Abstract
Aims
Net primary productivity is expected to increase in many forests as Earth warms, which can increase litter inputs to soils and affect carbon (C) and nitrogen (N) dynamics. Understanding how increasing litter inputs affect soil C and N cycling in tropical and subtropical forests is important because they represent some of the most productive ecosystems on Earth, suggesting that small changes in these cycles can have large effects.
Methods
To test the effects of increased litter inputs and the interactive effect between microbes and roots on soil C and N stocks and dynamics, we manipulated litter inputs and used trenching to exclude roots in a 40-year-old Cunninghamia lanceolata Lamb. (Chinese fir) plantation. At the site, we measured soil C and N pools, soil 13C and 15N natural abundance, and potential activities for C-, N-, and phosphorus-acquiring enzymes.
Results
After four years of experimental treatment, we found that increasing litter inputs reduced total soil N content by 26% relative to background litter inputs, but that increasing litter inputs did not affect soil C content in the plots with roots. In the plots without roots, both soil N and C did not change in response to litter inputs. In the plots with roots, soil δ15N increased with increasing litter inputs, but there was no effect in the plots without roots. We found a strong interactive effect between root and litter treatment on soil N pools and δ15N. The decline in soil N pools and increase in soil δ15N were associated with elevated potential enzyme activity for N-acquisition (N-acetyl glucosaminidase).
Conclusions
Adding litter did not have a significant effect on soil C pools, likely because potential soil C losses were offset by increasing litter-derived C inputs. In contrast to C, adding litter decreased N availability, likely through multiple pathways including gaseous N losses, NO3− leaching, root N uptake, and interactions between saprotrophic microbes and roots during the four-year litter addition experiment. Global changes that increase litter production may lower N pools and imbalance C and N cycling in subtropical coniferous forest ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Net primary productivity (NPP) is expected to change with changes in climate (Hickler et al. 2008; Cernusak et al. 2013; Klein et al. 2016), which may alter the quantity and quality of litter inputs to soils. Some studies suggest that NPP will increase due to a CO2 fertilization effect and higher temperatures (Raich et al. 2006; Hickler et al. 2008), whereas other studies suggest that NPP will decrease because of increased frequency of droughts as the climate changes (Gatti et al. 2014; Doughty et al. 2015). Regardless of the direction, changes in NPP may affect the quantity and quality of litter entering soils and soil carbon (C) and nitrogen (N) stocks.
Historically, litter quantity instead of quality was thought to determine whether C would persist in soils (Grandy and Neff 2008; Gentile et al. 2011; Wiesmeier et al. 2019). However, recent studies suggest that increasing litter inputs can instead destabilize soil C, increasing soil CO2 emissions (Sayer et al. 2011), while potentially decreasing soil C stocks (Fang et al. 2015a). Similarly, other studies have found that adding litter can lower soil C stocks through positive priming—i.e., an increase in microbial decomposition of SOM after adding C (Bingeman et al. 1953)—in subtropical forests but not in plantation forests (Liu et al. 2017; Chen and Chen 2018; Lyu et al. 2018). These inconsistent results are likely related to the quality of litterfall, which could affect the rate of SOM stabilization by affecting microbial substrate use efficiency. For instance, when microbial substrate use efficiency is high (high-quality litter of low C to N ratio), the microbial anabolism:catabolism ratio is also high (Cotrufo et al. 2013; Castellano et al. 2015). As a consequence, more microbial residues and less CO2 is produced per metabolized amount of plant litter. In contrast, when microbial substrate use efficiency is low (low-quality substrates of high C to N ratio) this may lead to microbial N limitation, encouraging microbes to invest resources into producing N-acquiring enzymes to mineralize SOM and access N (Jenkinson et al. 1985; Schimel and Bennett 2004), thereby lowering soil N stocks (Castellano et al. 2015). Thus, adding litter with high C/N may have a more pronounced effect on soil N stocks and cycling than on soil C.
While increasing litter inputs can decrease soil N stocks through microbial processes, increasing litter inputs can also change soil physical factors like temperature and moisture (Xu et al. 2013), which may also indirectly affect soil N availability (Sayer et al. 2012; Marklein et al. 2016). Increasing litter inputs may also indirectly affect soil N availability by increasing fine root proliferation and growth and, therefore, N uptake as demonstrated in subtropical and tropical forests (Liu et al. 2017; Rodtassana and Tanner 2018). This raises the question: does increasing litter inputs affect soil C and N availability through effects on microbial processes, roots, or their interactions?
Roots and root-associated microbes are known to influence soil C and N dynamics (Rasse et al. 2005; Talbot et al. 2008; McCormack et al. 2014). Soil microbes and root-associated mycorrhiza fungi play an important role in soil N cycling (Brzostek et al. 2015), by altering soil microbial communities and influencing N transformations (Coskun et al. 2017). For instance, N mineralization and nitrification are key processes producing ammonium (NH4+) and nitrate (NO3−) (Houlton et al. 2006; Fang et al. 2015b), which are key for plant nutrition. But these processes may also promote N loss because NO3− is a mobile anion and both NH4+ and NO3− can favor N loss via gaseous pathways such as nitric oxide (NO) and nitrous oxide (N2O) (Zhang et al. 2011, 2014; Shcherbak et al. 2014; Homyak et al. 2016), with annual losses of 5.6–30.1 kg of N per hectare via gaseous pathways (Fang et al. 2015b). Although plants do not directly participate in nitrification, they take up and assimilate both NO3− and NH4+, and therefore influence the soil N status (Krapp 2015). Moreover, it is becoming increasingly clear that roots can accelerate decomposition by releasing root exudates that fuel microbial growth and enzyme synthesis (Drake et al. 2011; Brzostek et al. 2013) and by providing easily decomposed C to free-living microbies to activate organic-matter-degrading enzymes in the soil (Read and Perez-Moreno 2003; Brzostek et al. 2015; Coskun et al. 2017). On the other hand, roots may also constrain decomposition by releasing C-rich exudates that cause rhizosphere microbes to immobilize nutrients inhibiting the growth and activity of enzyme-producing saprotrophs (Gadgil and Gadgil 1971; Lindahl et al. 2010; Fernandez and Kennedy 2016). How increasing litter inputs will affect root–microbe interactions and its effect on soil C and N cycling in forest ecosystems remains unclear.
To understand how roots influence C and N cycling, some previous girdling studies demonstrate that reducing belowground C fluxes reduces microbial respiration (Högberg et al. 2001; Giardina and Ryan 2002), and microbial extracellular enzyme activities (Weintraub et al. 2007). These studies have indicated that root-associated microbes, particularly mycorrhizal fungi, probably enhance decomposition rates (Brzostek et al. 2015). Ectomycorrhizal (ECM) fungi associated with roots are known to produce a suite of extracellular enzymes (Talbot et al. 2008) and often exude substantial amounts of C to soil. In contrast, arbuscular mycorrhizal (AM) tree roots generally lack the enzymatic capabilities of ECM trees and can only exude smaller amounts of C to soil than do ECM roots (Phillips and Fahey 2005; Yin et al. 2014). To date, however, most studies have focused on ECM-dominated stands, with few studies focusing on AM fungi. Tropics and subtropics have the largest area of plantation forests worldwide (Brockerhoff et al. 2008; Huang et al. 2013). In China, more than 32% of these plantation forests are dominated by coniferous species such as Chinese fir (Cunninghamia lanceolata) (see Fig. S1; FAO 2006) which are associated with AM fungi (Gai et al. 2006). Furthermore, Chinese fir is known to have higher ratios of C to N and lignin to N (i.e., lower N availability) in litter than broadleaved tree species (Lin et al. 2011; Wan et al. 2015). Thus, increased litter and root-derived C inputs from Chinese fir may have significant impact on microbial decomposition. Understanding the effects of the changing litter inputs on soil C and N retention is crucial for the management of subtropical plantation forests.
Here, we conducted a litter manipulation in combination with a root trenching experiment to understand the effects of increased litter inputs and the interactive effect between microbes and roots on soil C and N stocks and dynamics in a 40-year-old Chinese fir plantation. We used trenching to exclude roots and disentangle the effects of adding litter on soil C and N stocks since it is well documented that adding litter increases fine root growth in these forests (Li et al. 2016; Liu et al. 2017). We hypothesized that: (H1) adding litter would reduce soil N pools but have little effect on soil C pools, because potential C losses from decomposition would be offset by litter C inputs; and that (H2) adding litter with a high C/N would increase N-degrading enzyme activity as microbes invest resources into acquiring N by mining SOM.
Materials and methods
Site descriptions
Our study area is located at the Forest Ecosystem and Global Change Research Station (FEGCRS) (26°09′24′′N, 117°28′03′′E, 300 m a.s.l.), Sanming, Fujian, China. The area has a subtropical monsoonal climate with a mean annual temperature of 20.1 °C and a mean annual precipitation of 1670 mm, with precipitation mainly occurring from April to August. The parent material of the soil is classified as a sandy clay Ferric Acrisol according to the FAO/UNESCO classification (Lü et al. 2015; Lyu et al. 2017).
Experimental design
Plant litter inputs were manipulated using the Detritus Input and Removal Treatment (DIRT; Nadelhoffer et al. 2004) in June 2012. Briefly, three 20 m × 20 m plots were established in a 40-year-old Chinese fir plantation forest. Within each plot, 18 subplots (1 m × 1 m) were randomly divided into nine subplots and trenched to exclude roots. The remaining nine subplots were left unmanipulated. The root exclusion treatment was established by trenching the perimeter of the subplot to 0.6–0.8 m depth, and then inserting nylon mesh screen (0.149 mm) around the trenched plot to avoid roots from growing into the plots. Litter manipulation was conducted in both the plots with and without roots and included litter removal (No litter, L0), in-situ background rates of litterfall (Background litter, L1; 520 g m−2 yr.−1), and doubling litterfall (Double litter, L2; 1040 g m−2 yr.−1) treatments (Fig. S2). Above each litter removal plot, we installed a horizontal 1-mm nylon mesh screen (1 m × 1 m) 1 m above the ground to capture litterfall. The captured litterfall was then evenly spread onto the L2 plots biweekly to double background litterfall inputs.
Soil sampling and assays
In May 2016, after four years of adding litter and trenching, five soil cores (0–10 cm depth) were collected from each subplot (1 × 1 m) with a 3.5 cm diameter auger. All soil samples were kept in sealed plastic bags and processed within 2 h. Gravel, roots, and large organic residues were manually removed from the soil samples before passing through a 2 mm sieve. We measured total soil C and N using a CN elemental analyzer (Elementar Vario MAX, Hanau, Germany). Dissolved organic C (DOC), dissolved organic N (DON) and, inorganic N (NH4+ and NO3−) were extracted from the soil using 2 M KCl and measured using a continuous flow analyzer (Skalar san++, Netherlands) (Liu et al. 2017; Zhang et al. 2017). Soil microbial biomass C (MBC) and N (MBN) were determined by the fumigation-extraction technique (Vance et al. 1987) and extracted with 0.5 M K2SO4 and then used a TOC analyzer (Shimadzu VCPH/TNM-1, Tokyo, Japan) to determine C concentration of extraction and a continuous flow analyzer (Skalar san++, Netherlands) to determine N concentration of extraction. Isotopic analyses for C and N were conducted at the Stable Isotope Mass Spectrometry Laboratory at Fujian Normal University with an isotope ratio mass spectrometer (Finnigan MAT-253; Thermo Electron, San Jose, CA, USA) coupled to an automatic, online elemental analyzer (Flash EA1112; Thermofinnigan, San Jose, CA, USA).
We investigated soil enzymes known to play key roles in the mineralization of C, N, and P in soils, including β-glucosidase (βG), Cellobiohydrolase (CBH), phenol oxidase (PHO), peroxidase (PER), N-acetyl glucosaminidase (NAG) and Acid phosphatase (AP) (see Table S1 for their functions). These soil enzymes are divided into two groups: hydrolytic enzymes (βG, CBH, NAG, AP) and oxidative enzymes (PHO, PER). Soil sub-samples from each plot were assayed for potential activity of hydrolytic enzymes and oxidative enzymes according to Saiya-Cork et al. (2002). Briefly, suspensions of 1 g soil to 125 ml of acetate buffer at a concentration of 50 mol L−1 were prepared for each sample and agitated for 1 min using a Brinkmann Polytron PT 3000 homogenizer. The sample suspensions were continuously mixed in a magnetic stir plate during which 200 ml of the suspensions was portioned into 96-well microplates at 16 replicate wells per sample per assay. Further details can be found in Liu et al. (2017).
Statistical analyses
One-way ANOVA was used to test differences in soil properties, soil δ13C and δ15N, and extracellular enzyme activities among treatments. A paired t-test was used to test differences in parameters between root retention and root exclusion treatments. Where treatment effects were significant (p < 0.05), post-hoc comparisons were made using LSD test. The relationships between soil δ13C and δ15N, and extracellular enzyme activity were modelled with Pearson linear correlation coefficient. Linear mixed models (LMM) were used to test differences in the soil properties, soil δ13C and δ15N, and extracellular enzyme activities across root treatments (factor with two levels) and litter treatments (factor with three levels). In the fitted LMM, root treatments, and litter treatment and their interaction terms were modelled as fixed effects, block was modelled as a random effect, and a Type II Wald Chisquare test was used to evaluate the significance of tested effects. The LMM analysis was carried out by using the lmer function in the lme4 package and the ANOVA function in the car package in the statistical platform R 3.0.2. We also performed redundancy analysis (RDA) to determine which environmental factors were related to soil enzymes activity using the statistical platform R 3.0.2.
Results
Soil carbon and nitrogen pools
SOC and DOC concentrations did not vary with increasing litter inputs in the plots with roots (p > 0.05), but in the plots without roots, double litter inputs (L2) increased DOC up to 58% compared with no litter (L0), and up to 125% compared with background litter inputs (L1) (p < 0.05, Table 1).
In contrast to soil C, total soil N decreased with increasing litter inputs in the plots with roots; N decreased by up to 12% in L1 plots and up to 22% in L2 plots relative to L0 plots (p < 0.05; Table 1). Total soil N content remained constant in the plots without roots. Although neither litter nor root treatment alone affected total soil N, there was a root × litter treatment interaction on total soil N (F = 4.2, p = 0.048; Table S2). In the plots with roots, the concentration of NH4+ decreased with increasing litter inputs while the NO3− in L1 treatment was higher than that in L0 and L2 treatments. In the plots without roots, the concentrations of NO3− and NH4+ did not change with increasing litter inputs (Table 1). There was a root × litter interaction on NH4+ (F = 8.0, p = 0.008) but not for NO3− (F = 0.9, p = 0.45; Table S2). The concentration of DON decreased with increasing litter inputs in the plots with roots whereas it remained constant in the plots without roots (Table 1). There were no effects of root exclusion, litter addition, or their interaction on DON (Table S2).
Soil δ13C and δ15N
In the plots with roots, soil δ15N increased with increasing litter inputs (p < 0.001); soil δ15N was 16% higher in L1 and 60% higher in L2 relative to L1 (Fig. 1). Soil δ13C was significantly higher in L2 than that in L0 but there were no differences between L1 and L0 in the plots with roots. In the plots without roots, manipulating litter inputs had no effect both on soil δ13C and δ15N (Fig. 1). Overall, the results of LMM showed that manipulating litter had a significant and positive effect on soil δ15N in the presence of roots (F = 24.4, p < 0.003) but removing roots did not (F = 2.8, p = 0.126). We found a significant interaction between root × litter treatment on soil δ15N (F = 31.9, p < 0.001; Table S2).
Soil δ13C and δ15N in response to manipulating litterfall within plots with and without roots. L0: no litter; L1: background litter; L2: double litter. Different lowercase letters indicate significant differences between litter manipulation treatments within treatments without roots; different capital letters indicate significant differences between plots with and without roots within each litter manipulation treatment at α = 0.05. Error bars indicate standard deviation (n = 3)
Soil microbial biomass
Soil MBC was significantly lower in L1 and L2 than that in L0 both in plots with and without roots. In the plots with roots, soil MBN was up to 39% higher in L1 and 33% higher in L2 than that in L0 plots but there were no differences between L1 and L2 (Table 1). In the plots without roots, soil MBN was significantly lower in L1 and L2 than that in L0 inputs but there was no difference between L1 and L2.
Soil enzyme activity
In the plots with roots, the specific enzyme activities of PER and NAG were significantly higher in L2 than that in L1 and L0 while the βG and AP in L2 was significantly lower than in L1 but higher than L0 (Fig. 2). In the plots without roots, the PHO, PER and AP in L2 was significantly higher than that in L1 but there were no differences in βG, CBH and NAG. The results of LMM showed that litter treatment had a significant effect on βG (p = 0.004), CBH (p = 0.017), PHO (p = 0.013), PER (p = 0.004) and AP (p < 0.001), and the root treatment had significant effect on βG (p = 0.029) and AP (p = 0.037). Although both root and litter treatments did not affect NAG, there was a root × litter treatment interaction on NAG (F = 8.1; p = 0.008; Fig. 2). In the plots with roots, soil δ15N was significantly and positively correlated to the specific activity of PHO, PER and NAG, while no relationship was found between soil δ13C and specific enzyme activity (Table S3). In the plots without roots, there was no relationship between soil δ13C and δ15N and specific enzyme activities (Table S3).
Specific potential activity of six hydrolytic and oxidative enzymes involved in C, N, and P acquisition. L0: no litter; L1: background litter; L2: double litter. Error bars indicate standard deviation (n = 3). ns, no significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001. Cmic: microbial biomass C; βG: β-1, 4-glucosidase, CBH: Cellobiohydrolase; PHO: Phenol Oxidase; PER: Peroxidase; NAG: β-1, 4-N-acetylglucosaminidase; AP: Acid phosphatase. Different lowercase letters indicate significant differences between litter manipulation treatments within plots with and without roots; different capital letters indicate significant differences between plots with and without roots within each litter manipulation treatment at α = 0.05
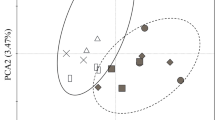
Redundancy analysis showed that there were significant differences in all six enzyme activities between the plots with and without roots (Fig. 3). Soil enzyme activity was significantly and positively related to the concentrations of NO3− and soil total N, with NO3− explaining 21% and total N explaining 14% of the variance in enzyme activity (Table S4). Furthermore, NO3− was negatively related to the enzyme activities in the plots with roots (Fig. 3).
Redundancy analysis ordination biplot of enzymes indicating the relationships between the variation of enzyme activities and environmental parameters. The result of conditional term effects are shown in Table S4. △ represents plots with roots (green dashed area); ◯ represent plots without roots (red dashed area). Symbols filled in red represent no litter inputs; symbols filled in green represent background litter inputs; symbols filled in blue represent double litter inputs
Discussion
Litter manipulation effect on soil carbon dynamics
Changes in litter production, in response to a changing climate, may lead to either increasing or decreasing soil C stocks depending on the balance between inputs from above- and belowground litter and outputs from decomposition (Sayer et al. 2011; Sokol and Bradford 2019). After a four-year litter manipulation, we show that adding litter did not change soil C stocks in either the plots with roots or without roots consistent with our first hypothesis (Table 1). This finding contrasts previous studies where adding litter increased surface soil C stocks in temperate and tropical forests (Sayer et al. 2007; Leff et al. 2012; Fekete et al. 2014), and other studies where adding litter decreased mineral soil C stocks in a temperate old-growth coniferous forest and a subtropical natural broadleaved forest (Crow et al. 2009; Liu et al. 2017).
It is possible that we did not detect changes in soil C stocks because adding litter did not induce a priming effect—the increase in soil respiration after adding litter (81 ± 10 g C ha−1 yr.−1) was similar to the rate of litter respiration (92 ± 11 g C ha−1 yr.−1), suggesting no additional contribution of soil to respiration (Li et al. 2016). But it is also possible that even though adding litter could have stimulated soil C decomposition via a smaller priming effect (Lyu et al. 2019), soil C stocks were compensated by C inputs from the added litter (Liang et al. 2018). In our study, soil DOC did not change after adding litter in the plots with roots, but it increased significantly after doubling litter inputs in plots without roots (Table 1), suggesting C litter inputs to the soil were important. This suggests we may have not detected changes in DOC in plots with roots because DOC inputs stimulated microbial activity (Leff et al. 2012; Fang et al. 2015a). Indeed, we found that the potential activity of C- and N-degrading enzymes increased in the plots with roots (Fig. 2), suggesting there was a microbial response to increased DOC supply. While adding litter actually decreased soil MBC relative to plots without litter, a lower microbial biomass does not imply microbes are unable to assimilate DOC. Thus, undetectable changes in soil C pools in response to adding litter to plots with roots could have been the result of a balance between C inputs and respiration, suggesting that increasing litter inputs do not affect soil C stocks in a Chinese fir forest.
Our results also suggest that manipulating litter affects soil processes and soil δ13C (Arai and Tokuchi 2010). Most biochemical processes, such as decomposition of SOC, favor the use of the lighter isotope (i.e., 12C), enriching the remaining substrate with 13C (Hobbie 2005). In our study, both litter addition and litter removal did not significantly affect soil δ13C (Fig. 1), indicating that there was no detectable decomposition of SOC in the absence of roots. However, when roots were present, soil δ13C increased with increasing litter mass, suggesting that an interaction between roots and microbes with increasing litterfall may stimulate decomposition of SOC. This further supports our earlier interpretation that increasing litter inputs may stimulate SOC decomposition while the decomposed soil C was compensated by C inputs from litter (Liang et al. 2018), since changes in C inputs and SOC decomposition would have strong effect on isotope fractionation (Arai and Tokuchi 2010). Overall, our results are consistent with our first hypothesis suggesting that adding litter has little effect on soil C pools in Chinse fir forests, because potential C losses from decomposition would be offset by litter C inputs.
Litter manipulation effect on soil N dynamics
In contrast to the effects of manipulating litter on soil C pools, litter manipulation significantly changed soil N pools and 15N natural abundance in the plots with roots (Table 1; Fig. 1). The δ15N value of an ecosystem N pool is controlled by both inputs and outputs (Brenner et al. 2001; Amundson et al. 2003). N inputs such as atmospheric N deposition and biological N fixation are generally 15N-depleted (Amundson et al. 2003). Therefore, increasing N inputs is consistent with decreasing soil δ15N so as long as N outputs remain constant. In contrast, N outputs such as plant net N uptake, denitrification, and leaching all discriminate against 15N (Houlton et al. 2006; Bai and Houlton 2009; Fang et al. 2015b), implying that increasing N outputs would increase δ15N in soils (Houlton et al. 2006).
In the plots with roots, the increase in soil δ15N with increasing litter inputs is consistent with a relatively open N cycle (i.e., greater N loss over N recycled) (Handley and Raven 1992; Högberg 1997). For example, microbial processes governing N trace gas emissions can discriminate against the heavy isotope increasing soil δ15N (Houlton et al. 2006; Zhang et al. 2014; Fang et al. 2015b; Homyak et al. 2016). Our results indicate that increases in litter addition might either increase microbial N immobilization (Homyak et al. 2008), or increase N losses through gaseous N emission, via NO or N2O (Zhang et al. 2014), and/or N leaching via hydrologic losses of NO3− (Dise et al. 2009). Because we did not observe differences in soil MBN between background litter and double litter addition treatments (Table 1), the decline in soil N availability is likely the result of ecosystem N losses instead of N uptake by microbes after adding litter.
To further understand the mechanisms controlling N loss, we show that adding litter did not change neither soil N pools nor δ15N in the plots without roots (Table 1; Fig. 1). This suggests that the effect of adding litter on soil N pools occurred through interactions between microbes, litter, and roots. Because adding litter increases root growth and biomass in these stands (Li et al. 2016; Liu et al. 2017), it is also possible that N pools decreased due to higher root uptake rates as observed in other studies (Gleeson and Good 2003; Zhang et al. 2017).
Chinese fir is an AM-associated plant, which generally cannot take up nutrients directly from litter or SOM, and depends on scavenging nutrients released by saprotrophic microbes (Phillips and Fahey 2005; Yin et al. 2014). By adding litter and reducing soil N availability, it is possible these AM trees eventually obtained N by mining SOM (Cheng et al. 2012). AM trees can acquire SOM-derived N by providing labile C (e.g. exudates, root litter debris) through mycorrhizal hyphae to saprotrophic microbes (Fig. 4; Phillips et al. 2013). As discussed earlier, in the presence of roots, the increased supply of DOC derived from adding litter may had been used by saprotrophic microbes to breakdown SOM and meet the N demand of AM fungi and roots. Consistent with this understanding, we found that soil δ15N was positively and linearly correlated to NAG, PHO and PER in plots with roots but not in the plots without roots (Table S3)—depolymerization of SOM by microbial extracellular enzymes facilitates root N uptake (Schimel and Bennett 2004). Similarly, changes in total N and NO3− also led to significant positive effects on soil enzyme activities, especially for PHO and PER (Fig. 3). Consistent with our hypothesis, these results suggest that litter-induced changes in N cycling in plots with roots may also result from depleting SOM-derived N stocks.
Conceptual framework illustrating the accelerated N cycle caused by plant and microbial interactions with increasing litter inputs in an arbuscular mycorrhizal (AM) tree plantation. Increasing litter inputs increase labile C supply to rhizosphere microbes, stimulating the mineralization of soil organic matter (SOM) for root N demands and leading to an open N cycle system, ultimately resulting decline in soil N stocks. The blue lines represent N inputs; the red lines represent microbial decomposition or mineralization of SOM as well as N outputs; and the green lines represents plant uptake; 0, non-significant effect; +, increased
Conclusions
Using a four-year field litter manipulation experiment combined with a root exclusion treatment, we found that increasing litter inputs did not significantly change soil C pools but significantly decreased soil N pools. It is possible that C pools did not change because adding litter did not cause a priming effect, though our findings suggest potential C losses from decomposition were offset by C inputs from litter (Lyu et al. 2018; Liang et al. 2018). In contrast, the increase in soil δ15N was only observed in the plots with roots but not in plots without roots, suggesting that an accelerating N cycle in these ecosystems requires interactions between roots, microbes, and litter. Smaller soil N pools and increasing soil δ15N, are likely the result of a combination of factors including gaseous N losses, NO3− leaching, root N uptake, and an interaction between saprotrophic microbes and AM-associated roots during a four-year litter addition experiment. While subtropical forests are characterized by having high N availability (Mo et al. 2006), N still limits the growth of Chinese fir (Zhang et al. 2017). Global changes favoring increased litter production may lead to a net loss of soil N in Chinese fir plantations, constraining plant available soil N.
References
Amundson R, Austin AT, Schuur EA, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Glob Biogeochem Cy 17:1031
Arai H, Tokuchi N (2010) Factors contributing to greater soil organic carbon accumulation after afforestation in a Japanese coniferous plantation as determined by stable and radioactive isotopes. Geoderma 157:243–251
Bai E, Houlton BZ (2009) Coupled isotopic and process-based modeling of gaseous nitrogen losses from tropical rain forests. Glob Biogeochem Cy 23:GB2011
Bingeman C, Varner J, Martin W (1953) The effect of the addition of organic materials on the decomposition of an organic soil. Soil Sci Soc Am J 17:34–38
Brenner DL, Amundson R, Baisden WT, Kendall C, Harden J (2001) Soil N and 15N variation with time in a California annual grassland ecosystem. Geochim Cosmochim Acta 65:4171–4186
Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J (2008) Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv 17:925–951
Brzostek ER, Greco A, Drake JE, Finzi AC (2013) Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 115:65–76
Brzostek ER, Dragoni D, Brown ZA, Phillips RP (2015) Mycorrhizal type determines the magnitude and direction of root–induced changes in decomposition in a temperate forest. New Phytol 206:1274–1282
Castellano MJ, Mueller KE, Olk DC, Sawyer JE, Six J (2015) Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob Chang Biol 21:3200–3209
Cernusak LA, Winter K, Dalling JW, Holtum JA, Jaramillo C, Körner C, Leakey AD, Norby RJ, Poulter B, Turner BL (2013) Tropical forest responses to increasing atmospheric CO2: current knowledge and opportunities for future research. Funct Plant Biol 40:531–551
Chen X, Chen HY (2018) Global effects of plant litter alterations on soil CO2 to the atmosphere. Glob Chang Biol 24:3462–3471
Cheng L, Booker FL, Tu C, Burkey KO, Zhou LS, Shew HD, Rufty TW, Hu SJ (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087
Coskun D, Britto DT, Shi W, Kronzucker HJ (2017) How plant root exudates shape the nitrogen cycle. Trends Plant Sci 22:661–673
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Chang Biol 19:988–995
Crow SE, Lajtha K, Bowden RD, Yano Y, Brant JB, Caldwell BA, Sulzman EW (2009) Increased coniferous needle inputs accelerate decomposition of soil carbon in an old-growth forest. For Ecol Manag 258:2224–2232
Dise NB, Rothwell JJ, Gauci V, Van der Salm C, De Vries W (2009) Predicting dissolved inorganic nitrogen leaching in European forests using two independent databases. Sci Total Environ 407:1798–1808
Doughty CE, Metcalfe D, Girardin C, Amézquita FF, Cabrera DG, Huasco WH, Silva-Espejo J, Araujo-Murakami A, da Costa M, Rocha W (2015) Drought impact on forest carbon dynamics and fluxes in Amazonia. Nature 519:78–82
Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJ (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett 14:349–357
Fang X, Zhao L, Zhou G, Huang W, Liu J (2015a) Increased litter input increases litter decomposition and soil respiration but has minor effects on soil organic carbon in subtropical forests. Plant Soil 392:139–153
Fang Y, Koba K, Makabe A, Takahashi C, Zhu W, Hayashi T, Hokari AA, Urakawa R, Bai E, Houlton BZ, Xi D (2015b) Microbial denitrification dominates nitrate losses from forest ecosystems. PNAS 112:1470–1474
FAO (2006) Global forest resources assessment 2005: progress towards sustainable forest management. Food and Agriculture Organization of the United Nations, Rome
Fekete I, Kotroczo Z, Varga C, Nagy PT, Varbiro G, Bowden RD, Toth JA, Lajtha K (2014) Alterations in forest detritus inputs influence soil carbon concentration and soil respiration in a central-European deciduous forest. Soil Biol Biochem 74:106–114
Fernandez CW, Kennedy PG (2016) Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? New Phytol 209:1382–1394
Gadgil RL, Gadgil PD (1971) Mycorrhiza and litter decomposition. Nature 233:133
Gai JP, Christie P, Feng G, Li XL (2006) Twenty years of research on community composition and species distribution of arbuscular mycorrhizal fungi in China: a review. Mycorrhiza 16:229–239
Gatti L, Gloor M, Miller J, Doughty C, Malhi Y, Domingues L, Basso L, Martinewski A, Correia C, Borges V (2014) Drought sensitivity of Amazonian carbon balance revealed by atmospheric measurements. Nature 506:76–80
Gentile R, Vanlauwe B, Six J (2011) Litter quality impacts short- but not long-term soil carbon dynamics in soil aggregate fractions. Ecol Appl 21:695–703
Giardina CP, Ryan MG (2002) Total belowground carbon allocation in a fast-growing Eucalyptus plantation estimated using a carbon balance approach. Ecosystems 5:487–499
Gleeson SK, Good RE (2003) Root allocation and multiple nutrient limitation in the New Jersey pinelands. Ecol Lett 6:220–227
Grandy AS, Neff JC (2008) Molecular C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci Total Environ 404:297–307
Handley LL, Raven JA (1992) The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ 15:965–985
Hickler T, Smith B, Prentice IC, Mjöfors K, Miller P, Arneth A, Sykes MT (2008) CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Glob Chang Biol 14:1531–1542
Hobbie EA (2005) Using isotopic tracers to follow carbon and nitrogen cycling in fungi. In: Dighton J, White JF, Oudemans P (eds) The Fungal Community: Its Organization and Role in the Ecosystem. Taylor & Francis, Boca Raton, pp 361–381
Högberg P (1997) Tansley review no. 95 15N natural abundance in soil–plant systems. New Phytol 137:179–203
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Homyak PM, Yanai RD, Burns DA, Briggs RD, Germain RH (2008) Nitrogen immobilization by wood-chip application: protecting water quality in a northern hardwood forest. Forest Ecol Manag 255:2589–2601
Homyak PM, Blankinship JC, Marchus K, Lucero DM, Sickman JO, Schimel JP (2016) Aridity and plant uptake interact to make dryland soils hotspots for nitric oxide (NO) emissions. PNAS 113:E2608–E2616
Houlton BZ, Sigman DM, Hedin LO (2006) Isotopic evidence for large gaseous nitrogen losses from tropical rainforests. PNAS 103:8745–8750
Huang ZQ, He ZM, Wan XH, Hu ZH, Fan SH, Yang YS (2013) Harvest residue management effects on tree growth and ecosystem carbon in a Chinese fir plantation in subtropical China. Plant Soil 364:303–314
Jenkinson DS, Fox RH, Rayner JH (1985) Interactions between fertilizer nitrogen and soil nitrogen-the so-called priming effect. Eur J Soil Sci 36:425–444
Klein T, Bader MKF, Leuzinger S, Mildner M, Schleppi P, Siegwolf RT, Körner C (2016) Growth and carbon relations of mature Picea abies trees under 5 years of free-air CO2 enrichment. J Ecol 104:1720–1733
Krapp A (2015) Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces. Curr Opin Plant Biol 25:115–122
Leff JW, Wieder WR, Taylor PG, Townsend AR, Nemergut DR, Grandy AS, Cleveland CC (2012) Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob Chang Biol 18:2969–2979
Li XJ, Liu XF, Xiong DC, Lin WS, Lin TW, Shi YW, Xie JS, Yang YS (2016) Impact of litterfall addition and exclusion on soil respiration in Cunninghamia lanceolata plantation and secondary Castanopsis carlesii forest in mid-subtropical China. Chin J Plant Ecol 40:447–457
Liang J, Zhou Z, Huo C, Shi Z, Cole JR, Huang L, Konstantinidis KT, Li X, Liu B, Luo Z, Penton CR, Schuur EAG, Tiedje JM, Wang Y, Wu L, Xia J, Zhou J, Luo Y (2018) More replenishment than priming loss of soil organic carbon with additional carbon input. Nat Commun 9:3175
Lin C, Yang Y, Guo J, Chen G, Xie J (2011) Fine root decomposition of evergreen broadleaved and coniferous tree species in midsubtropical China: dynamics of dry mass, nutrient and organic fractions. Plant Soil 338:311–327
Lindahl BD, de Boer W, Finlay RD (2010) Disruption of root carbon transport into forest humus stimulates fungal opportunists at the expense of mycorrhizal fungi. Isme J 4:872–881
Liu XF, Lin TC, Yang ZJ, Vadeboncoeur MA, Lin CF, Xiong DC, Lin WS, Chen GS, Xie JS, Li YQ, Yang YS (2017) Increased litter in subtropical forests boosts soil respiration in natural forests but not plantations of Castanopsis carlesii. Plant Soil 418:141–151
Lü MK, Xie JS, Wang C, Guo JF, Wang MH, Liu XF, Chen YM, Chen GS, Yang YS (2015) Forest conversion stimulated deep soil C losses and decreased C recalcitrance through priming effect in subtropical China. Biol Fertil Soils 51:857–867
Lyu MK, Xie JS, Ukonmaanaho L, Jiang MH, Li YQ, Chen YM, Yang ZJ, Zhou YX, Lin WS, Yang YS (2017) Land-use change exerts a strong impact on deep soil C stabilization in subtropical forests. J Soils Sediments 17:2305–2317
Lyu MK, Xie JS, Vadeboncoeur MA, Wang MH, Qiu X, Ren YB, Jiang MH, Yang YS, Kuzyakov Y (2018) Simulated leaf litter addition causes opposite priming effects on natural forest and plantation soils. Biol Fertil Soils 54:925–934
Lyu MK, Nie YY, Giardina CP, Vadeboncoeur MA, Ren YB, Fu ZQ, Wang MH, Jin CS, Liu XM, Xie J (2019) Litter quality and site characteristics interact to affect the response of priming effect to temperature in subtropical forests. Funct Ecol. https://doi.org/10.1111/1365-2435.13428
Marklein AR, Winbourne JB, Enders SK, Gonzalez DJ, van Huysen TL, Izquierdo JE, Light DR, Liptzin D, Miller KE, Morford SL, Norton RA (2016) Mineralization ratios of nitrogen and phosphorus from decomposing litter in temperate versus tropical forests. Glob Ecol Biogeogr 25:335–346
McCormack ML, Adams TS, Smithwick EAH, Eissenstat DM (2014) Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecology 95:2224–2235
Mo J, Brown S, Xue J, Fang Y, Li Z (2006) Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 282:135–151
Nadelhoffer KJ, Boone RD, Bowden RD, Canary JD, Kaye J, Micks P, Ricca A, Aitkenhead JA, Lajtha K, McDowell WH (2004) The DIRT experiment: litter and root influences on forest soil organic matter stocks and function. In: Foster D, Aber J (eds) Forests in time: the environmental consequences of 1000 years of change in New England. Yale University Press, New Haven, pp 300–315
Phillips RP, Fahey TJ (2005) Patterns of rhizosphere carbon flux in sugar maple (Acer saccharum) and yellow birch (Betula allegheniensis) saplings. Glob Chang Biol 11:983–995
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol 199:41–51
Raich JW, Russell AE, Kitayama K, Parton WJ, Vitousek PM (2006) Temperature influences carbon accumulation in moist tropical forests. Ecology 87:76–87
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems – a journey towards relevance? New Phytol 157:475–492
Rodtassana C, Tanner EVJ (2018) Litter removal in a tropical rain forest reduces fine root biomass and production but litter addition has few effects. Ecology 99:735–742
Saiya-Cork K, Sinsabaugh R, Zak D (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Sayer EJ, Powers JS, Tanner EVJ (2007) Increased litterfall in tropical forests boosts the transfer of soil CO2 to the atmosphere. PLoS One 2:e1299–e1299
Sayer EJ, Heard MS, Grant HK, Marthews TR, Tanner EVJ (2011) Soil carbon release enhanced by increased tropical forest litterfall. Nat Clim Chang 1:304–307
Sayer EJ, Wright SJ, Tanner EVJ, Yavitt JB, Harms KE, Powers JS, Kaspari M, Garcia MN, Turner BL (2012) Variable responses of lowland tropical forest nutrient status to fertilization and litter manipulation. Ecosystems 15:387–400
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Shcherbak I, Millar N, Robertson GP (2014) Global meta-analysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. PNAS 111:9199–9204
Sokol NW, Bradford MA (2019) Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat Geosci 12:46–53
Talbot JM, Allison SD, Treseder KK (2008) Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct Ecol 22:955–963
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wan X, Huang Z, He Z, Yu Z, Wang M, Davis MR, Yang Y (2015) Soil C: N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 387:103–116
Weintraub MN, Scott-Denton LE, Schmidt SK, Monson RK (2007) The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 154:327–338
Wiesmeier M, Urbanski L, Hobley E, Lang B, von Lützow M, Marin-Spiotta E, van Wesemael B, Rabot E, Ließ M, Garcia-Franco N, Wollschläger U (2019) Soil organic carbon storage as a key function of soils-a review of drivers and indicators at various scales. Geoderma 333:149–162
Xu S, Liu L, Sayer E (2013) Variability of above-ground litter inputs alters soil physicochemical and biological processes: a meta-analysis of litterfall-manipulation experiments. Biogeosciences 10 (11):7423–7433
Yin H, Wheeler E, Phillips RP (2014) Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biol Biochem 78:213–221
Zhang JB, Zhu TB, Cai ZC, Müller C (2011) Nitrogen cycling in forest soils across climate gradients in eastern China. Plant Soil 342:419–432
Zhang JB, Yu YJ, Zhu TB, Cai ZC (2014) The mechanisms governing low denitrification capacity and high nitrogen oxide gas emissions in subtropical forest soils in China. J Geophys Res Biogeosci 119:1670–1683
Zhang QF, Xie JS, Lyu MK, Xiong DC, Wang J, Chen YM, Li YQ, Wang MK, Yang YS (2017) Short-term effects of soil warming and nitrogen addition on the N: P stoichiometry of Cunninghamia lanceolata in subtropical regions. Plant Soil 411:395–407
Acknowledgments
The research was funded by the National key research and development program (No. 2016YFD0600204) and the National Natural Science Foundation of China (Nos U1505233, 31870604 and U1405231).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maokui Lyu and Xiaojie Li co-first authors.
Electronic supplementary material
ESM 1
(DOCX 328 kb)
Rights and permissions
About this article
Cite this article
Lyu, M., Li, X., Xie, J. et al. Root–microbial interaction accelerates soil nitrogen depletion but not soil carbon after increasing litter inputs to a coniferous forest. Plant Soil 444, 153–164 (2019). https://doi.org/10.1007/s11104-019-04265-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04265-w