Abstract
Ecosystem nutrient economies are commonly studied from the perspective of primary productivity in relation to nutrient availability, but a plant-specific view limits our ability to predict broader-scale patterns related to nutrient economics, such as ecosystem carbon turnover and storage controlled by soil microbial communities. Viewing nutrient economics from the heterotrophic decomposer community perspective can provide an additional perspective on ecosystem nutrient economics. We used microbial investment in extracellular enzyme production and ecoenzymatic stoichiometry theory as a conceptual framework to study nutrient economics in forest soils of the Northeast United States. Two years after experimentally increasing soil pH and phosphorus availability to offset the effects of chronic acid and nitrogen deposition, we found that belowground microbial communities were consistently investing more energy into phosphorus acquisition relative to nitrogen, in both space and time, suggesting that ecosystem demand for phosphorus might be high relative to nitrogen. Moreover, soil age and weathering, quantified by comparing glaciated vs non-glaciated regions, might not be useful predictors of nutrient economics in these forest systems. This study offers important insight into controls on ecosystem nutrient dynamics and soil microbial resource investment, information that can inform efforts to model soil microbial interactions and ecosystem carbon dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecosystem nutrient economies are often viewed through the lens of prevailing conceptual models of ecosystem development and nutrient limitation, where limits to primary productivity are associated with ecosystem age and/or nutrient sources and losses (Vitousek and Farrington 1997). This approach to understanding the nutrient economy of terrestrial and aquatic ecosystems reduces complexity and has provided profoundly important insights into the nature and consequence of nutrient limitation. However, a primary production-centric view can limit our ability to predict broader-scale emergent properties of nutrient economics, such as ecosystem carbon (C) turnover and storage.
For example, temperate forest ecosystems of Eastern North America, especially those found on glacial parent material, are traditionally considered nitrogen (N)-limited with a nutrient economy largely focused on N acquisition and retention (Aber 1992). This view arises from both conceptual models (Vitousek and Farrington 1997) and empirical data (LeBauer and Treseder 2008), with much of the supporting evidence coming from fertilization and foliar nutrient concentration studies (e.g., Crowley et al. 2012; Finzi 2009). However, this perspective is complicated by the fact that human activities have rapidly increased the inputs of both nitrogen (N) and acidic compounds to most forest ecosystems in Eastern North America, resulting in significant N enrichment and soil acidification (Aber 1992; Lovett and Goodale 2011). Conceptually, increased N availability and acidification should shift ecosystems towards phosphorus (P) limitation because allochthonous terrestrial P sources are lacking and P can be occluded in unavailable pools at low pH (Walker and Syers 1976). Recent work by Goswami et al. (2018) suggests that, depending on site conditions and successional status, P can limit productivity in northern hardwood forests at Hubbard Brook. Nevertheless, many previous studies have failed to demonstrate such P limitation on primary production in temperate forests (Finzi 2009; Wood et al. 1984) and it is broadly accepted that increasing N availability via anthropogenic N deposition has increased both above and belowground ecosystem C storage in the Northeast US (Frey et al. 2014; Thomas et al. 2010), lending further support for a generally N-dominated nutrient economy.
We suggest that this prevailing view is influenced by a predominately plant-oriented perspective and methodology and that a more complete understanding of the overall forest nutrient economy requires a view from the base of the ecosystem and not just the top. Soil microbial decomposition of plant litter is a primary control on nutrient and C turnover in forest ecosystems and evaluating nutrient economics from this perspective takes into consideration plant species composition and litter quality, an outcome of plant functional traits (Cornwell et al. 2008; Rinkes et al. 2013), in addition to relative nutrient availability and the stoichiometry and metabolic characteristics of the microbial community (Sinsabaugh and Follstad Shah 2012). Recent work further suggests that litter traits of dominant forest trees induce plant-soil feedbacks that can support vastly different forest nutrient and C economies (Bahram et al. 2020; Phillips et al. 2013). Overall, this implies soil microbial control over long-term nutrient availability and forest C balance (Fernandez-Martinez et al. 2014). Although much work has been done to examine how soil microbes respond to nutrient availability and demand, the relationship between soil microbial processes and the underlying nutrient economy and stoichiometry of forest ecosystems has received less attention.
Soil microbial investment in nutrient acquisition through extracellular enzyme production is an example of such a metric (Sinsabaugh et al. 2008). Soil microbes should respond to increased nutrient availability by down-regulating the production of specific nutrient acquiring enzymes. For instance, acid phosphatase, an extracellular enzyme that mineralizes organic P, should have reduced activity when P availability increases (Moorhead et al. 2013). This approach has been taken in many studies of soil nutrient dynamics (e.g., Saiya-Cork et al. 2002; Weintraub et al. 2007) and has been used to elucidate nutrient limitation signals in acid forest soils (DeForest et al. 2012; Kluber et al. 2012).
Further insight can be obtained by examining stoichiometric relationships (activity ratios) among soil extracellular enzymes as an index of both nutrient and C availability and microbial effort allocated towards obtaining multiple resources (Moorhead et al. 2016; Sinsabaugh et al. 2008). Derived from ecoenzymatic stoichiometry theory (Sinsabaugh and Follstad Shah 2012), this approach utilizes C to P and C to N-acquiring enzyme ratios to quantify relative N, P, and/or C limitation. Because the trajectory of ratio change over time provides insight into the relative importance of N, P, and/or C limitation (Fanin et al. 2016; Moorhead et al. 2016), we suggest this is a particularly useful tool for addressing spatial (i.e., environmental setting and biologic community effects) and temporal (seasonal nutrient demand) nutrient economy dynamics, from the microbial perspective.
Here, we present belowground nutrient cycling and microbial process/activity results after two and three years of experimentally increasing P availability in six previously studied hardwood forest stands in eastern Ohio, USA (DeForest and Scott 2010; DeForest et al. 2012; Kluber et al. 2012; Shaw and DeForest 2013). We demonstrate the first use of extracellular enzyme activity (EEA) ratios/stoichiometry and ecoenzyme vector analysis (Moorhead et al. 2016) to evaluate a forest nutrient economy in an experimental context at the ecosystem level. Forests of eastern Ohio are ideal for studying nutrient economics due to a history of chronic N deposition and acidification (DeForest and McCarthy 2011) and relatively similar overstory plant communities across latitudinal differences in climate, geology, and soil age (glaciated versus unglaciated). By comparing EEA and nutrient cycling across these site differences and sampling across seasons (varying nutrient demand and environmental conditions), our study was designed to transcend site-specific or temporally anomalous patterns. We expected greater nutrient response magnitudes (more P and N limitation) in unglaciated sites than glaciated sites with younger, less weathered soils, and that increasing P availability would generally shift the belowground nutrient economy towards N acquisition and away from P, regardless of site age.
Materials and methods
Study sites
This experiment took place in two different physiographic regions of eastern Ohio, USA: glaciated Till Plains in the north and unglaciated Allegheny Plateau in the south (see Online Resource 1). Glaciated sites are located at The Holden Arboretum (two forest stands) and the Case Western Reserve University field station (one forest stand). Annual total precipitation averages ~ 120 cm with an average temperature of ~ 8.0 °C, and soils are silty loam Hapludalfs or loamy Endoaqualfs with a pre-treatment soil pH of ~ 4.3 (0–5 cm mineral soil depth). All three forest stands in the unglaciated region are publicly owned and managed by the Ohio Department of Natural Resources. Annual precipitation at these sites is ~ 100 cm and average temperature is ~ 10.7 °C. Soils are loamy Hapludalfs, silty loam Dystrudepts, and silty loam Hapludults with a pre-treatment soil pH of ~ 4.7 (0–5 cm mineral soil depth). The overstory forest communities in both regions are oak-beech-maple-dominated.
Experimental design
In August 2009, 72 20 × 40 m plots were established in a randomized complete block design with two regions (glaciated and unglaciated), three forests in each region (i.e. blocks), four treatments (control, elevated pH, elevated P, and elevated pH + P) and three replicate treatment plots in each forest (see Online Resource 1). Pelletized lime was added to the elevated pH plots to raise the pH to a target range of 5.8 to 6.2 to immobilize reactive Al (DeForest and Scott 2010). Soil pH was measured in early spring to determine the amount of lime needed to reach the target pH range by taking a composite of 10 0–5 cm cores from each plot and then calculating a regional lime demand by averaging across all plots and forest stands in each region. On average, the glaciated and unglaciated sites were amended with 11.4 and 7.3 Mg ha−1 pelletized lime, respectively over the 3 years of application. Elevated P plots were amended with pelletized calcium dihydrogen phosphate (triple super phosphate (TSP)) at a rate of 4.9 kg P ha−1 in autumn 2009, 24.6 kg P ha−1 in spring 2010 (just prior to leaf out) and 24.6 kg P ha−1 in spring 2011. The phosphate addition levels were determined by averaging P sorption values from each forest block using methods outlined in Tiessen et al. (1991). The elevated pH + P plots were amended with both lime and TSP.
In each treatment plot, we collected a composite of 15 2-cm dia. mineral soil cores to a 5 cm depth (the sites have no measurable O horizon). Samples were transported cold, passed through a 2-mm sieve, and stored at 4 °C until analyzed (within 48 h). In order to capture seasonal and annual responses to our treatments, sampling and analyses were repeated five times over a one-year period (November 2010 (fall), February 2011 (winter), May 2011 (spring, just prior to TSP application), July 2011 (early summer), and September 2011 (late summer)).
Soil variables and nutrient pools
A soil subsample was dried at 105 °C to determine moisture content and then pulverized for determining total C and N content on an elemental analyzer (Costech Analytical, Valencia, CA USA). pH was determined using a 1:2 dilution of field moist soil and deionized (DI) water. Inorganic N was extracted by shaking field moist samples on a rotary shaker in a 1:5 dilution of soil and 1 M KCl for 1 h, centrifuging at 3000 rpm for 10 min, and drawing off the supernatant. Supernatant was analyzed colorimetrically in 96 well-plate format following methods detailed by DeForest and Scott (2010), where ammonium (NH4+) was determined using a salicylate-hypochlorite procedure (Kempers and Zweers 1986) and nitrate (NO3−) plus nitrite (reported as NO3−) using a VCl3/Griess procedure (Miranda et al. 2001). To estimate P availability, we measured readily available inorganic P (referred to here as resin P) using anion exchange membranes (AEM; GE Infrastructure: Water & Process Technologies, Watertown, MA, USA) following methods outlined in DeForest et al. (2012) and Shaw and DeForest (2013). Briefly, a 2 × 6 cm AEM strip, charged with NaHCO3, was placed in a 50 ml Oakridge high-density centrifuge tube with 5 g of field moist sieved soil and 20 ml of DI water. Samples were then shaken (175 rpm) horizontally for 4 h. AEM strips were removed and rinsed with DI water to remove debris and then shaken in a flask with 25 ml of 0.5 M HCl for 18 h. Extract P content was determined colorimetrically using the Murphy–Riley ascorbic acid method (Kuo 1996) with a 200 µl sample volume, 110 µl working solution volume, and 18–20 h incubation time (Shaw and DeForest 2013). Absorbance was determined at 880 nm on a Synergy HT (BioTek, Winooski, VT, USA) microplate spectrophotometer.
Nutrient cycling, EEA, and activity ratios
Microbial N turnover was estimated using net N mineralization and nitrification assays (Robertson et al. 1999). Field moist soil subsamples were incubated at 20 °C for 14 days near field capacity in the lab and subsequently extracted with 1 M KCl and analyzed for NH4+ and NO3− concentration (see method for nutrient pools). The difference between initial and final ion concentrations were then used to calculate net N mineralization and nitrification rates. P mineralization was determined by incubating moist soil for 14 days at 22 °C and then comparing resin P concentration to that of the initial pre-incubation concentration (Shaw and DeForest 2013).
Potential soil EEA associated with C, N, and P cycling were estimated by homogenizing 5 g field moist soil in 500 ml 50 mM acetate buffer (pH 5) and adding fluorogenic methylumbelliferone (MUF)-linked model substrates (DeForest 2009; DeForest et al. 2012). For this study, we chose one primary C-acquiring enzyme (ß-1,4-glucosidase(BG)), two N-acquiring enzymes (β-N-acetylglucosaminidase (NAG) and leucine aminopeptidase LAP)), and two P-acquiring enzymes (phosphomonoesterase (PM) and phosphodiesterase (PD)). We modified the common single-measurement method using NaOH (Saiya-Cork et al. 2002) because of methodological issues associated with NaOH additions and the fact that soil properties, environmental conditions, and seasonal effects can influence EEA and assay reaction time (DeForest 2009; Paz-Ferreiro et al. 2011). We measured well-plate fluorescence repeatedly over time to select the best incubation period for each enzyme following the procedure of Shaw and DeForest (2013). NAG and PM activity were estimated at four measurement times between 1 and 2.5 h while BG and PD were measured four times between 2 and 3.5 h. EEA was calculated by the slope of product formed over time and expressed on a soil C mass basis (nM g soil C−1 h−1) in order to normalize data across glaciated and unglaciated sites with drastically different soil C content. LAP, due to the slow but linear development of fluorescence in our assays, was estimated using a single time point read at 24 h, but expressed in the same units.
To understand how soil microbial communities responded to our treatments over time, we examined changes in the relationship between N and P-acquiring enzymes relative to C-acquiring enzymes (enzyme stoichiometry (Sinsabaugh et al. 2008)). Enzyme activity ratios generated seasonal treatment trajectories with respect to nutrient and C demand. Differences in the relationship between enzymatic C:N and C:P activity across treatment, site, and season, as measured by ratios of BG:(NAG + LAP) and BG:(DP + MP), were determined by following the approaches of Moorhead et al. (2013, 2016) and Fanin et al. (Fanin et al. 2016). Enzyme activity vector lengths and angles, relative to the origin of the plots of these two enzyme-activity ratios, were calculated for each pair of observations using a variation of methods described by (Fanin et al. 2016; Moorhead et al. 2016). We calculated angles with the equation:
where EEAR is the EEA activity ratio indicated in subscript. Vector length was calculated using the Pythagorean Theorem: vector length equals the length of the hypotenuse (c) where the EEA ratios represent the two sides (a and b) of a right triangle. As vector length increases, there is greater C limitation relative to nutrient limitation, implied by the greater BG versus nutrient acquisition activities. As vector angle increases (steeper), P is more limiting relative to N. For example: 90° represents extreme P limitation, 0° represents extreme N limitation, and 45° is considered co-limitation. We use this interpretation to provide an illustrative (Online Resource 2 provides graphical example) and quantitative metric of relative C limitation and relative P versus N limitation (Moorhead et al. 2013, 2016).
Data analysis
Statistical analyses were conducted using the statistical software package R v2.15 (Team 2017). Treatment, site, and seasonal differences in soil chemistry and microbial activity were determined by linear mixed-effect models in the context of ANOVA using the nmle (v3.1) package (Pinheiro et al. 2013). Site, treatment, and season were fixed effects and plot within forest stand was the random effect. Because our measurements were repeated seasonally and therefore sensitive to temporal autocorrelation, we accounted for repeated measures using autoregressive variance–covariance structure of the first order (AR1). Aikaki Information Criterion (AIC) was used to determine the optimal variance components and random structure for each model (Zuur et al. 2009). When appropriate, data were log-transformed (logln (n + 1)). Post-hoc pairwise comparisons were conducted using the multicomp (v1.2) package (Bretz et al. 2011).
Correlative relationships among microbial activity and chemistry/environment were explored using Principle Components Analysis (PCA) on ecoenzyme rates with soil microbial process rates and chemistry as vectors. PCA was performed using the prcomp function in R. Data were log-transformed as before, centered, and scaled. Relationships between the first two principle components and environmental variables were explored using Pearson correlations and illustrated with vector overlays (McCune and Grace 2002).
Results
Nutrient cycling, EEA, and activity ratios
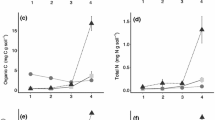
For glaciated soils, net N mineralization significantly increased in response to raising pH, but this response was most apparent in the spring and early summer (Fig. 1a, b and Online Resource 3). These seasonally high rates also drove both higher average net N mineralization rates in the glaciated (6.4 mg N kg soil−1 d−1) versus unglaciated sites (2.4 mg N kg soil−1 d−1) and site by season and treatment by season interactions. In spring and early summer, net N mineralization was greater in plots with elevated pH and elevated pH + P, but seasonal pattern was only significant for the glaciated sites. Overall, these N mineralization patterns are significant but need to be considered in context as they were driven primarily by the nitrification component of net N mineralization and not ammonification. Nitrification patterns alone were complex and exhibited significant site, treatment, and seasonal effects, including a three-way interaction (Fig. 1e, f and Online Resource 3). The seasonal pattern was again significant for the glaciated sites but not the unglaciated sites. Pairwise comparisons indicated that elevating pH significantly increased nitrification rates in the glaciated and unglaciated sites, particularly in the growing season.
Soil nutrient cycling processes and EEA by treatment at each sampling season for glaciated (left) and unglaciated (right) soils. Sampling seasons were fall (Fall), winter (Win), spring (Spr), early summer (eSum), and late summer (lSum). Significant treatment effects on net N mineralization and nitrification rates in the unglaciated sites are masked by the Y-axis scale but can be viewed separately in Online Resource 5
Net P mineralization patterns exhibited significant site, treatment, and seasonal effects (Fig. 1c, d and Online Resource 3). The seasonal pattern was significant in glaciated and unglaciated sites, but only for some treatments. Although more pronounced in the glaciated sites, raising soil pH, both in the elevated pH and elevated pH + P treatments, increased overall net P mineralization and decreased seasonal variability. However, all of our net P mineralization numbers were negative (i.e. P immobilization), indicating that P demand exceeded gross P mineralization rates in these systems, although increasing soil pH brings those numbers closer to zero (Fig. 1c, d).
EEA often responded significantly to site and treatment, but overall patterns appeared to be driven by seasonal rate variation (Fig. 1g–p and Online Resource 3). Even though the functionally similar P acquisition enzymes (PM and PD) had comparable significant seasonal responses, they exhibited dissimilar site and treatment responses. Both enzymes followed a pattern of decreasing activity toward early summer and late summer rebound. However, when averaged across season and by treatment PM did not respond to treatment and was 42% greater (P < 0.01) in the unglaciated sites compared to glaciated sites—a pattern driven by greater winter and spring activity in the unglaciated site. In contrast, PD exhibited stronger treatment than site differences. Treatment by season pairwise comparisons suggested that all treatments suppressed PD activity relative to controls in some seasons but not others (Fig. 1o, p).
Enzyme stoichiometric relationships indicated that soil processes in the glaciated sites were generally more C-limited than unglaciated sites, both regions were overall primarily P-limited, and treatments had little effect on nutrient economics (Figs. 2, 3; Table 1). Vector length, an indicator of relative C-limitation, showed a significant site by season by treatment interaction, in which glaciated sites were more C-limited than unglaciated sites during the fall and winter (Figs. 2, 3; Table 1). The treatment interaction was primarily driven by seasonal differences in the glaciated sites for one treatment (elevated pH) and not an overall trend (Table 1). Vector angles, indicators of relative P versus N-limitation, showed no significant site, season, or treatment responses, but the steep angles did illustrate that both the glaciated and unglaciated study systems are relatively more P-limited than N-limited (all angles greater than 45°; Table 1; Fig. 3).
Enzyme stoichiometry ratios for C:N versus C:P acquiring enzymes. Separate panels represent individual sampling seasons (A–E) and all data (F). The dashed 1:1 line indicates the line along which enzyme activity vector angles equal to 45° and N and P are considered co-limited. Location of a point above or below the 1:1 line indicates relative P and N limitation, respectively. The distance of a point from the origin (i.e., enzyme activity vector length) indicates relative C limitation, with greater distance indicating greater C limitation. See Online Resource 2 for further illustration of the enzyme space concept
Soil variables and nutrient pools
Soil moisture content varied seasonally, but was always higher in the glaciated sites (Fig. 4a, b and Online Resource 4) with gravimetric water contents of 62% (glaciated) and 28% (unglaciated) when averaged across season and treatment. In both glaciated and unglaciated sites, soil pH was significantly higher (1.5–2 pH units) in the elevated pH and elevated pH + P treatments than the elevated P and control treatments (Fig. 4c, d and Online Resource 4), a pattern consistent with our treatment goals. However, pH did show significant seasonal variability, particularly in the elevated pH and elevated pH + P treatments as our late summer lime addition worked its way through the system.
Soil variables and nutrient pool data by treatment in fall (Fall), winter (Win), spring (Spr), early summer (eSum), and late summer (lSum) for glaciated (left) and unglaciated (right) soils. Significant treatment effect on NH4+ pools in the unglaciated sites are masked by the Y-axis scale but can be viewed separately in Online Resource 5
Soil C and N content across season and treatment was consistently 2–3 times higher in glaciated (76.4 g C kg soil−1, 4.5 g N kg soil−1) than unglaciated (28.1 g C kg soil−1, 1.8 g N kg soil−1) sites, and 2–3 years of experimental treatments did not appear to directly influence total C and N pools in either site (Fig. 4e–h and Online Resource 4). Site by season interactions were observed for both C and N, but were driven by slight seasonal variation in glaciated soil C and N pools and plot-scale variability. A similar seasonal pattern and site by season interaction was observed for soil C:N, but neither site nor treatment differences were significant (Fig. 4i, j and Online Resource 4).
Extractable soil NH4+ pools were greatest in the winter and early growing season but decreased significantly during early and late summer for glaciated sites (Fig. 4k, l, and Online Resource 4). Unglaciated sites behaved somewhat differently and NH4+ pools were still the smallest during early summer, but were greatest in the late summer and fall. With the exception of autumn and late summer, glaciated soils overall had greater NH4+ pools than unglaciated soils. For both sites, the elevated pH treatment had greater NH4+ pools than the control treatment but not greater than the elevated P and elevated pH + P treatment, which were not different from the control treatment. Overall, treatment effects on NH4+ pools were small. Soil NO3− pools varied seasonally in both glaciated and unglaciated sites (Fig. 4m, n and Online Resource 4), but in general were significantly greater in the glaciated (19.1 mg kg soil−1) versus unglaciated (5.8 mg kg soil−1) sites. The significant site by season interaction further illustrated the seasonal and within-site variability of the NO3− pool, but our experimental treatments had no significant influence on seasonal or within-site NO3− pools. In contrast, readily available P pools (resin P) were both different between glaciated and unglaciated sites and significantly affected by our treatments; those effects significantly interacted with both season and site, although independently (Fig. 4o, p and Online Resource 4). As expected, resin P was highest in the elevated P treatments in both glaciated and unglaciated sites. Resin P pools were 19.2 mg kg soil−1 (glaciated) and 14.0 mg kg soil−1 (unglaciated) averaged across elevated P plots compared to 7.7 mg kg soil−1 (glaciated) and 4.5 mg kg soil−1 (unglaciated) averaged across control plots. In the glaciated sites all treatments were significantly different from each other across seasons, and elevating pH reduced P availability relative to the controls even in the elevated pH + P treatment. Elevated pH treatment plots in the unglaciated site were not significantly different than controls.
Environmental controls on EEA
Principle Components Analysis of EEA data across site, season, and treatment (Fig. 5) revealed that early summer data separate out along Principle Component (PC) 1 (58% of variation) and sites separate out along PC2 (11% of variation), but treatments did not exhibit distinct groupings. Environmental variables, which appear to be site-related, were primarily correlated with PC2 (Table 2). A smaller subset of variables also exhibited correlations with PC1; these tended to be environmental factors that were high in early summer in the glaciated plots and associated primarily with N cycling. Surprisingly, pH and variables associated with P cycling were not correlated with either PC (Table 2).
Ordination of plot data based on EEA per g of soil C (nmol g C−1 h−1) using principal components analysis. Component 1 accounts for 58% of the variation and is correlated with BG (r = − 0.45), DP (r = − 0.50), MP (r = − 0.49), NAG (r = − 0.46), and LAP (r = − 0.31). Component 2 accounts for 11% of the variation and was correlated with BG (r = 0.23), PD (r = -0.10), PM (r = − 0.27), NAG (r = − 0.40), and LAP (r = 0.84). Cut off for inclusion of environmental variable vectors was r ≥ 0.3 for at least one axis
Pearson correlations among environmental variables, soil chemistry, and microbial activity revealed that many environmental variables were correlated with each other and EEA rates. Similar to our PCA results, neither pH, resin P, nor P mineralization were strongly correlated with EEA (Online Resources 6 and 7). Soil pH was positively correlated with nitrification, net N mineralization and P mineralization, but negatively correlated with resin P regardless of whether elevated pH also included elevated P. With the exception of a weak significant correlation with NH4+ concentration, BG activity was not correlated with environmental factors or EEA. NAG activity was often negatively correlated with environmental variables and N cycling. LAP was negatively correlated with C:N and positively correlated with NH4+ concentration. PM activity was generally negatively associated with soil C content and N availability and cycling, but not related to P availability and cycling. PD exhibited no significant environmental or process correlations.
Discussion
The forest nutrient economy
Despite 3 years of experimentally increasing soil pH (~ 1.5 units) and at least doubling readily available soil P concentrations, nutrient cycling and enzyme stoichiometry data suggest that these forest soil microbial communities might persistently exhibit greater investment in P acquisition relative to N. This supports initial findings from previous studies from this experimental system (DeForest et al. 2012; Kluber et al. 2012; Shaw and DeForest 2013), but also provides a temporally rigorous view of underlying biogeochemistry that reveals complex interactions and coupling of C, N, and P cycling. P limitation, as conceptualized by ecoenzymatic stoichiometry theory (Sinsabaugh and Follstad Shah 2012), might be an underlying condition that is not realized in aboveground plant production responses due to soil microbial compensation and a shift from primarily inorganic to organic P acquisition (Turner 2008); this hypothesis is supported by reports of soil microbial P limitation in other eastern deciduous forests (Fiorentino et al. 2003; Gress et al. 2007; Minick et al. 2011).
Moreover, our data suggests that this P-focused nutrient economy is a chronic state. The microbial communities in our forests apparently have acclimated to our experimental treatments (Fig. 1); EEA suppression patterns seen previously (e.g., DeForest et al. 2012; Kluber et al. 2012) were not sustained over time and P remains in high microbial demand. It is possible our treatments provided only a transient decrease in ecosystem P demand or that treatments alleviated other limits on productivity, such as pH stress or calcium limitation, and the P availability increase was offset by concomitant increased P demand. Although we would expect less consistent patterns due to the spatial distribution of plant functional groups (Phillips et al. 2013) or temporal changes in nutrient demand relative to C quality and stage of litter decomposition (Moorhead et al. 2013), our observed patterns were surprisingly consistent (Fig. 2).
Our findings suggest that plant-based metrics of nutrient economics alone do not provide a complete picture of nutrient economics at the ecosystem or landscape level; plant tissue concentrations and element ratios might not be context-specific enough to draw broad conclusions and the temporal scales at which forest trees respond to fertilization can limit interpretations. For example, Crowley et al. (2012) documented regional patterns of foliar chemistry in relation to N deposition rates to infer patterns of nutrient limitation, but did not include specific site data such as soil nutrient status and chemistry or fertilization responses. Finzi (2009) similarly examined northern hardwood forest responses to N and P fertilization to conclude that widespread P limitation is not apparent. However, the latter study measured aboveground responses two years after fertilization and forest tree NPP responses can take a decade or more to emerge (Pregitzer et al. 2008). Furthermore, although nutrient limitation is essentially defined as increased growth or process rates when that nutrient becomes more available, plant response to nutrient limitation might not manifest as change in aboveground growth. A broad study of Canadian Acer saccharum stands suggests that P is more limiting than N or Ca on acidic soils, but that P deficiency has not led to decreased forest health or productivity (Casson et al. 2012). In contrast, a four-year fertilization experiment at Hubbard Brook found conclusive evidence that relative basal area increment responds positively to P fertilization, but that response varied with respect to stand age and site conditions (Goswami et al. 2018). An assessment of soil microbial metrics, including those measured here, might augment foliar approaches and aboveground growth responses because nutrient demand is examined more directly and microbial communities respond rapidly to changes in nutrient and substrate availability. Thus, combining perspectives might reveal a more complete and temporally explicit picture of ecosystem-level nutrient limitation.
General treatment effects
Our elevated pH treatments clearly altered soil pH (Fig. 4 and Online Resource 4), but had a minimal effect on nutrient pools and no observed influence on overall nutrient economics from the microbial demand perspective (Fig. 2). This response is counter to the observation that pH exerts a strong control on soil microbial communities (Fierer and Jackson 2006; Rousk et al. 2010), soil EEA patterns (Sinsabaugh et al. 2008), and soil P availability (Walker and Syers 1976). Interpreting these results is complicated, in part, because our elevated pH treatment is fundamentally a multi-treatment that increased pH, Ca, and potentially P availability, although we did not find that our liming or P treatments altered foliar Ca concentrations after three years of treatment (unpublished data). Given the low pH of our soils, we had the biogeochemical expectation that elevating pH would increase inorganic P availability, but we did not observe such an effect in either of our sites. A similar response to lime addition was seen in Canadian forests (Gradowski and Thomas 2008). In contrast, 1 year after increasing soil pH by 1 unit with non-lime Ca, Fiorentino et al. (2003) found that soil resin P increased 2 to tenfold. We found that elevated pH, particularly in the glaciated sites, actually reduced resin P relative to controls. We reason that P demand is so high in our sites that rapid immobilization masked any effect of elevated pH on inorganic P availability. Resin P did not increase with elevated pH even when P availability was also elevated (elevated pH + P treatment). This implies either that added Ca shuttles our P addition into non-resin pools or that these systems have a large inorganic P demand and therefore rapid immobilization at higher pH. Our net P mineralization and enzyme stoichiometry findings support the latter dynamic and suggest complex interactions between nutrient limitation and soil pH and/or Ca availability. Interpretation of these results would be greatly improved by incorporating P into microbial litter decay models (e.g., Moorhead et al. 2012; Moorhead and Sinsabaugh 2006; Schimel and Weintraub 2003).
Elevating P availability increased resin P concentrations and reduced P mineralization rates (Fig. 1); however, these patterns disappeared in the elevated pH + P treatment. Furthermore, we saw no effect of elevated P on other microbial processes or enzyme stoichiometry, suggesting that N cycling was not influenced by P availability. We did not demonstrate a general P effect on phosphatase activity and only a slight effect on PD activity in some seasons, potentially due to reduction in demand for less available phosphate diesters (Turner 2008) under P fertilization. Similar patterns were observed in a study at Hubbard Brook. Groffman and Fisk (2011) amended plant-free organic soil with P and Ca and found that P, but not Ca, increased resin P and neither treatment influenced microbial biomass, respiration, C-metabolizing EEA, potential net N mineralization, or nitrification. They did find that P addition reduced phosphatase enzyme activities similar to our previous work (DeForest et al. 2012; Kluber et al. 2012; Shaw and DeForest 2013). Overall, these results are perplexing as our enzyme stoichiometry and P mineralization data clearly indicate chronic belowground P demand yet our P additions had little impact on net nutrient cycling processes and appeared to be immobilized or made unavailable under elevated pH. This could be due to the seasonal aspect of our measurements compared to the single annual sampling from our initial study (DeForest et al. 2012), but further research from these sites does suggest that the lack of suppression we observed in 2011 was transient and suppression resumes in the following years (Deforest et al. 2021). We have no data to suggest that variations in weather (e.g., cold vs. warm or dry vs. wet) drove this response. It is possible that some aspect of microbial/plant acclimation to pH or multiple nutrient, mineral, or organic interactions with pH that have not been investigated in fertilization experiments are driving this pattern and requires attention in future studies.
We did observe lime-induced net N mineralization, nitrification, and P mineralization increases 2–3 years after treatment (Fig. 1). DeForest et al. (2012) demonstrated similar patterns in these sites after only 1 year, but only for the unglaciated sites and suggested that it was associated with a threshold response to exceeding a treatment pH ≥ 6, a pattern observed in boreal forest soils (Geisler et al. 1998). Our sites reached that threshold (Fig. 4c, d) and exhibited a similar response (Fig. 1a–f). Other studies have made the opposite observation where both liming and non-lime Ca additions decreased N cycling rates (Melvin et al. 2013; Minick et al. 2011; Persson et al. 1995), suggesting that our observations are either a transient treatment response or the result of complex N-P interactions. However, additional research from our study sites demonstrates that suppression of P-acquiring enzymes activity has persisted after 6 years, suggesting that some soil microbial responses related to P cycling are not transient (Deforest et al. in revision).
Site influences on nutrient economics
Interactions between geology and soil age often give rise to distinct geographic patterns of nutrient economics where P limitation is associated with older, highly weathered soils (Vitousek and Farrington 1997; Walker and Syers 1976). Our two study regions are clearly separated by soil age (glaciated ~ 15,000 years and unglaciated ~ 125,000 up to 2,500,000 years) and we expected clear biogeochemical cycling differences associated with lower ambient pH and greater C and nutrient pools in the glaciated sites. Instead, we saw only subtle EEA site differences and enzyme stoichiometry values that suggest high P demand in both sites (Fig. 2). However, some important site differences do emerge. First, enzyme ratio plots demonstrate site clustering and separation with glaciated site values shifted more toward C limitation than unglaciated sites. Enzyme vector lengths confirm this pattern (Table 1). Because glaciated sites had twice as much soil C as unglaciated sites, we suggest this response is due to either soil C quality driving C dynamics and not quantity, or greater N deposition and N availability in the glaciated sites, consistent with N saturation theory (Aber 1992). We also saw site-dependent clustering in ordinations of controls on EEA (Fig. 5), as well as seasonal separation within sites during the period of highest nutrient demand (early summer). This coincides with the period of minimum C limitation in the glaciated sites and maximum N limitation in the unglaciated sites and could be due to root exudation shifting the primary C source away from SOM. Overall, our EEA site patterns and lack of treatment effect did not follow—and sometimes invert—the expected relationships between EEA, SOM content, and pH reported by Sinsabaugh et al. (2008). However, EEA patterns (Fig. 2) are consistent with the relative relationship between C:P acquiring enzymes and mean annual temperature and C:N acquiring enzymes and mean annual precipitation observed in that paper, suggesting climatic controls on background EEA.
Implications for ecosystem C balance and future directions
Although aboveground plant responses to nutrient availability have important implications for overall net ecosystem C balance and storage (Elser et al. 2007), the function of soil heterotrophs might ultimately play a disproportionately larger role in controlling net ecosystem C exchange because extracellular control of organic matter turnover is a rate-controlling step in the global C cycle (Sinsabaugh and Follstad Shah 2012). This is particularly true if soil microbes can alleviate potential nutrient limitation at the primary producer level. It remains unknown if P, N, or both (co-limitation (Davidson and Howarth 2007)), or some other factor limits aboveground NPP in our experimental system. However, enzyme stoichiometry data, based on our criteria, consistently suggest chronic soil P limitation in forests across eastern Ohio, a pattern supported by P mineralization results for all treatments (Fig. 1). It is possible that N and P limitation are highly coupled across plant and microbial guilds, yet this question has not been addressed specifically and should be a focus of future research. The relative availability of soil N and P, in conjunction with plant trait-related differences in litter quality, influence not only litter decay rates (Bahram et al. 2020; Cornwell et al. 2008; Phillips et al. 2013), but also net soil C sequestration by altering both the realized microbial C use efficiency (CUE) at the physiological level and the composition and function of the decomposer community. Overall, we predict that microbial compensation for changes in nutrient availability comes at a cost of reduced CUE to the community when decomposers must invest more energy in litter decomposition to acquire limited nutrients. The need for such investments is likely to have consequences for net ecosystem C storage (Sinsabaugh and Follstad Shah 2012) but such patterns have not been quantified.
Studies in Hawaiian soils have demonstrated that plant responses, soil EEA, litter turnover, and microbial responses to C and nutrient additions are N-limited (younger soils) or co-limited by N and P (older soils) (Hobbie and Vitousek 2000; Reed et al. 2010), suggesting that P-limited systems respond to N with increased NPP. However, Hawaiian soils receive little N or acid deposition relative to eastern North America and those results might be a function of relative N vs. P limitation. A study of hardwood forest litter decomposition (Rinkes et al. 2013) suggested only minor P limitation on EEA and only varying degrees of C and N limitation over time (Moorhead et al. 2013). Direct comparisons between these findings and ours should be viewed cautiously since our study of mineral surface soil lacking organic horizons addressed different soil components (litter, O horizon, or mineral) than those studies, which has implications for process rates and soil chemistry. Nevertheless, this evidence implies that future work should consider soil C dynamics in relation to nutrient co-limitation (with respect to different microbial functional groups or ecological guilds) and incorporate both P and N into experimental designs to better understand coupled N-P cycling in the context of the metabolic and stoichiometric theories of ecological systems.
Conclusions
Chronic acid and N deposition has the potential to alter C and nutrient dynamics in hardwood forests of the Northeast US. This comprehensive seasonal study of soil microbial activity in response to experimentally altered soil pH and P availability provides a theoretical framework for studying forest nutrient economies. Our findings suggest nutrient economics can be dominated by P acquisition when viewed from the perspective of soil heterotrophs. Thus, soil microbes might compensate for acidification and increasing N availability by investing more energy into P relative to N acquisition. However, soil age and weathering are not useful predictors of belowground nutrient economic status in these forest systems. Three years of increasing soil pH and P availability has not alleviated apparent P demand, even though treatment effects on absolute EEA rates mostly disappeared compared to previous studies. This suggests our treatments had a transient effect on microbial enzyme dynamics, P demand increased, or there are aspects of C-N-P interactions and microbial decomposition that are yet to be understood. Regardless, this study offers a glimpse into the controls on ecosystem nutrient economics and utilizes a potentially powerful new enzyme activity analysis tool for understanding soil microbial resource investment that can inform efforts to model soil microbial interactions, litter decomposition, and ultimately belowground ecosystem C turnover and storage (e.g.,Wang et al. 2013).
Data availability (data transparency)
EDI Data Portal (DOI link forthcoming).
References
Aber JD (1992) Nitrogen cycling and nitrogen saturation in temperate forest ecosystems. Trends Ecol Evol 7:220–224
Bahram M et al (2020) Plant nutrient-acquisition strategies drive topsoil microbiome structure and function. New Phytol. https://doi.org/10.1111/nph.16598
Bretz F, Hothorn T, Westfall PH (2011) Multiple comparisons using R. CRC Press, Boca Raton
Casson NJ, Eimers MC, Watmough SA (2012) An assessment of the nutrient status of sugar maple in Ontario: indications of phosphorus limitation. Environ Monit Assess 184:5917–5927. https://doi.org/10.1007/s10661-011-2390-2
Cornwell WK et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. https://doi.org/10.1111/j.1461-0248.2008.01219.x
Crowley KF et al (2012) Do nutrient limitation patterns shift from nitrogen toward phosphorus with increasing nitrogen deposition across the northeastern United States? Ecosystems 15:940–957. https://doi.org/10.1007/s10021-012-9550-2
Davidson EA, Howarth RW (2007) Environmental science - Nutrients in synergy. Nature 449:1000–1001. https://doi.org/10.1038/4491000a
DeForest JL (2009) The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol Biochem 41:1180–1186. https://doi.org/10.1016/j.soilbio.2009.02.029
DeForest JL, McCarthy BC (2011) Diminished soil quality in an old-growth, mixed mesophytic forest following chronic acid deposition. Northeast Nat 18:177–184
DeForest JL, Scott LG (2010) Available organic soil phosphorus has an important influence on microbial community composition. Soil Sci Soc Am J 74:2059–2066. https://doi.org/10.2136/sssaj2009.0426
DeForest JL, Smemo KA, Burke DJ, Elliott HL, Becker JC (2012) Soil microbial responses to elevated phosphorus and pH in acidic temperate deciduous forests. Biogeochemistry 109:189–202. https://doi.org/10.1007/s10533-011-9619-6
DeForest JL, Dorkoski R, Freedman ZB, Smemo KA (2021) Multi-year soil microbial and extracellular phosphorus enzyme response to lime and phosphate addition in temperate hardwood forests. Plant Soil. https://doi.org/10.1007/s11104-021-04947-4
Elser JJ et al (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x
Fanin N, Moorhead D, Bertrand I (2016) Eco-enzymatic stoichiometry and enzymatic vectors reveal differential C, N, P dynamics in decaying litter along a land-use gradient. Biogeochemistry 129:21–36. https://doi.org/10.1007/s10533-016-0217-5
Fernandez-Martinez M et al (2014) Nutrient availability as the key regulator of global forest carbon balance. Nat Clim Change 46:471–476. https://doi.org/10.1038/nclimate2177
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631. https://doi.org/10.1073/pnas.0507535103
Finzi AC (2009) Decades of atmospheric deposition have not resulted in widespread phosphorus limitation or saturation of tree demand for nitrogen in southern New England. Biogeochemistry 92:217–229. https://doi.org/10.1007/s10533-009-9286-z
Fiorentino I, Fahey TJ, Groffman PM, Driscoll CT, Eagar C, Siccama TG (2003) Initial responses of phosphorus biogeochemistry to calcium addition in a northern hardwood forest ecosystem. Can J for Res 33:1864–1873. https://doi.org/10.1139/x03-111
Frey SD, Ollinger S, Nadelhoffer K, Bowden R, Brzostek E, Burton A, Caldwell BA, Crow S, Goodale CL, Grandy AS, Finzi A, Kramer MG, Lajtha K, LeMoine J, Martin M, McDowell WH, Minocha R, Sadowsky JJ, Templer PH, Wickings K (2014) Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121:305–316. https://doi.org/10.1007/s10533-014-0004-0
Geisler R, Högberg M, Högberg P (1998) Soil chemistry and plants in fennoscandian boreal forest as exemplified by a local gradient. Ecology 79:119–137
Goswami S, Fisk MC, Vadeboncoeur MA, Garrison-Johnston M, Yanai RD, Fahey TJ (2018) Phosphorus limitation of aboveground production in northern hardwood forests. Ecology 99:438–449. https://doi.org/10.1002/ecy.2100
Gradowski T, Thomas SC (2008) Responses of Acer saccharum canopy trees and saplings to P, K and lime additions under high N deposition. Tree Physiol 28:173–185. https://doi.org/10.1093/treephys/28.2.173
Gress SE, Nichols TD, Northcraft CC, Peterjohn WT (2007) Nutrient limitation in soils exhibiting differing nitrogen availabilities: what lies beyond nitrogen saturation? Ecology 88:119–130. https://doi.org/10.1890/0012-9658(2007)88[119:nlised]2.0.co;2
Groffman PM, Fisk MC (2011) Phosphate additions have no effect on microbial biomass and activity in a northern hardwood forest. Soil Biol Biochem 43:2441–2449. https://doi.org/10.1016/j.soilbio.2011.08.011
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877. https://doi.org/10.1890/0012-9658(2000)081[1867:nlodih]2.0.co;2
Kempers AJ, Zweers A (1986) Ammonium determination in soil extracts by the salicylate method. Commun Soil Sci Plan 17:715–723. https://doi.org/10.1080/00103628609367745
Kluber LA, Carrino-Kyker SR, Coyle KP, DeForest JL, Hewins CR, Shaw AN, Smemo KA, Burke DJ (2012) Mycorrhizal response to experimental pH and P manipulation in acidic hardwood forests. PLoS ONE 7:e48946. https://doi.org/10.1371/journal.pone.0048946
Kuo S (1996) Phosphorus. In: Sparks DL (ed) Methods of soil analysis, part 3, chemical methods. SSSA and ASA, Madison, pp 869–919
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379. https://doi.org/10.1890/06-2057.1
Lovett GM, Goodale CL (2011) A new conceptual model of nitrogen saturation based on experimental nitrogen addition to an oak forest. Ecosystems 14:615–631. https://doi.org/10.1007/s10021-011-9432-z
McCune B, Grace J (2002) Analysis of ecological communities. MJM Software Design, Gleneden Beach
Melvin AM, Lichstein JW, Goodale CL (2013) Forest liming increases forest floor carbon and nitrogen stocks in a mixed hardwood forest. Ecol Appl 23:1962–1975. https://doi.org/10.1890/13-0274.1
Minick KJ, Fisk MC, Groffman PM (2011) Calcium and phosphorus interact to reduce mid-growing season net nitrogen mineralization potential in organic horizons in a northern hardwood forest. Soil Biol Biochem 43:271–279. https://doi.org/10.1016/j.soilbio.2010.10.009
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide-Biol Chem 5:62–71. https://doi.org/10.1006/niox.2000.0319
Moorhead DL, Lashermes G, Sinsabaugh RL (2012) A theoretical model of C- and N-acquiring exoenzyme activities, which balances microbial demands during decomposition. Soil Biol Biochem 53:133–141. https://doi.org/10.1016/j.soilbio.2012.05.011
Moorhead DL, Rinkes ZL, Sinsabaugh RL, Weintraub MN (2013) Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: informing enzyme-based decomposition models. Front Microbiol 4:1–12. https://doi.org/10.3389/fmicb.2013.00223
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174
Moorhead DL, Sinsabaugh RL, Hill BH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7. https://doi.org/10.1016/j.soilbio.2015.10.019
Paz-Ferreiro J, Trasar-Cepeda C, Leirós M, Seoane S, Gil-Sotres F (2011) Intra-annual variation in biochemical properties and the biochemical equilibrium of different grassland soils under contrasting management and climate. Biol Fert Soils 47:633–645. https://doi.org/10.1007/s00374-011-0570-4
Persson T, Rudebeck A, Wirén A (1995) Pools and fluxes of carbon and nitrogen in 40-year-old forest liming experiments in Southern Sweden. Water Air Soil Pollut 85:901–906. https://doi.org/10.1007/bf00476944
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199:41–51. https://doi.org/10.1111/nph.12221
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2013) nlme: linear and nonlinear mixed effects models. R package version 3.1–108
Pregitzer KS, Burton AJ, Zak DR, Talhelm AF (2008) Simulated chronic nitrogen deposition increases carbon storage in Northern Temperate forests. Glob Change Biol 14:142–153. https://doi.org/10.1111/j.1365-2486.2007.01465.x
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reed SC, Vitousek PM, Cleveland CC (2010) Are patterns in nutrient limitation belowground consistent with those aboveground: results from a 4 million year chronosequence. Biogeochemistry 106:323–336. https://doi.org/10.1007/s10533-010-9522-6
Rinkes Z, DeForest J, Grandy AS, Moorhead D, Weintraub M (2013) Interactions between leaf litter quality, particle size, and microbial community during the earliest stage of decay. Biogeochemistry. https://doi.org/10.1007/s10533-013-9872-y
Robertson GP, Wedin D, Groffman PM, Blair JM, Holland EA, Nadelhoffer K, Harris D (1999) Soil carbon and nitrogen availability: nitrogen minerlaization, nitrification, and soil respiration potentials. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 258–271
Rousk J, Baath E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351. https://doi.org/10.1038/ismej.2010.58
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Shaw AN, DeForest JL (2013) The cycling of readily available phosphorus in response to elevated phosphate in acidic temperate deciduous forests. Appl Soil Ecol 63:88–93. https://doi.org/10.1016/j.apsoil.2012.09.008
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Ann Rev Ecol Evol Sys 43:313–343. https://doi.org/10.1146/annurev-ecolsys-071112-124414
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264. https://doi.org/10.1111/j.1461-0248.2008.01245.x
Thomas RQ, Canham CD, Weathers KC, Goodale CL (2010) Increased tree carbon storage in response to nitrogen deposition in the US. Nat Geosci 3:13–17
Tiessen H, Frossard E, Mermut AR, Nyamekye AL (1991) Phosphorus sorption and properties of ferruginous nodules from semiarid soils from Ghana and Brazil. Geoderma 48:373–389. https://doi.org/10.1016/0016-7061(91)90055-X
Turner BL (2008) Resource partitioning for soil phosphorus: a hypothesis. J Ecol 96:698–702. https://doi.org/10.1111/j.1365-2745.2008.01384.x
Vitousek PM, Farrington H (1997) Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37:63–75
Walker TW, Syers JK (1976) Fate of phosphorus during pedogenesis. Geoderma 15:1–19. https://doi.org/10.1016/0016-7061(76)90066-5
Wang G, Post WM, Mayes MA (2013) Development of microbial-enzyme-mediated decomposition model parameters through steady-state and dynamic analyses. Ecol Appl 23:255–272. https://doi.org/10.1890/12-0681.1
Weintraub MN, Scott-Denton LE, Schmidt SK, Monson RK (2007) The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 154:327–338
Wood T, Bormann FH, Voigt GK (1984) Phosphorus cycling in a northern hardwood forest: biological and chemical control. Science 223:391–393. https://doi.org/10.1126/science.223.4634.391
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
The National Science Foundation supported this work (DEB 0918681 and DEB 0918167). We thank The Holden Arboretum, the Ohio DNR, and Case Western Reserve University for study site access. David Burke and Sarah Carrino-Kyker helped with samples collection, and Charlotte Hewins contributed to both sample collection and laboratory analyses.
Funding
National Science Foundation (DEB 0918681 and DEB 0918167).
Author information
Authors and Affiliations
Contributions
KS, LK, and JD conceptualized and designed the study. KS, LK, JD, and AS collected samples, performed laboratory and data quality analyses. SP and KS statistically analyzed and interpreted the data. KS, SP, and JD wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Additional information
Responsible Editor: Marie-Anne de Graaff
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smemo, K.A., Petersen, S.M., Kluber, L.A. et al. Temporal soil enzyme patterns provide new insights into the nutrient economy of acidic hardwood forests. Biogeochemistry 155, 97–112 (2021). https://doi.org/10.1007/s10533-021-00814-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-021-00814-7