dynamics rest on the assumption that enzyme production is sensitive to resource stoichiometry, allowing decomposer organisms to target those resources most in demand (Sinsabaugh and Moorhead 1994). For example, in northern hardwood forests, nitrogen fertilization tends to shift enzyme allocation away from lignin oxidation (and the mining of N, Moorhead and Sinsabaugh 2006) toward hydrolysis of more labile carbon compounds via hydrolytic enzymes (Saiya-Cork et al. 2002; Sinsabaugh et al. 2002; Waldrop et al. 2004; Zak et al. 2008; Cusack et al. 2011). Elsewhere, N additions to N-poor Hawaiian forests caused an increase in phosphatase enzyme activity (Olander and Vitousek 2000).
While these studies support optimal allocation theory, evidence from both broad-scale syntheses and small-scale laboratory studies suggests links between resource availability and enzyme activities are complex. In a 2009 meta-analysis, Sinsabaugh et al. observed a convergence of C, N, and P-mineralizing enzyme activities on a ratio of 1:1:1 across broad ecosystem types encompassing diverse resource stoichiometries. This result may imply that the flexibility of decomposers to alter enzyme production in response to different resource environments is limited by physiological or metabolic constraints. Other studies have observed that enzymes targeting specific bio-molecules display varied responses to resource levels, with production of some enzymes induced by supply of the decomposition product, while others are inhibited by the end product as optimal allocation models predict (Mcgill and Cole 1981; Sinsabaugh and Moorhead 1994; Geisseler and Horwath 2009; Hernandez and Hobbie 2010). These results highlight areas of uncertainty in our understanding of soil enzyme regulation in the context of resource availability.
As with a number of other components of ecosystem function (Townsend et al. 2008; Malhi et al. 2009; Hedin et al. 2009; Randerson et al. 2009), our understanding of soil extracellular enzyme dynamics is poor in tropical forests. And yet, tropical forests are both a key driver of biosphere–atmosphere feedbacks to global change (Townsend et al. 1992; Field et al. 1998; Bonan 2008) and are experiencing rapid changes in climate, atmospheric conditions and direct human disturbance (Clark et al. 2003; Wright 2005; Lewis et al. 2009; Chai and Tanner 2010; Miettinen et al. 2012). All of these changes have the potential to substantially alter the amount of organic matter delivered to soils (Guariguata and Ostertag 2001; Murty et al. 2002; Nemani et al. 2003; Taylor 2012). In turn, the fate of such organic matter inputs will depend on soil enzyme activities.
There are reasons to suspect tropical soil enzymes might display different enzymatic patterns and linkages with soil resources than their temperate counterparts. For instance, at the global scale total soil C appears to be a robust predictor of hydrolytic enzyme activities (Sinsabaugh et al. 2008). However, current enzyme databases are dominated by temperate sites. In contrast to high-latitude forests, tropical C cycling is rapid (Parton et al. 2007; Cusack et al. 2009), partly due to significant transfers of soluble C and nutrients from the litter layer to soil during the early stages of litter decay (Allison and Vitousek 2004; Cleveland and Townsend 2006; Wieder et al. 2009). Such fluxes regulate short-term variations in soil respiration (Cleveland and Townsend 2006; Cleveland et al. 2010). As such, one might expect stronger links between enzyme activities and transfers of dissolved OM from litter to soil than with total soil C pools. Moreover, organic matter cycling in tropical forests is more likely to be constrained by P rather than N availability (Vitousek and Sanford 1986; Herbert and Fownes 1995; Cleveland et al. 2002; Reich and Oleksyn 2004; Cleveland et al. 2011). Heterotrophic microbes in P-limited environments with high N availability should be able to allocate more cellular resources to the procurement of P (Marklein and Houlton 2012), leading to different ratios of C, N and P mineralizing enzymes than those observed in temperate soils.
Here we used two forms of resource manipulation—a long-term leaf litter manipulation and a throughfall reduction experiment—to explore links between soil resources and soil enzyme activities in a wet lowland tropical forest. Previous work in these experimental plots has revealed links between litter-to-soil dissolved organic carbon (DOC) transfers and organic matter decomposition (Wieder et al. 2009; Cleveland et al. 2010), litter P content and rates of mass loss (Wieder et al. 2009) as well as changes in soil resource stoichiometry with changes in OM quantity (Nemergut et al. 2010; Leff et al. 2012b). Specifically, the doubling of leaf litter inputs leads to an increase in dissolved and total soil organic matter C to N ratios. As such, we predicted that: (1) DOC concentrations would exhibit strong links with enzyme activities, (2) enzyme allocation patterns would generally support belowground P limitation, but (3) relative allocation to enzymes that mineralize C, N and P would change due to altered resource abundance and stoichiometry across the treatment plots. We tested these predictions by measuring the potential enzyme activity of six hydrolytic enzymes as well as a suite of soil biogeochemical properties.
Methods
Site description
The study was conducted in a primary, lowland wet tropical forest of high species diversity located on the Osa Peninsula, southwestern Costa Rica, near the town of Progresso in the Drake River Valley (8° 43′ N, 83° 37′ W). This forest, part of the Golfo Dulce Forest Reserve, receives ~5,000 mm rain per year and has a mean annual temperature of 26 °C. Annual temperature fluctuations are small but precipitation is highly seasonal—a pronounced dry season occurs at this site from December through March. The soils are classified as Ultisols and are derived from basaltic parent material (Berrange and Thorpe 1988). Prior work at the site has demonstrated exceptionally high rates of litterfall and decomposition, with a strong influence of the seasonal rainfall pattern on carbon fluxes (Cleveland et al. 2006; Cleveland and Townsend 2006). For more detailed descriptions of this site, see Cleveland et al. (2006, 2002) and Bern et al. (2005).
Leaf litter and throughfall manipulations
In April 2007, we initiated a leaf litter manipulation experiment with three treatments: litter removal (0×), double litter addition (2×) and control (1×). At monthly intervals, all fine litterfall was raked and removed from the 0× plots, weighed, homogenized and redistributed onto the 2× plots. Control plots received no litter manipulation (n = 10 per treatment). Each treatment plot was 3 × 3 m and plots were established in an area of the forest with minimal topographic variation. For more details regarding this experiment, see Wieder et al. (2011) and Leff et al. (2012b).
In September of 2007, a throughfall exclusion experiment was established in close proximity to the leaf litter manipulations. Throughfall exclusion shelters were constructed by cutting 5 cm diameter polyvinylchloride (PVC) pipes in half longitudinally and mounting them on 2.4 × 2.4 m aluminum frames (Cleveland et al. 2010; Wieder et al. 2009). The PVC was mounted at 5 or 15 cm intervals in order to shield one-quarter or one-half of incoming throughfall, and plots were not trenched as the intent of the experiment was to focus on changes in litter-to-soil transfers of dissolved organic matter. In the present study, we focused on plots that received a 50 % through-fall reduction (−50 %; n = 10). The throughfall exclusions were dismantled in 2009, while the leaf litter manipulations are ongoing.
Enzyme assays
Sub-samples of frozen soil from litter manipulations and −50 % throughfall plots were assayed for the activity of six hydrolytic extracellular enzymes. A previous test of sample storage methods for tropical soil enzyme activities indicated that soil freezing has only modest effects on overall activities, and no disproportionate effects on any given class of enzymes, thus making it a reasonable method for comparisons across enzyme types and treatments (Turner and Romero 2010). Assays were performed on soil samples collected at three time-points known from prior work to be especially important in understanding the biogeochemistry of this site: the early wet season (June 2008), the peak wet season (October 2008) and the dry season (March 2009). Soil samples were assayed using standard fluorometric microplate methods (Sinsabaugh et al. 2002; Saiya-Cork et al. 2002). The activity of one P, two N, and three C-mineralizing enzymes were examined using fluorescently labeled substrates (Table 1). Since substrates were added in non-limiting quantities, the assays are a measure of the potential activity of the residual soil enzyme pool, not in situ degradative activity (Wallenstein and Weintraub 2008).
Briefly, frozen soil was thawed for 30 min, then 1.0 g was combined with 125 ml of 50 μM sodium acetate buffer (pH 5.0) and homogenized with a Virtex 45 tissue homogenizer (Virtex Inc., Yonkers, NY, USA) for 1 min. Soil slurries were continuously stirred on a magnetic stir plate while 200 μl aliquots were dispensed into black 96-well assay plates, with 16 analytical replicate wells per sample. After plating all suspensions, 50 μl of 200 μM substrate solutions (Sigma-Aldrich, St. Louis, MO, USA) were added to the appropriate wells (Table 1). In addition to sample wells, negative control wells received 200 μl buffer plus 50 μl substrate solution, sample control wells received 200 μl sample plus 50 μl buffer, standard reference wells received 200 μl buffer plus 50 μl standard (10 μM 4-methylumbelliferone or 7-amino-4-methylcoumarin), and quench wells received 200 μl sample plus 50 μl reference standard, all in analytical replicates of eight. Plates were incubated at room temperature for 3–6.5 h, depending on the enzyme being assayed.
In order to optimize fluorescence, reactions were terminated by raising the pH in each well with 10 μl of 1 M sodium hydroxide (NaOH). The time between NaOH additions and fluorescence readings was short (circa 8 min) and consistent for all samples and enzymes (German et al. 2011). Fluorescence was measured at 365 nm excitation and 460 nm emission using a Fluoroskan II microplate fluorometer (Thermo Labsystems, Franklin, MA, USA). Enzyme activity was determined after correcting for sample quenching and fluorescence in sample controls and negative controls.
Soil biogeochemical analyses
We monitored a host of soil biogeochemical parameters throughout the duration of the experiments in order to assess the impacts of changes in leaf litter quantity and throughfall on nutrient cycling (Cleveland et al. 2010; Wieder et al. 2011). For this study, we focused on total, extractable, and dissolved soil nutrient pools with hypothesized links to soil extracellular enzyme activities (EEA). For soil nutrient analyses, surface soils (0–10 cm) were collected fresh, transported on ice to the University of Colorado Boulder within 72 h, sieved at 4 mm, and analyzed for gravimetric soil moisture.
Total soil C and N concentrations were determined using oven-dried soils that were ground and analyzed via combustion on a Carlo Erba EA 1110 elemental analyzer (CE Elantech, Lakewood, NJ, USA). Total soil P was measured using a hot sulfuric acid (H2SO4) and hydrogen peroxide (H2O2) digest of 0.5 g of soil on a block digester with a sequential heating regime (maximum temperature = 360 °C for 3 h; Tiessen and Moir 1993). Reference soils were included to test for digest efficiency, and total P was quantified by colorimetric analysis of PO4 3− concentrations (ascorbic acid method) on an Alpkem autoanalyzer (OI Analytical, College Station, TX, USA).
Inorganic N was extracted from fresh surface soils using 2 M potassium chloride. Net rates of N mineralization were determined on a sub-sample of soil incubated at 25 °C under field moisture conditions for 25 days. Net N mineralization was calculated as the difference between initial and day-25 total inorganic N concentrations (Hart et al. 1994). Ammonium (NH4 +) and nitrate (NO3 −) were measured colorimetrically using an Alpkem autoanalyzer (OI Analytical, College Station, TX, USA). Extractable P was determined on air-dried, ground soils using 0.5 M sodium bicarbonate (NaHCO3), following a partial Hedley fractionation method (Tiessen and Moir 1993). After an 18 h extraction, supernatants were decanted and digested with ammonium persulfate and sulfuric acid at 120 °C for 1 h. NaHCO3-extractable P was quantified by colorimetric analysis of PO4 3− concentrations (ascorbic acid method) on an Alpkem autoanalyzer.
We monitored dissolved organic matter fluxes from the litter layer to the soil using zero-tension surface lysimeters installed in experimental plots. For more detailed information on surface lysimeter construction, see Cleveland et al. (2010). Water from the lysimeters was collected bi-weekly and frozen. A sub-sample was sent to the University of Colorado for analysis of DOC and total dissolved nitrogen (TDN) concentrations on a total C–N analyzer (Shimadzu TOCvcpn, Kyoto, Japan).
Microbial biomass (MB) C and N were determined on fresh soils extracted with 0.5 M K2SO4 with and without exposure to a 5-day chloroform fumigation (Brookes et al. 1985; Beck et al. 1997). Total C and N from extracts were measured via combustion using a total C–N analyzer (Shimadzu TOCvcpn, Kyoto, Japan). MB was calculated as the difference between fumigated and unfumigated samples, without correction for extraction efficiency.
Statistical analyses
We calculated enzyme activities in three ways: nmol substrate converted per gram soil per hour (nmol g−1 h−1), per gram soil C per hour (nmol g soil C−1 h−1) and per milligram MB C per hour (nmol mg MB-C−1 h−1). We took this course because we knew that soil and microbial C could be important determinants of soil enzyme activities (Sinsabaugh et al. 2008) and that they changed in response to the treatments (Nemergut et al. 2010; Wieder et al. 2011; Leff et al. 2012a).
Prior to statistical analyses, enzyme activities were natural log transformed (ln) to satisfy assumptions of normality and homoscedasticity. We used analysis of variance (ANOVA) of linear mixed effects models, with treatment and season as fixed factors and plot as a random effect, to determine the effects of litter or throughfall treatment on soil EEA. Since the interaction between the two fixed factors was insignificant in all cases, we removed the interaction term from enzyme statistical models and focused on the effects of the treatments rather than season. Quantile–quantile (qq) plots were used to check models for random distribution of residuals. In order to characterize enzyme allocation patterns, we calculated the ratios of enzymatic acquisition of P relative to C (BG:aP), N relative to C [BG:(NAG + LAP)] and P relative to N [(NAG + LAP):aP] and conducted ANOVA of mixed effects models to test for treatment effects on these ratios as above.
To determine the effect of the treatments on soil biogeochemical properties, we used ANOVA of mixed effects models with treatment and season as fixed factors and plot as a random effect. In the cases where interactions between treatment and season were significant, the interaction was included in the model structure. We restricted our analyses to time-points when both biogeochemical properties/processes and enzyme activities were measured (i.e. June 2008, October 2008 and March 2009). For time-integrated biogeochemical measurements from these manipulations, see Cleveland et al. (2010), Wieder et al. (2011) and Leff et al. (2012b). Soil environmental data were ln or square root transformed if distributions did not meet assumptions of normality prior to analysis. In order to explore links with soil variables and enzyme activities, we used Pearson’s product-moment correlations. All statistical analyses were conducted in R version 2.9.2 (R Core Development Team, Vienna Austria) and significant differences were determined at P < 0.05.
Results
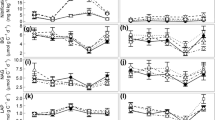
Leaf litter manipulation caused substantial shifts in EEA while throughfall reduction did not. Relative to controls, higher potential activity per gram soil was detected in 2× plots for five of the six enzymes measured, with NAG displaying the largest response (Fig. 1a; ESM Table 1). Litter removal resulted in significantly lower activities per gram soil for NAG, BG, and CBH, with strongest declines in the two C-degrading enzymes. When normalized per gram soil C and MB C, EEA revealed a slightly different picture. NAG activity still exhibited a sizable increase in 2× plots even when normalized by soil C and MB C. However, aP activity was not significantly higher (Fig. 1b, c; ESM Table 1). On the other hand, aP activity per gram soil C and per milligram MB C was significantly higher in 0× plots relative to controls while NAG activity was unchanged. The activity of the cellulose-degrading enzyme CBH increased significantly in 2× plots and decreased significantly in 0× plots even when normalized by soil C. The other two C-degrading enzymes showed more subtle responses: BG tended to increase and decrease with litter addition and removal (respectively) but BX displayed higher activity in both 2× and 0× plots per milligram MB-C (Fig. 1c). We detected no significant changes in the activity of LAP in experimental plots and no differences in soil enzyme activities in response to the −50 % treatment.
Response ratios of enzymes activities (treatment/control) per gram soil (a), per gram soil carbon (b), and per milligram microbial biomass carbon (c; −50 % fifty percent = throughfall reduction, 0× = litter removal, 2× = double litter). Horizontal dashed line indicates 1:1 value. Stars indicate significant differences between treatment and control values based on results of linear mixed effects models (ESM Table 1). One star means treatment values were lower than controls while two stars indicate treatment values were higher than controls
Similar to the EEA trends above, enzyme stoichiometry was markedly impacted by the leaf litter treatments but not by reduction in throughfall. The mean BG:aP ratio of enzyme activity in control plots was 0.22. This ratio did not change significantly in double-litter plots but declined to 0.13 in litter removal plots (F = 10.19, P < 0.001; Fig. 2). Enzyme N to P stoichiometry also showed robust response to the treatments. Control plot (NAG + LAP):aP ratios averaged 0.151. Litter removal caused this ratio to fall to 0.117 and litter addition caused it to increase to 0.212 (F = 12.88, P < 0.001; Fig. 2). Ratios of BG:(NAG + LAP) did not change significantly with treatment.
Litter manipulation altered many soil biogeochemical properties, which helps contextualize enzymatic responses. Notably, litter addition resulted in elevated surface soil C concentrations (+30 %) and a higher soil C:N ratio (13.8 vs. 11.7 in 1× plots; Table 2). Litter removal caused declines in soil C and N, but as they were of similar magnitude, soil C:N in 0× plots did not change. We did not detect changes in total soil P or extractable P in the treatment plots. Mean extractable NH4 + concentration was significantly higher with litter addition, but net N mineralization rates were highest in control plots while tending to decline when litter was either added or removed (Table 2). This trend was only marginally significant but the largest decline was in double litter plots (P = 0.081). None of the above soil properties were significantly altered by the throughfall reduction treatment.
The concentration of DOC in litter-to-soil fluxes was strongly affected by both litter and throughfall treatments, with a near tripling of [DOC] in −50 % plots, a doubling of [DOC] in 2× plots and a 59 % reduction in 0× plots compared to controls. However, the ratio of DOC to TDN was similar in −50 % plots while it significantly increased and decreased in 2× and 0× plots respectively (Table 2). Both MB C and gravimetric soil moisture declined significantly with leaf litter removal but did not change in response to other treatments. The lack of a strong response in soil moisture to the throughfall reduction was intentional, as the plots were not trenched to focus the manipulation on rainfall-driven variations in litter-to-soil transfers of dissolved organic matter.
Many of these soil variables displayed some degree of positive correlation with enzyme activities. Concentrations of total soil C displayed stronger positive association with enzyme activities than concentrations of soluble C (Table 3). While soil C was more strongly correlated with individual enzymes, the soil C:N ratio was the strongest correlate of two of the three enzyme ratios. We did not detect any direct evidence from our soil chemical data of end-product inhibition, as inorganic N and P concentrations did not correlate negatively with N and P mineralizing enzyme activities or enzyme ratios. However, we did detect a positive correlation between rates of net N mineralization and BG:(NAG + LAP) activity and a negative correlation between rates of net N mineralization and (NAG + LAP):aP. MB N was more strongly associated with enzyme activities then MB C, and soil moisture displayed significant positive correlations with five of the six enzymes assayed.
Discussion
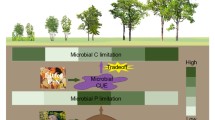
Here we used two forms of resource manipulation to examine how tropical soil enzyme activities and ratios shift with OM inputs. We found leaf litter addition and removal had notable impacts on soil EEA while throughfall reduction did not. This was most likely due to robust links between total soil C and enzyme activities in the experimental plots but a lack of strong correlation between DOC and enzymes. As soil C changed markedly with litter manipulation but was not altered by throughfall reduction, the observed enzyme response per gram soil to the treatments was not surprising. Given the importance of DOC concentrations and fluxes for regulating soil respiration and litter decomposition rates in our study site (Cleveland et al. 2006, 2010; Wieder et al. 2009), we hypothesized that [DOC] would exhibit significant correlations with soil enzyme activities. This hypothesis was not supported; instead we found that five of the six soil enzymes measured showed strong positive correlations with soil C (Table 3), similar to what has been observed in other biomes (Sinsabaugh et al. 2008). The lack of correlation between [DOC] and enzyme activities (Table 3) and DOC fluxes and enzyme activities (data not shown) may be due to the high variability of DOC pools in space and time. Litter-to-soil DOC fluxes vary with litter composition, litter layer thickness, rainfall intensity, and other variables (Montano et al. 2007; Wieder et al. 2009; Fujii et al. 2011). As such, it may be a more fruitful strategy for soil decomposer microbes to attune enzymes, which can have long life-spans within the soil environment (Burns 1982; Allison 2006), with total soil OM, essentially integrating dissolved OM fluxes over time.
As the proximal agents of organic matter mineralization, soil enzymes can provide valuable information on belowground nutrient limitation (Sinsabaugh et al. 2008; Sinsabaugh and Follstad Shah 2012). As we predicted, enzyme stoichiometries suggest strong P constraints in this tropical soil community. Our control plot BG:aP (0.22 ± 0.02) and (NAG + LAP):aP (0.15 ± 0.01) ratios are much lower than the global soil averages of 0.62 and 0.44, respectively (Sinsabaugh et al. 2009), with enzyme allocation favoring P acquisition. These allocation patterns indicate that soil organisms direct more effort toward acquiring and cycling P relative to C and N in our tropical site compared to extra-tropical ones. Though they diverge from global means, our enzyme ratios are quite comparable to the handful of tropical sites where similar measurements have been made (Sinsabaugh et al. 2008). While other factors may play a role, these allocation patterns are an indirect yet compelling line of evidence for elevated heterotrophic P demand in the dominant soil orders of lowland tropical forest soils (Ultisols and Oxisols; Sanchez et al. 1982), and complement studies that have observed microbial responses to the addition of P in such soils (Cleveland et al. 2002; Ilstedt and Singh 2005; Cleveland and Townsend 2006; Reed et al. 2010).
That said, the changes in soil enzyme allocation patterns we observed following leaf litter manipulation highlight the potential for OM inputs to impact not only nutrient supply, but relative nutrient limitation. The significant drop in the BG:aP activity ratio (Fig. 2) and the increase in aP activity per unit soil C and MB C (Fig. 1b, c; ESM Table 1) upon litter removal suggest P constraints are further exacerbated when leaf litter inputs are curtailed. The importance of leaf litter as a source of P has been noted in other tropical litter manipulations (Wood et al. 2009) and is not surprising given the changes in P pools and P availability in highly weathered soils (Walker and Syers 1976; Crews et al. 1995; Turner and Engelbrecht 2011). Although we did not observe significant changes in chemically extractable or total P pools in treatment plots (similar to Sayer et al. 2012), the shifts in enzyme activities suggest demand for P was elevated upon leaf litter removal.
By contrast, leaf litter addition seemed to shift enzymatic investment toward the acquisition of nitrogen. The significant increase in the (NAG + LAP):aP ratio in 2× plots (Fig. 2) indicates a change in enzyme allocation away from P and toward N-mineralization. The large increase in NAG activity per unit soil C and MB C (Fig. 1b, c; ESM Table 1) also supports this interpretation. This enzymatic reallocation points towards increasing N demand, which is probably linked to increases in the C:N ratio of both total and dissolved organic matter pools in double litter plots (Table 2). In extra-tropical ecosystems exposed to free-air CO2 enrichment, C-rich conditions cause elevated production of detritus and higher tissue C:N ratios. Over time, this appears to trigger progressive nitrogen limitation (PNL; Luo et al. 2004; Reich et al. 2006). Many tropical forests have large N-stocks and a high capacity for biological N-fixation (Cleveland et al. 1999; Reed et al. 2007; Hedin et al. 2009), thus the nature and timing of increasing N constraints under C-rich conditions is not likely to mirror temperate systems. But while the common paradigm for lowland tropical forests suggests a relative abundance of N (Vitousek and Matson 1988; Martinelli et al. 1999; Hedin et al. 2009), the importance of N limitation seems to increase in wetter tropical forests (Austin and Vitousek 1998; Houlton et al. 2006; Nardoto et al. 2008; Posada and Schuur 2011). Our study site lies in the wetter end of the lowland tropical forest rainfall spectrum, and multiple lines of evidence suggest N may not cycle in excess even under baseline conditions (Cleveland and Townsend 2006; Wieder et al. 2012; Taylor 2012). Thus, the relative increase in N-specific enzyme activity under higher OM inputs suggests the potential for PNL to develop fairly quickly in at least some lowland forests. As well, the links we observed between net N mineralization and enzyme stoichiometry (Table 3) indicate that changes in N availability mediated by altered OM inputs may have cascading consequences for belowground nutrient cycling.
Enzymes that degrade OM to provide energy (C) versus nutrients (N and P) may be regulated by resource availability in different ways. While evidence suggests inverse relationships between N and P availability and N and P mineralizing enzyme activities (McGill and Cole 1981; Sinsabaugh and Moorhead 1994; Olander and Vitousek 2000), C-degrading enzymes may in fact be stimulated by elevated concentrations of their substrates and end-products (Hernandez and Hobbie 2010). Our results support the interpretation that C-degrading enzymes are up-regulated when their substrates are abundant, not scarce. In litter addition plots, the activity of the cellulose-degrading enzyme CBH was significantly higher than control plot levels, even when concentrations of soil C and MB C were accounted for. This result suggests shifts in the importance of cellulose as a substrate with increases in litter quantity. In 0× plots that received essentially no leaf litter for an extended period, C-mineralizing enzyme activities were largely suppressed. This trend was especially pronounced for CBH and is not surprising given the lack of plant-derived cellulose that would result from litter removal. These findings are consistent with laboratory incubations that showed an increase in cellulase activity following additions of cellulose (Geisseler and Horwath 2009).
Aside from direct regulation by soil resource availability, it is also possible that changes in the composition of the soil microbial community with litter addition and removal (Nemergut et al. 2010; Leff et al. 2012a) may have mediated some of the observed changes in enzyme activities and allocation patterns. For example, litter-rich conditions in 2× plots may have promoted the proliferation of decomposer microbes that specialize on holocellulose (Stursova et al. 2012), with consequent increases in cellulase production. The fact that we observed tighter links with EEA and MB N versus MB C suggests that functional aspects of the microbial community may be important to consider. Recent conceptual models have highlighted the importance of fungi, both free living and mycorrhizal, in a C-rich world (Pendall et al. 2004; Carney et al. 2007; Johnson 2010). While it is possible that shifts in the relative abundance of bacteria and fungi impacted the enzyme patterns we observed in response to litter manipulation, both qPCR and fluorescent microscopy indicated a low abundance of soil fungi, regardless of treatment, within the study soils (Nemergut and Weintraub, unpublished data).
Finally, while soil resources and their ratios clearly exert significant control on enzyme activities, so too do climate variables (Freeman et al. 2001; Stursova et al. 2006; Wallenstein et al. 2009; Brzostek and Finzi 2011). In our study system, soil moisture content exhibited positive correlations with five of the six enzymes measured (Table 3). This result is notable in that overall moisture content in these high-clay but well-drained soils shows only minor temporal variation (Cleveland and Townsend 2006). Even so, we observed higher soil enzyme activities during the wet season when soils were slightly wetter than our dry season sampling date (data not shown). Differences in soil moisture between 0× and control plots likely also played a role. These results imply that variation in soil moisture conditions may be important for depolymerization rates, even in very wet tropical forests. Such variation can occur not only because of seasonal rainfall patterns, but via spatial variation in factors such as litter depth and topography. The mechanisms that underlie links with soil moisture and enzyme activities are not known but may include direct effects of water availability on microbial activity or indirect effects related to changes in soil oxygen levels (Silver et al. 1999) and/or the flux of labile C and nutrients (sensu Cleveland et al. 2006, 2010), although as noted above soluble C was not a significant predictor of EEA.
Taken as a whole, our study reinforces the growing conceptual understanding of enzyme regulation, in which soil C is an important predictor of enzyme activities (Sinsabaugh et al. 2008) yet soil nutrient availability and resource ratios regulate enzyme allocation patterns. Our results are broadly consistent with the theoretical framework underlying optimal allocation models (Sinsabaugh and Moorhead 1994; Treseder and Vitousek 2001), but also suggest that factors beyond resource-driven feedbacks—e.g. climate variability—may be important even at local scales. Our results also support the idea that patterns in enzyme allocation to C versus N versus P acquisition will be distinct in tropical forests relative to higher latitude ecosystems and shifts in OM inputs can impact the balance between relative N and P limitation. Finally, the data presented here add to a body of literature (e.g. Waldrop et al. 2004; Carney et al. 2007; Allison et al. 2010; Cusack et al. 2011) that suggests soil enzyme activities are a useful tool for assessing changes in belowground nutrient limitation and OM cycling with environmental change, both within and beyond tropical ecosystems.
References
Aber JD, Melillo JM (1980) Litter decomposition—measuring relative contributions of organic-matter and nitrogen to forest soils. Can J Bot (Revue Canadienne De Botanique) 58(4):416–421
Allison SD (2006) Soil minerals and humic acids alter enzyme stability: implications for ecosystem processes. Biogeochemistry 81(3):361–373
Allison SD, Vitousek PM (2004) Extracellular enzyme activities and carbon chemistry as drivers of tropical plant litter decomposition. Biotropica 36(3):285–296
Allison S, Wallenstein M, Bradford M (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Austin A, Vitousek P (1998) Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 113:519–529
Beck T, Joergensen RG, Kandeler E, Makeschin F, Nuss E, Oberholzer HR, Scheu S (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biochem 29(7):1023–1032
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manag 133(1–2):13–22
Bern CR, Townsend AR, Farmer GL (2005) Unexpected dominance of parent-material strontium in a tropical forest on highly weathered soils. Ecology 86(3):626–632
Berrange JP, Thorpe RS (1988) The geology, geochemistry and emplacement of the cretaceous tertiary ophiolitic Nicoya Complex of the Osa Peninsula, southern Costa-Rica. Tectonophysics 147(3–4):193–220
Bonan GB (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320(5882):1444–1449
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil-nitrogen—a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842
Brzostek ER, Finzi AC (2011) Substrate supply, fine roots, and temperature control proteolytic enzyme activity in temperate forest soils. Ecology 92(4):892–902
Burns RG (1982) Enzyme-activity in soil—location and a possible role in microbial ecology. Soil Biol Biochem 14(5):423–427
Burns RG, Dick RP (2002) Enzymes in the environment: activity, ecology and applications. Marcel Dekker, New York
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc Natl Acad Sci USA 104:4990–4995
Chai SL, Tanner E (2010) Are we losing the best parts of our protected areas in tropical mountains? Biotropica 42:739–747
Clark D, Piper S, Keeling C, Clark D (2003) Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. Proc Natl Acad Sci USA 100:5852–5857
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci USA 103(27):10316–10321
Cleveland CC, Townsend AR, Schimel D et al (1999) Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Biogeochemistry 13:623–645
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5(7):680–691
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87(2):492–503
Cleveland CC, Wieder WR, Reed SC, Townsend AR (2010) Experimental drought in a tropical rain forest increases soil carbon dioxide losses to the atmosphere. Ecology 91(8):2313–2323
Cleveland C, Townsend A, Taylor P et al (2011) Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol Lett 14:939–947
Crews T, Kitayama K, Fownes J et al (1995) Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76:1407–1424
Cusack DF, Chou WW, Yang WH, Harmon ME, Silver WL, Team L (2009) Controls on long-term root and leaf litter decomposition in neotropical forests. Glob Change Biol 15(5):1339–1355
Cusack DF, Silver WL, Torn MS, Burton SD, Firestone MK (2011) Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92(3):621–632
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281(5374):237–240
Freeman C, Ostle N, Kang H (2001) An enzymic ‘latch’ on a global carbon store—a shortage of oxygen locks up carbon in peatlands by restraining a single enzyme. Nature 409(6817):149
Fujii K, Hartono A, Funakawa S et al (2011) Fluxes of dissolved organic carbon in three tropical secondary forests developed on serpentine and mudstone. Geoderma 163:119–126
Geisseler D, Horwath WR (2009) Relationship between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia 53(1):87–98
German DP, Weintraub MN, Grandy AS, Lauber CL et al (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397
Guariguata MR, Ostertag R (2001) Neotropical secondary forest succession: changes in structural and functional characteristics. For Ecol Manag 148:185–206
Hart SC, Stark JM, Davidson EA, Firestone MK (1994) Nitrogen mineralization, immobilization and nitrification. In: Weaver RW (ed) Methods of soil analysis, part 2: microbiological and biochemical properties. Soil Science Society of America, Madison, pp 985–1018
Hedin LO, Brookshire E, Menge D, Barron A (2009) The nitrogen paradox in tropical forest ecosystems. Annu Rev Ecol Evol Syst 40:613–635
Herbert DA, Fownes JH (1995) Phosphorus limitation of forest leaf area and net primary production on a highly weathered soil. Biogeochemistry 29:223–235
Hernandez DL, Hobbie SE (2010) The effects of substrate composition, quantity, and diversity on microbial activity. Plant Soil 335(1–2):397–411
Houlton BZ, Sigman D, Hedin LO (2006) Isotopic evidence for large gaseous nitrogen losses from tropical rainforests. Proc Natl Acad Sci USA 103:8745–8750
Ilstedt U, Singh S (2005) Nitrogen and phosphorus limitations of microbial respiration in a tropical phosphorus-fixing acrisol (ultisol) compared with organic compost. Soil Biol Biochem 37:1407–1410
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647
Leff J, Nemergut D, Grandy A et al (2012a) The effects of soil bacterial community structure on decomposition in a tropical rain forest. Ecosystems 15:284–298
Leff J, Wieder W, Taylor P et al (2012b) Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob Change Biol 18:2969–2979
Lewis S, Lopez-Gonzalez G, Sonké B et al (2009) Increasing carbon storage in intact African tropical forests. Nature 457:1003–1006
Luo Y, Su B, Currie WS et al (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731
Malhi Y, Aragão L, Metcalfe DB et al (2009) Comprehensive assessment of carbon productivity, allocation and storage in three Amazonian forests. Glob Change Biol 15:1255–1274
Marklein A, Houlton B (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704
Martinelli L, Piccolo M, Townsend A et al (1999) Nitrogen stable isotopic composition of leaves and soil: tropical versus temperate forests. Biogeochemistry 46:45–65
Mcgill WB, Cole CV (1981) Comparative aspects of cycling of organic C, N, S and P through soil organic-matter. Geoderma 26(4):267–286
Miettinen J, Shi C, Liew SC (2012) Two decades of destruction in Southeast Asia’s peat swamp forests. Front Ecol Environ 10:124–128
Montano NM, García-Oliva F, Jaramillo VJ (2007) Dissolved organic carbon affects soil microbial activity and nitrogen dynamics in a Mexican tropical deciduous forest. Plant Soil 295:265–277
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76(2):151–174
Murty D, Kirschbaum M, Mcmurtrie R, Mcgilvray H (2002) Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Glob Change Biol 8:105–123
Nardoto GB, Ometto JPHB, Ehleringer JR et al (2008) Understanding the influences of spatial patterns on N availability within the Brazilian amazon forest. Ecosystems 11:1234–1246
Nemani RR, Keeling CD, Hashimoto H et al (2003) Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300:1560–1563
Nemergut DR, Cleveland CC, Wieder WR, Washenberger CL, Townsend AR (2010) Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol Biochem 42(12):2153–2160
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 49(2):175–190
Parton W, Silver WL, Burke IC et al (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315(5814):361–364
Pendall E, Bridgham S, Hanson PJ et al (2004) Below-ground process responses to elevated CO2 and temperature: a discussion of observations, measurement methods, and models. New Phytol 162:311–322
Posada JM, Schuur EAG (2011) Relationships among precipitation regime, nutrient availability, and carbon turnover in tropical rain forests. Oecologia 165:783–795
Randerson JT, Hoffman FM, Thornton PE et al (2009) Systematic assessment of terrestrial biogeochemistry in coupled climate-carbon models. Glob Change Biol 15:2462–2484
Reed SC, Cleveland CC, Townsend AR (2007) Controls over leaf litter and soil nitrogen fixation in two lowland tropical rain forests. Biotropica 39:585–592
Reed SC, Vitousek PM, Cleveland CC (2010) Are patterns in nutrient limitation belowground consistent with those aboveground: results from a 4 million year chronosequence. Biogeochemistry 106:323–336
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA 101:11001–11006
Reich PB, Hobbie SE, Lee T et al (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34(9):1309–1315
Sanchez PA, Bandy DE, Villachica JH, Nicholaides JJ (1982) Amazon basin soils: management for continuous crop production. Science 216:821–827
Sayer EJ, Joseph Wright S, Tanner EVJ et al (2012) Variable responses of lowland tropical forest nutrient status to fertilization and litter manipulation. Ecosystems 15:387–400
Silver WL, Lugo AE, Keller M (1999) Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry 44(3):301–328
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–333
Sinsabaugh RL, Moorhead DL (1994) Resource-allocation to extracellular enzyme-production—a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26(10):1305–1311
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60(1):1–24
Sinsabaugh RL, Lauber C, Weintraub M et al (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462(7274):795–798
Stursova M, Crenshaw CL, Sinsabaugh RL (2006) Microbial responses to long-term N deposition in a semiarid grassland. Microb Ecol 51:90–98
Stursova M, Zifčáková L, Leigh MB et al (2012) Cellulose utilisation in forest litter and soil: identification of bacterial and fungal decomposers. FEMS Microb Ecol 80:735–746
Taylor PG (2012) Carbon and nutrient cycling in tropical forests: climatic, hydrologic and stoichiometric controls. Dissertation. University of Colorado, Boulder
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis Publishers, Boca Raton, pp 75–86
Townsend A, Vitousek P, Holland E (1992) Tropical soils could dominate the short-term carbon cycle feedbacks to increased global temperatures. Clim Change 22:293–303
Townsend A, Asner G, Cleveland C (2008) The biogeochemical heterogeneity of tropical forests. Trends Ecol Evol 23:424–431
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82(4):946–954
Turner BL, Engelbrecht BMJ (2011) Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 103:297–315
Turner B, Romero T (2010) Stability of hydrolytic enzyme activity and microbial phosphorus during storage of tropical rain forest soils. Soil Biol Biochem 42:459–465
Vitousek P, Matson P (1988) Nitrogen transformations in a range of tropical forest soils. Soil Biol Biochem 20:361–367
Vitousek PM, Sanford RJ (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36(9):1443–1451
Walker T, Syers J (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Wallenstein MD, Weintraub MN (2008) Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol Biochem 40(9):2098–2106
Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Change Biol 15(7):1631–1639
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90(12):3333–3341
Wieder WR, Cleveland CC, Townsend AR (2011) Throughfall exclusion and leaf litter addition drive higher rates of soil nitrous oxide emissions from a lowland wet tropical forest. Glob Change Biol. doi:10.1111/j.1365-2486.2011.02426.x
Wieder WR, Cleveland CC, Taylor PG et al (2012) Experimental removal and addition of leaf litter inputs reduces nitrate production and loss in a lowland tropical forest. Biogeochemistry. doi:10.1007/s10533-012-9793-1
Wood T, Lawrence D, Clark D, Chazdon R (2009) Rain forest nutrient cycling and productivity in response to large-scale litter manipulation. Ecology 90:109–121
Wright SJ (2005) Tropical forests in a changing environment. Trends Ecol Evol 20:553–560
Zak DR, Holmes WE, Burton AJ, Pregitzer KS, Talhelm AF (2008) Simulated atmospheric NO3− deposition increases soil organic matter by slowing decomposition. Ecol Appl 18(8):2016–2027
Acknowledgments
We thank F. Campos Rivera, the Organización para Estudios Tropicales (OET) and the Ministerio de Ambiente, Energia y Telecommunicaciones (MINAET) for assisting with research permits and providing logistical support in Costa Rica, Marleny Jimenez and the Drake Bay Wilderness Camp for their generous access to field sites and W. Cambronero Castro for assistance in the field. We thank A. King and S. Schmidt for their support in conducting enzyme analyses. National Science Foundation Grants (DEB-0515744 and DEB-0852916) supported this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Weintraub, S.R., Wieder, W.R., Cleveland, C.C. et al. Organic matter inputs shift soil enzyme activity and allocation patterns in a wet tropical forest. Biogeochemistry 114, 313–326 (2013). https://doi.org/10.1007/s10533-012-9812-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-012-9812-2