Abstract
Lipid biomarkers [fatty acids (FAs), sterols and alcohols] and total organic carbon (TOC) were analyzed in 48 surface (0–2 cm) sediment samples collected twice (winter 2008/2009 and summer/2009) in two transects ranging from 25 to 3,000 m depths. This sampling array encompassed the major upwelling region in the southeastern Brazilian continental margin, where the river influence is probably minimal. The objectives were (1) to evaluate the sources, transport and major areas of organic matter (OM) accumulation in the continental margin and (2) to identify the fraction of OM that is potentially available to secondary benthic producers. As expected from the regional oceanographic characteristics, lipids derived from primary and secondary autochthonous producers (0.073–5.3 mg gTOC−1) made the major fraction of the sedimentary OM, whereas lipids from allochthonous sources (0.043–0.40 mg gTOC−1) and from bacteria (<0.01–0.43 mg gTOC−1) were of relatively less importance. The accumulation of OM in the sediments was highly dependent on the coupling of physical (hydrodynamics) and biological (response to upwelling) factors. It was found that while some restricted areas in the shelf was a sink of labile OM, the export of this material to the upper and middle slope (400–1,000 m depths) can represent an important source of bioavailable OM to the deep sea benthic community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The knowledge of the origin, composition and distribution of organic matter (OM) in continental margins is relevant for several reasons. It is well known that continental margins are highly productive environments in response to the relative high availability of nutrients derived from upwelling events, continental runoff and ground-water discharge (Fasham 2003; Gattuso et al. 1998). However, the comprehension of the pelagic and benthic ecology in aquatic systems seems to be dependent on the quality rather than on the quantity of OM available for primary and secondary producers (Pusceddu et al. 2009 and references therein). In addition, continental shelves account for a significant fraction of total OM burial in marine sediments (Hedges and Keil 1995), thus acting as a sink for atmospheric CO2 (Chen 2004; Frankignoulle and Borges 2001). However, the magnitude of this process is primarily controlled by the export to and/or accumulation in the sediment of the autochthonous and labile fraction of this OM.
Several geochemical indicators, broadly divided into elemental ratios, isotopic composition and molecular markers, can be considered to ascertain and characterize the sources of OM in aquatic sediments. Among the molecular markers, lipids are largely used because of their source specificity and higher resistance to bacterial degradation in comparison to other classes of organic compounds (see review in Volkman 2006). The assignment of lipids from several classes [e.g., fatty acids (FAs), sterols and/or alcohols] to distinct sources of OM allow the identification of the relative contributions of autochthonous and allochthonous inputs to the sedimentary record (e.g., Grossi et al. 2003; Mejanelle and Laureillard 2008; Schmidt et al. 2010; Volkman et al. 2008; Waterson and Canuel 2008).
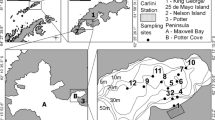
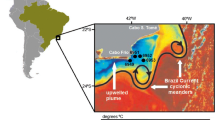
The Campos Basin (20–24°S; 39–42°W), in the southeastern Brazilian continental margin (SEBCM), is the most important region of offshore petroleum production in the country, and also has ecological significance because of the presence of areas of relatively high productivity in the water column. This is caused by upwelling events of the cold and nutrient-rich South Atlantic Central Water (SACW), whose core is in Cabo Frio (23°S) during spring-summer months, but their influence may extend for over hundreds of km in the shelf (Campos et al. 2000; Lorenzzetti and Gaeta 1996; Silveira et al. 2000; Valentin et al. 1987). There are evidences of the role of the Cabo Frio upwelling upon the primary and secondary productions of OM over both spatial and temporal scales (De Leo and Pires-Vanin 2006; Gonzalez-Rodriguez et al. 1992; Guenther et al. 2008; McManus et al. 2007; Sumida et al. 2005; Valentin et al. 1986).
However, the available information about the influence of the upwelling events on the quality and quantity of OM that accumulates in the continental margin sediments of the SEBCM have only recently been investigated in further detail. For example, Yoshinaga et al. (2008) considered a suite of lipid biomarkers to characterize the OM in sediments from the upwelling region of Cabo Frio and a southward non-upwelling region. Although with only four sampling stations on the shelf (i.e., at 40, 100, 250 and 500 m water depth), these authors observed a complex pattern of OM deposition in response to physical forcing (i.e., upwelling and eddies formation), whose influence on the biogeochemistry of OM was observed not only in the platform, but also in the shelf-break. Additionally, Carreira et al. (2010) also used lipid biomarkers to show that sediments in the slope (750–1,950 m depth) located 150 km northeastern of Cabo Frio have lower contents of OM compared to shallow regions, but still with a significant bioavailable fraction (particularly in the upper slope of 750–1,050 m).
In order to take a step forwards on the comprehension of the biogeochemistry of OM in the upwelling region of Cabo Frio, following the previous studies cited above, here we also considered lipid biomarkers (FAs, sterols and alcohols) but enhanced the spatial resolution by the collection of samples in 24 stations and in two periods along two sediment transects off Cabo Frio (23°S). Each transect extends from the inner continental shelf down to the deep ocean (i.e., from 25 to 3,000 m water depths). The objectives were (1) to increase the knowledge about the sources, transport and major areas of OM accumulation in the continental margin and (2) to identify the fraction of OM that is potentially available to secondary benthic producers. This study is part of a larger project entitled “Habitats Project—Campos Basin Environmental Heterogeneity” conducted by “CENPES/PETROBRAS”.
Methods
Study area and sampling procedures
The Campos Basin, in the southeastern Brazilian continental margin (SEBCM), extends from the Vitoria High (20°S) to the Cabo Frio High (24°S), covering an area of approximately 100,000 km2. The shelf has a mean width of 100 km and the mean depth of the shelf-break is 110 m, ranging from 80 m in the southern-sector to 130 m in the northern sector. The continental slope is 40 km wide with a mean inclination of 2.5 degrees, and is characterized by a complex system of submarine channels and canyons. The transition from the slope to the continental rise is marked by the presence of the São Paulo Plateau, extending from 2,000 to 3,500 m depths with a low gradient (1:100) (Viana et al. 1998).
The inner shelf is influenced by the Coastal Water [temperature (T) >15 °C; salinity (S) <35; depth <30 m], resulting from the dilution of oceanic waters with the continental drainage. In the outer shelf and continental slope the Tropical Water (T > 20 °C; S > 36.4; depth = 0–200 m), which comprises the southward branch of the Brazil Current (BC), is observed in surface waters, while the South-Atlantic Central Water (SACW; T < 20 °C; 35 < S < 36.4) is observed between 200 and 500 m depth (Calado et al. 2010; Marone et al. 2010). The interaction between these three water masses, coupled to a complex circulation pattern, marked by the meandering of the BC, are responsible for the sedimentation patterns in the shelf and slope from the SEBCM (Mahiques et al. 2004). In deeper portions of the basin, the Antarctic Intermediate Water (2 °C < T < 6 °C, S ~34.2, 550–1,200 m depth) and the North-Atlantic Deep Water (3 °C < T < 4 °C, 34.6 < S < 35, 1,500–3,000 m depth) are observed (Reid 1989).
The northeasterly trade winds, more frequent during spring-summer months (September–March), favor the Ekman offshore transport of surface waters and result in the intrusion of SACW over the shelf, which may reach the surface, particularly at Cabo Frio (Franchito et al. 2008; Moreira da Silva 1973; Valentin and Kempf 1977). The upwelling of Cabo Frio induces periods of enhanced primary production (2.0–14.0 μgC L−1 h−1) (Gonzalez-Rodriguez et al. 1992) in surface waters (<50 m depth)—which contrast with the overall oligotrophic characteristic of the shelf waters (Marone et al. 2010)—but the presence of SACW in the sub-surface is also responsible for the maxima chlorophyll contents that are observed at the base of the photic zone (~50 m depth) over large areas of the SEBCM (Rossi-Wongtschowski and Madureira 2006 and references therein). In addition, it must be considered the effect of the BC meandering and eddies formation—resulted from the interaction between the current flow and shelf topographic features—upon shelf-break upwelling of SACW (Campos et al. 2000; Castelao et al. 2004) and the consequences of these processes on the primary production and transport of materials along the continental margin (Marone et al. 2010).
The sediment samples were collected onboard the R/Vs Gyre, Miss Emma McCall and/or Luke Thomas in the winters of 2008 and 2009 and in the summer of 2009, using grabs similar to a box-corer that allowed the recovery of undisturbed 0–2 cm layer of the sediment. In each of the 24 sampling stations in the two transects, ranging from 25 to 3,000 m depths (Fig. 1), it was collected samples from at least three deployments of the box-corer. The samples were stored at −20 °C, freeze dried in the laboratory and the three independent samples in each station were combined to make one composite sample. A total of 48 homogenized samples was analyzed.
Total organic carbon analysis
Total organic carbon (TOC) was determined using a Carlo Erba EA 1110 elemental analyzer after removal of inorganic carbon with 0.1 M HCl (Hedges and Stern 1984). Sub-samples (2–5 mg) were weighed (precision = ±0.01 mg) in tin containers, folded and placed in the instrument. Quantification was performed by instrument response factor relative to the standard cystine. Analytical precision was better than 3 % (±standard deviation; n = 4) for TOC and TN and accuracy was verified by analyzing a reference material (PACS-2, National Research Council of Canada; reference value for total C). TOC data were relative to bulk sediment, after correction for carbonate content.
Lipid biomarker analysis
Sediment samples (~5.0 g, dry weight) were Soxhlet extracted with dichloromethane and methanol (9:1, v:v) for 24 h, after addition of about 2,500 or 5,000 ng each of 5α-androstan-3β-ol, C19 and C21 FAMEs (fatty acid methyl esters) and 1-nonadecanol as surrogate standards. Bulk extracts were saponified (110 °C for 2 h) using aqueous 1 M KOH to isolate neutral lipids (sterols and alcohols) and FAs. Analyses of sterols and alcohols (as TMS-derivatives after reaction with BSTFA at 85 °C for 1 h) were performed using gas chromatography coupled to mass spectrometry (Finnigan Focus DSQ GC/MS system) while FAs (as methyl esters or FAMEs after reaction with BF3/MeOH at 85 °C for 2 h) were analyzed by gas chromatography with flame ionization detection (Hewlett-Packard 6890). The GC/MS system was operated in the EI (70 eV) and scan mode (50–550 amu). A DB-5 type column (5 % methyl-phenyl siloxane, 30 m × 0.32 mm × 0.25 μm film) was used in both instruments. Quantification in the GC/MS was performed using a calibration curve (100–5,000 ng mL−1) with commercial standards (octadecanol, cholest-5-en-3β-ol, 5α-cholestan-3β-ol, 24-methylcholest-5-en-3β-ol, 24-ethylcholesta-5,22E-dien-3β-ol and 24-ethylcholest-5-en-3β-ol) and by considering the peak areas of key ions (m/z 129 or 215 for sterols and m/z 103 for n-alcohols) and relative response factors to the internal standard 5α-cholestane (m/z 217). Similar response factors of key ions were assumed for structurally related compounds whose standards were unavailable. Compound identifications in the GC/MS were based on the full-scan mass spectra obtained from the standards available (see above) or by comparison with those found in the literature for the other compounds. FAMEs were identified and quantified using a standard mixture (C37FAMEs, Supelco®) and deuterated tetracosane as internal standard. Confirmatory analysis of FAMEs was performed by GC/MS. Laboratory blanks showed no contamination during sample processing. Mean recoveries of surrogates were 85.9 ± 24.9 % and 81.6 ± 20.0 for C19 and C21 FAME, respectively, 79.1 ± 17.7 % for sterols and 81.5 ± 19.6 % for alcohol (data were not corrected for recovery).
Results
Total organic carbon and total lipids
The mean TOC contents, considering all samples collected in the two periods (winter and summer), was 6.9 ± 4.3 mg g−1 for transect A and 8.1 ± 5.7 mg g−1 for transect B (Table 1). The cross-shelf variation in the TOC concentrations was similar in the slope of the two transects. Higher values (TOC ~16–17.5 mg g−1) were measured in the middle slope stations (700–1,000 m water depths) and at 400 m depth in transect B. A steady decrease was observed from 1,900 to 3,000 m depths, reaching values around 2.00 mg g−1. On the shelf, the TOC contents were generally low (<5.0 mg g−1), with the exception of stations at 75 and 100 m in transect A (TOC = 10.6–13.6 mg g−1) and at 25 m in transect B (TOC ~ 12 mg g−1).
The concentration of total lipids (sum of 38 FAs, 16 n-alcohols, phytol and 14 sterols) ranged from 0.7 to 36.5 μg g−1 (Table 1), with an overall mean (i.e., considering the samples from both sampling periods) of 9.05 ± 5.92 μg g−1 for transect A and 15.13 ± 10.65 μg g−1 for transect B. These means are statistically different (Student-t test, p < 0.05), but there is no significant difference in total lipid concentration between the sampling periods (winter 2008/2009 and summer/2009) for a particular transect.
Lipid classes: FAs, sterols and alcohols
All classes of lipids followed, in general, the same spatial distribution as observed for the total lipids in both sampling periods. The FAs occurred as compounds between 14 and 30 carbon atoms (C14–C30) and were the most abundant class of lipids, with a mean concentration of 8.1 ± 5.7 μg g−1 (or 70.0 ± 14.0 % of total lipids; Table 1). Saturated and short-chain FAs (SCFAs, <C22) contributed with 53.3 ± 14.3 % of total FAs, with the C16:0 and C18:0 as the most abundant compounds. The saturated and long-chain FAs (LCFAs, ≥C22) accounted for only 9.1 ± 4.3 % of total FAs, being the C22:0 and C24:0 the most abundant ones. Highest concentrations of LCFAs (1.24–4.46 μg g−1) were measured in the winter at stations B06 to B09, in the slope (400–1,300 m depths) of transect B, but relatively enriched contents of LCFAs was also measured in the slope of transect A in both samplings and in transect B during the summer (Table 1). Both the SCFAs and the LCFAs exhibited strong even-over-odd carbon number predominance. The mono-unsaturated FAs (MUFAs), with C16, C18 and C20 chain-lengths, represented 29.9 ± 7.6 % of total FAs, with a predominance of C18:1 (1.2 ± 0.9 μg g−1; sum of cis and trans isomers) and C16:1 (1.0 ± 0.8 μg g−1). The polyunsaturated FAs (C20:2, C20:3, C20:4, C20:5, C22:2, C22:6; PUFAs) and the branched FAs (iso- and anteiso- C15 and C17 and 10-methyl-C16; BRANCH) accounted, respectively, for 5.2 ± 3.4 % and 6.7 ± 4.7 % of total FAs. PUFAs contents higher than 10 % of total FAs were measured in both transects and in both samplings at 100 m (stations A4 and B4) and 150 m (stations A5 and B5) water depths, in the shelf-break, while the BRANCH were enriched (i.e., ~15–20 % of total FA) at 1,000 and 1,300 m depths during the winter in both transects.
The sterols occurred as C27 to C30 compounds, with mean total concentration of 3.3 ± 3.6 μg g−1 or 21.5 ± 12.5 % of total lipids (Table 1). On the shelf, highest concentrations of total sterols were measured at 75 and 100 m in transect A (stations A03 and A04) and at 25 m in transect B (station B01). Each of these stations showed very similar concentrations of total sterols in the two samplings. In the slope, the highest total sterols concentrations were measured between 400 and 1,000 m depths in transect B (stations B06, B07 and B08), particularly in the winter sampling. A similar trend in the slope was measured in transect A, but at relatively lower concentrations compared to transect B. At 2,500 and 3,000 m depths (stations labeled 11 and 12, respectively), in the continental rise, many sterols were below detection limit in both transects. Cholest-5-en-3β-ol (cholesterol, 27Δ5) and 24-ethylcholest-5-en-3β-ol (29Δ5) were the most abundant sterols, representing 31.6 ± 13.7 % and 25.3 ± 12.1 % of total sterols, respectively. The 27Δ5 was more abundant on the shelf stations (stations <150 m depth), while in the slope and rise (stations from 400 to 3,000 m depths) the 29Δ5 predominated.
The other unsaturated sterols [cholesta-5,22E-dien-3β-ol (27Δ5,22); 24-methylcholesta-5,22E-dien-3β-ol (28Δ5,22); 24-methylcholesta-5,24(28)-dien-3β-ol (28Δ5,24(28)) 24-methylcholest-5-en-3β-ol (28Δ5); 24-ethylcholesta-5,22E-dien-3β-ol (29Δ5,22) and 4α,23,24-trimethyl-5α-cholest-22-en-3β-ol (30Δ22)] accounted for 6–11 % of total sterols and, in general, they showed the same distribution pattern as observed for the total sterols concentrations (see above). The stanols 5α-cholestan-3β-ol (27Δ0) and 24-ethyl-5α-cholestan-3β-ol (29Δ0) were also relatively abundant (around 6 % of total sterols), while other reduced sterols (27Δ22, 28Δ22 and 29Δ22) were minor components (Table 1).
The alcohols (including phytol) accounted for only 8.5 ± 7.3 % of the total lipids, with a mean total concentration of 0.85 ± 0.72 μg g−1 (Table 1). The saturated and even-numbered short-chain compounds (SCOH, <C22) were the most abundant (Table 1), with C18 (22.1 ± 17.0 % of the total alcohols) and C16 (14.0 ± 11.0 % of total alcohols) as the major individual compounds. Phytol (3,7,11,15-tetramethyl-2-hexadecen-1-ol), including the sum of free and bound fractions (since our analytical procedure includes a saponification step), accounted for 30.4 ± 24.7 % of total alcohols. Higher concentrations of phytol were measured in the transect B during winter, at 25 m (station B01) and between 400 and 1,000 m in the slope (stations B04, B05 and B06). Saturated and long-chain alcohols (LCOH, ≥C22), with a strong even-over-odd carbon number predominance, contributed with 27.3 ± 12.3 % of total alcohols. Although at low concentrations, the alcohols with C24, C26 and C28 were the most abundant LCOH and were present at similar proportions, around 7 % of the total alcohols. Relatively enriched contents of LCOH were measured at 25 m (shelf) and between 400 and 1,300 m depths (slope) of transect B (stations B01, B06, B07, B08 and B09, respectively).
Discussion
Bulk composition of OM
The mean concentration of TOC and total lipids obtained in the present study (respectively, 6.9 ± 4.3 mg g−1 and 9.05 ± 5.92 μg g−1 for transect A and 8.1 ± 5.7 mg g−1and 15.13 ± 10.65 μg g−1 for transect B) are comparable to previous data for the same region (Carreira et al. 2010; Yoshinaga et al. 2008) and are in the upper range of OM contents observed in the Brazilian continental margin (Jennerjahn et al. 2010). The upwelling causes nutrient injection in the photic zone and consequently there is a higher primary production in Cabo Frio compared to other areas in the SEBCM, which is marked by an overall oligotrophic condition (Rossi-Wongtschowski and Madureira 2006 and reference therein).
It is noteworthy the high and significant correlation (r 2 = 0.66; p < 0.001) between TOC and total lipids contents, considering the entire data set. In Fig. 2 it can be observed a group of stations from transect A [A03 (75 m), A04 (100 m), A07 (700 m) and A08 (1,000 m)] and transect B [B01 (25 m), B06 (400 m), B07 (700 m), B08 (1,000 m) and B09 (1,300 m)] with high contents, in the two samplings, of both total lipids (12.6 μg g−1 < total lipids < 36.5 μg g−1) and TOC (10 mg g−1 < TOC < 18 mg g−1). In all those stations, the sediment was composed by >85 % of silt and mud (C.E. Rezende, UENF, personal communication). On the other hand, very low concentration of total lipids and TOC were measured in some stations on the shelf—A01 (25 m), A02 (50 m), B02 (50 m) and B03 (75 m)—as well as in the deep-sea stations—label 11 and 12 of transect A and B, corresponding to 2,500 and 3,000 m depths, respectively. For the stations on the shelf, the low concentrations can be explained by the presence of coarse sediments (80–90 % sand; C. E. Rezende, UENF, personal communication), suggesting low tendency for OM accumulation. The presence of muddy sediments in the deep sea stations (i.e., 2,500 and 3,000 m depths) would favor accumulation of OM, and thus the low concentration of TOC and total lipids observed at these stations (Fig. 2) may be ascribed to distance of the main sources of lipids (see later). This result may also be associated with the low primary production observed in offshore waters of SEBCM (Rossi-Wongtschowski and Madureira 2006 and references therein) and/or the selective degradation of OM during its transport through the water column (Wakeham and Canuel 2006).
In Cabo Frio (23°S)—as also observed in other regions in the SEBCM, like Vitória (20°S), Cabo São Tomé (22°S) and Cabo Santa Marta (28°S)—the transport of materials and the sedimentation patterns seem to be largely influenced by the BC meandering and Ekman dynamics associated with NE winds (Mahiques et al. 2004; Marone et al. 2010). Based on these hydrodynamic patters, it has been recently outlined that a large fraction of OM produced on the shelf or imported from continental sources is probably transported offshore by the prevailing currents and eddy formation and is deposited in the shelf-break and in the slope (Marone et al. 2010). Our data for total lipid concentrations provided additional evidence of this process since it showed the upper and middle slope between 400 and 1,000 m depths as important areas of OM accumulation. On the other hand, our data of total lipids also show that in the shelf, between the 25 and 100 m isobaths, there are important areas of OM accumulation.
Source assignments of OM based on lipid biomarkers
In order to further evaluate the nature of the sedimentary OM in the continental margin, lipids were grouped as follows: (1) phytoplankton or primary producers: sum of the sterols 27∆5,22, 28∆5, 28∆5,22, 28∆5,24(28), 29∆5 and 30∆22, the FAs C16:1, C18PUFA and C20PUFA and phytol; (2) zooplankton and/or fauna: sum of 27∆5 and C18:1 and C22:6 FAs; (3) allochthonous or terrestrially derived: sum of LCFAs and LCOH; (4) bacteria: sum of the branched iso and anteiso C15 and C17 and 10-methyl-C16 FAs. In all cases, the TOC-normalized concentration of lipids was considered.
The association of individual compounds to a specific group was based on known source-assignments of lipids (e.g., McCallister et al. 2006; Bianchi and Canuel 2011) but also considered previous results for the Campos Basin (Carreira et al. 2010; Yoshinaga et al. 2008) and the calculated correlation coefficients obtained between individuals and/or groups of lipids. This approach may be constrained by the multiple sources of a particular lipid (Bianchi and Canuel 2011). One example is the C22:6 PUFA, which was included in the group of zooplankton/fauna but can also be produced by dinoflagellates (Yoshinaga et al. 2008 and references therein), but the C22:6 PUFA is present at low concentrations. More relevant is the abundant sterol 29Δ5, which was considered in our samples as derived from autochthonous sources because of its high correlation with the autochthonous sterols 27Δ5 (r = 0.78, p < 0.01) and 28Δ5,22 (r = 0.95, p < 0.01) (Schefüβ et al. 2004; Volkman 2006). However, the sterol 29Δ5 was also relatively well correlated with LCFA (r = 0.48, p < 0.01) and with LCOH (r = 0.61, p < 0.01), suggesting an additional contribution from terrestrial sources in the samples considered in the present work. Based on the limitations of source assignments of OM based on our approach of grouping the lipids, the discussion that follows is focused more in the evaluation of the changes in the contribution of a particular group among the samples rather than considering each group as a quantitative indicator of a particular source of OM.
Lipids from autochthonous sources
The predominance of lipids from primary production (PP) and zooplankton/fauna (ZF) over those derived from terrestrial sources (allochthonous) and from bacteria (Fig. 3) suggest an overall contribution of autochthonous sources to the total pool of sedimentary OM in the studied region. This was consistent with the relatively enhanced productivity levels in this sector of the SEBCM in response to upwelling events, as already observed in other studies (Jennerjahn et al. 2010; Yoshinaga et al. 2008). The low abundance of allochthonous lipids indicates a relatively small influence of Paraíba do Sul River outflow, located 150 km to the north of Cabo Frio, which is the main source of continental material to the shelf in the Campos Basin (Ekau and Knoppers 1999).
High TOC-normalized concentrations of PP lipids were measured during the winter sampling at stations from transect B on the outer shelf (B04, 100 m) and on the shelf-break (B05, 150 m) (1.36–1.43 mg gTOC−1), although down on the slope (stations B06 to B09) the concentrations of PP lipids were also elevated (0.52–1.22 mg gTOC−1). The shelf in transect B is narrower, characterized by smooth gradients until 60 m and steep gradients after 150 m depth. These topographical features might have enhanced the transport and accumulation of autochthonous OM on the shelf-break and in the slope of transect B in comparison to transect A. Intermediate concentrations of PP lipids (0.45–0.82 mg gTOC−1) were also measured in many shelf stations on both the winter and summer samplings (Fig. 3).
The distribution of TOC-normalized concentration of PP lipids differed from that based on absolute concentration of total lipids (i.e., μg of lipid per g of dry sediment), as previously discussed. This was caused by the elevated concentrations of TOC at stations B06 to B09 (11.2–17.6 mg g−1 in both samplings) in comparison to the values measured at stations B04 and B05 (2.6–4.4 mg g−1 in both samplings). In addition, the nearshore stations A01 and B01 (both at 25 m depth) were particularly affected by the normalization of lipid concentrations, but due to different reasons. Station A01 had low concentration of total lipids in μg g−1 (Table 1) but showed highest TOC-normalized concentrations of PP lipids in both samplings. However, at station B01, with elevated lipid concentration, the TOC content was one order of magnitude higher than measured in station A01, and consequently the normalized concentration of lipids was small (Fig. 3).
As the PP lipids represented the major fraction of the total lipids in the near-shore and shelf-break stations, it suggested a rapid transport of OM produced in the water column to the sediment. This process was apparently favored by the local shallow depths and the occurrence of coastal and shelf-break upwelling events. On the other hand, with increasing depth in the slope, the PP lipids contents were still significant, although they represented a lower fraction of the total sedimentary OM. The higher susceptibility of the PP lipids to bacterial degradation (Wakeham and Canuel 2006) could be responsible for the decreasing concentration of this group of lipids during the transport of OM from productive areas in the shelf of upper slope to the final accumulation in the slope sediments.
The concentration of ZF lipids was comparable to the values for the PP lipids (Fig. 3). In fact, the two groups of lipids were highly correlated (r = 0.94, p < 0.01). The presence in relative high concentrations of lipids that can be (not exclusively) ascribed to zooplankton, particularly 27Δ5 and C18:1 FA (C22:6 FA was a minor component), suggests that the OM produced in the water columns was efficiently reworked by zooplankton organisms before its final burial in the sediment in the studied region. The role of zooplankton seemed to be enhanced in the shelf stations (<150 m), where the mean concentration of the ZF lipids (considering all samples in the two transects) was 0.56 ± 0.47 mg gTOC−1 whereas in the slope stations (400–1,300 m) it was only 0.24 ± 0.01 mg gTOC−1. This is consistent with the effective grazing of phytoplankton by mesozooplanktonic species of Copepoda (Gonzalez-Rodriguez et al. 1992) that occurs during the period of day to weeks of an upwelling event (Valentin 1984) and agrees with previous results for the region (Yoshinaga et al. 2008). However, it cannot be ruled out possible contributions of primary producers to the group of ZF lipids (Volkman 2006; Bianchi and Canuel 2011), as previously pointed out.
The relatively enhanced concentration of lipids associated with primary and secondary producers observed in the winter sampling (Fig. 3), although with mean values for the two samplings periods statistically similar (at p < 0.01), was opposite to results obtained by Yoshinaga et al. (2008) in the same region. This discrepancy suggests that the temporal variation in the production and export of OM in the Cabo Frio region is complex and needs to be further investigated, for instance, by the deployment of sediment traps in the region. The complexity of OM distribution in the studied sediments may be associated with the episodic nature of the Cabo Frio upwelling events, whose upwelling/downwelling cycles are short (days to weeks) and dependent on the balance between blowing of trade winds and passage of cold fronts in the region (e.g., Gonzalez-Rodriguez et al. 1992). Another factor that may have influenced the variability of sedimentary OM accumulation is the occurrence of eddies and meanders of the Brazil Current (BC). These are meso-scale quasi-stationary and frontal cyclonic and anticyclonic phenomena, most frequently observed during Austral spring-summer time along the 100 and 150 m isobaths, that are formed due to the interaction of the southward flow of the BC, the abrupt change in the orientation of the coastline (from N–S to E–W) and the topographic features of the shelf (short and steep in the north and large and smooth in the south) at Cabo Frio (Campos et al. 2000; Lorenzzetti and Gaeta 1996; Marone et al. 2010; Silveira et al. 2008). The cyclonic eddies and meanders of the BC can induce upwelling event at the shelf-break (Calado et al. 2010; Campos et al. 2000) and can also enhance the offshore transport of materials (Marone et al. 2010). In fact, this process was considered to explain the high density of benthic invertebrate larvae observed in the water column in the slope of Cabo Frio region (Yoshinaga et al. 2010).
Lipids from allochthonous and bacterial sources
The low contribution of allochthonous lipids compared to PP and Z/F lipids probably reflected, as mentioned before, the distance of more than 150 km of Paraíba do Sul River, the major source of continentally derived OM to our sampling region. However, the allochthonous lipids were relatively enriched in some shelf stations, particularly at B03 (75 m) in both sampling periods, as well as at A01 (25 m) during the summer sampling and at B04 (100 m), in the winter sampling (Fig. 3). Possible explanations for these results can be inputs from small local rivers (not shown on Fig. 1) and/or atmospheric transport. A more regional sampling array is necessary to investigate these possibilities in further detail.
The bacterial lipids were the less important group of lipids (Fig. 3). However, they were more correlated with PP lipids (r = 0.84, p < 0.01) and ZF lipids (r = 0.83, p < 0.01) than with allochthonous lipids (r = 0.41, p < 0.01). These results suggest that the bacterial activity was closed linked to higher availability of labile OM (i.e., sum of PP and ZF lipids).
Evaluation of the quality of OM to benthic secondary producers
The quality or nutritional value of the sedimentary OM, basically represented by its percentage of fresh or labile components, is one of the most important variables that control benthic abundance and diversity, especially in open sea sediments (Fontainer et al. 2008; Pusceddu et al. 2009). These authors estimated the nutritional value of OM by bulk properties of OM, like total concentrations of proteins, carbohydrates and lipids. Alternatively, the proportion of chlorophyll-a to phaeopigments was also considered as a measured of OM “freshness” (Quintana et al. 2010).
Here, we evaluated the quality of OM by a different approach, i.e., by considering specific lipid biomarkers. In this sense, we used the relative contributions of PP lipids as indicators of bioavailable OM (i.e., with high nutritional value), while the allochthonous lipids represented the refractory fraction of OM (i.e., with low nutritional value). We stress out that this approach is qualitative rather than quantitative, since it is dependent on the number of compounds considered in each group and thus only relative changes among the stations can be evaluated. It can be observed that in transect A (Fig. 4), the proportion of PP lipids ranged from 40 to 50 % in all stations from 25 to 1,300 m depths, but it decreased to below 30 % towards deeper stations. In the samples from transect B, in addition to higher concentrations of PP lipids in comparison to transect A (see Fig. 3), the PP lipids ranged from 50 to 60 % at stations in the upper to middle slope (400–1,000 m depths), with percentages slightly higher than those observed in the shelf stations. On the other hand, the contribution of allochthonous lipids to total lipids steadily increases with depth in the two transects and reaches maxima values at stations from 1,900 to 3,000 m, excluding the sample at 75 m in transect B (Fig. 4).
Based on the percentage distribution of PP and allochthonous lipids discussed above, the quality of OM did not show major changes from 25 to 1,300 m depths, whereas the refractory fraction of OM become more significant from 1,900 to 3,000 m depths. Therefore, since the bioavailable fraction of OM—as suggested by the distribution of PP lipids—seems to be similar throughout the shelf and slope sediments, the TOC-normalized concentration of PP lipids might be more important than organic carbon content as the main chemical variable to be considered in the ongoing benthic ecology studies under the Habitats project.
Conclusion
The consideration of lipid biomarkers (FAs, sterols and alcohols) in surface sediments of cross-margin transects allowed the inference of the sources of OM and the characterization of the spatial variation of sedimentary OM accumulation at Cabo Frio, a portion of the SEBCM directly influenced by upwelling events. The OM in the sediments on the shelf and slope (down to 1,000 m depth) were essentially derived from primary and secondary producers and thus contained an important reactive fraction that is available to benthic organisms. In the deep portion (1,900–3,000 m depths), the OM content was low with an increased fraction of refractory OM with potentially low nutritional value.
Our data showed that some areas in the continental shelf, which is more directly influenced by nutrient enrichment caused by ventilation processes, act as a sink of OM. However, the presence of labile lipids in the upper and middle slope (400–1,000 m depths) in relatively high concentrations was probably derived from the offshore transport of materials associated with the meandering of the Brazil Current, since the primary productivity in the offshore waters is generally low (Rossi-Wongtschowski and Madureira 2006). In addition, our data, in conjunction with previous information for the same area, contributed to show that the production and transport of OM at Cabo Frio is complex and highly dependent on natural variability of major physical process (i.e., upwelling, meandering, Ekman transport) that determined the general hydrodynamic of the continental shelf.
References
Bianchi TS, Canuel EA (2011) Chemical biomarkers in aquatic ecosystems. Princeton University Press, Princeton
Calado L, da Silveira ICA, Gangopadhyay A, de Castro BM (2010) Eddy-induced upwelling off Cape São Tomé (22° S, Brazil). Cont Shelf Res 30(10–11):1181–1188
Campos EJD, Velhote D, Silveira ICA (2000) Shelf break upwelling driven by Brazil Current cyclonic meanders. Geophys Res Lett 27(6):751–754
Carreira RS, Araújo MP, Costa TLF, Ansari NR, Pires LCM (2010) Lipid biomarkers in deep sea sediments from the Campos Basin, SE Brazilian continental margin. Org Geochem 41:879–884
Castelao RM, Campos EJD, Miller JL (2004) A Modelling study of coastal upwelling driven by wind and meanders of the Brazil Current. J Coast Res 20(3):662–671
Chen C-TA (2004) Exchanges of carbon in the coastal oceans. In: Field CB, Raupach MR (eds) SCOPE 62: the global carbon cycle: integrating humans, climate, and the natural world. Island Press, Washington, pp 341–351
De Leo FC, Pires-Vanin AMS (2006) Benthic megafauna communities under the influence of the South Atlantic Central Water intrusion onto the Brazilian SE shelf: a comparison between an upwelling and a non-upwelling ecosystem. J Mar Syst 60(3–4):268–284
Ekau W, Knoppers BA (1999) An introduction to the pelagic system of the North-East and East Brazilian shelf. Arch Fish Mar Res 47(2/3):113–132
Fasham MJR (2003) Ocean biogeochemistry. The role of the ocean carbon cycle in global change. Springer, Berlin
Fontainer C, Jorissen JJ, Lansard B, Mouret A, Buscail R, Schimdt S, Kerhervé P, Buron F, Zaragosi S, Hunault G, Ernoult E, Artero C, Anschutz P, Rabouille C (2008) Live foraminefera from the open slope between Grand Rhône and Petit Rhône Canyons (Gulf of Lions, NW Mediterranean). Deep Sea Res 55:1532–1553
Franchito SH, Oda TO, Rao VB, Kayano MT (2008) Interaction between coastal upwelling and local winds at Cabo Frio, Brazil: an observational study. J Appl Meteor Clim 47(6):1590–1598
Frankignoulle M, Borges AV (2001) European continental shelf as a significant sink for atmospheric carbon dioxide. Glob Biogeochem Cycles 15(3):569–576
Gattuso J-P, Frankignoulle M, Wollast R (1998) Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu Rev Ecol Syst 29:405–434
Gonzalez-Rodriguez E, Valentin JL, André DL, Jacob SA (1992) Upwelling and downwelling at Cabo Frio (Brazil): comparison of biomass and primary production responses. J Plankton Res 14(2):289–306
Grossi V, Caradec S, Gilbert F (2003) Burial and reactivity of sedimentary microalgal lipids in bioturbated Mediterranean coastal sediments. Mar Chem 81(1–2):57–69
Guenther M, Paranhos R, Rezende CE, Gonzalez-Rodrigues E, Valentin JL (2008) Dynamics of bacterial carbon metabolism at the entrance of a tropical eutrophic bay influenced by tidal oscillation. Aquat Microb Ecol 50:123–133
Hedges JI, Keil RG (1995) Sedimentary organic matter preservation: an assessment and speculative synthesis. Mar Chem 49:81–115
Hedges JI, Stern JH (1984) Carbon and nitrogen determinations of carbonate-containing solids. Limnol Oceanogr 29(3):657–663
Jennerjahn TC, Knoppers BA, Souza WFL, Carvalho CE, Mollenhauer G, Hüber M, Ittekkot V (2010) The tropical Brazilian continental margin. In: Liu K-K, Atkinson L, Quiñones R, Talaue-MacManus L (eds) Carbon and nutrient fluxes in continental margins: a global synthesis. Springer, Berlin, pp 427–442
Lorenzzetti JA, Gaeta SA (1996) The Cape Frio upwelling effect over the South Brazil Bight northern sector shelf waters: a study using AVHRR images. Int Arch Photogramm Remote Sens XXXI(part B7):448–453
Mahiques MM, Tessler MG, Maria Ciotti A, da Silveira ICA, Sousa SHME, Figueira RCL, Tassinari CCG, Furtado VV, Passos RF (2004) Hydrodynamically driven patterns of recent sedimentation in the shelf and upper slope off Southeast Brazil. Cont Shelf Res 24(15):1685–1697
Marone E, Knoppers BA, Souza WFL, Silveira ICA, Godoi SS (2010) The Brazil current: physical-biogeochemical domains. In: Liu K-K, Atkinson L, Quiñones R, Talaue-MacManus L (eds) Carbon and nutrient fluxes in continental margins: a global synthesis. Springer, Berlin, pp 153–170
McCallister SL, Bauer JE, Ducklow HW, Canuel EA (2006) Sources of estuarine dissolved and particulate organic matter: a multi-tracer approach. Org Geochem 37(4):454–468
McManus G, Costas B, Dam H, Lopes R, Gaeta S, Susini S, Rosetta C (2007) Microzooplankton grazing of phytoplankton in a tropical upwelling region. Hydrobiologia 575(1):69–81
Mejanelle L, Laureillard J (2008) Lipid biomarker record in surface sediments at three sites of contrasting productivity in the tropical North Eastern Atlantic. Mar Chem 108(1–2):59–76
Moreira da Silva PC (1973) A ressurgência de Cabo Frio (I). Boletim do Inst de Pesq da Marinha 78:60
Pusceddu A, Dell′Anno A, Fabiano M, Danovaro R (2009) Quantity and bioavailability of sediment organic matter as signatures of benthic trophic status. Mar Ecol Prog Ser 375:41–52
Quintana CO, Yoshinaga MY, Sumida PYG (2010) Benthic responses to organic matter variation in a subtropical coastal area off SE Brazil. Mar Ecol 31(3):457–472
Reid JL (1989) On the total geostrophic circulation of the South Atlantic Ocean: flow patterns, tracers, and transports. Prog Oceanogr 23:149–244
Rossi-Wongtschowski CL, Madureira LAS (2006) O ambiente oceanográfico da plataforma continental e do talude da região sudeste-sul do Brasil. EdUSP, São Paulo
Schefüß E, Versteegh GJM, Jansen JHF, Sinninghe Damste JS (2004) Lipid biomarkers as major source and preservation indicators in SE Atlantic surface sediments. Deep Sea Res I 51(9):1199–1228
Schmidt F, Hinrichs K-U, Elvert M (2010) Sources, transport, and partitioning of organic matter at a highly dynamic continental margin. Mar Chem 118(1–2):37–55
Silveira IMO, Schmidt ACK, Campos EJD, Godoy SS, Ikeda Y (2000) A Corrente do Brasil ao largo da costa brasileira. Revista Brasileira de Oceanografia 48(2):171–183
Silveira ICA, Lima JAM, Schmidt ACK, Ceccopieri W, Sartori A, Francisco CPF, Fontes RFC (2008) Is the meander growth in the Brazil Current system off Southeast Brazil due to baroclinic instability? Dyn Atmos Oceans 45(3–4):187–207
Sumida PYG, Yoshinaga MY, Ciotti AM, Gaeta SA (2005) Benthic response to upwelling events of the SE Brazilian coast. Mar Ecol Prog Ser 291:35–42
Valentin JL (1984) Analyse des paramétres hydrobiologiques dans la remotée de Cabo Frio (Brésil). Mar Biol 82:259–276
Valentin JL, Kempf M (1977) Some characteristics of the Cabo Frio upwelling. Coast Upwelling Ecosyst Anal Newslett 6(2):18–19
Valentin JL, Silva NML, Monteiro-Ribas WM, Mureb MA, Bastos CTBT, Tenenbaum DR, André DL, Jacob SA, Pessoti E (1986) Le plancton dans l’upwelling de Cabo Frio (Brésil): microrépartition spatio-temporelle à une station fixe. Ann Inst Océanogr 62(1):117–135
Valentin JL, André DL, Jacob SA (1987) Hydrobiology in the Cabo Frio (Brazil) upwelling: two dimensional structure and variability during a wind cycle. Cont Shelf Res 7(1):77–88
Viana AR, Faugeres JC, Kowsmann RO, Lima JAM, Caddah LFG, Rizzo JG (1998) Hydrology, morphology and sedimentology of the Campos continental margin, offshore Brazil. Sediment Geol 115(1–4):133–157
Volkman JK (2006) Lipid markers for marine organic matter. In: Volkman JK (ed) Handbook of environmental chemistry, volume 2: reactions and processes 2 (N). Springer, Berlin, pp 27–70
Volkman JK, Revill AT, Holdsworth DG, Fredericks D (2008) Organic matter sources in an enclosed coastal inlet assessed using lipid biomarkers and stable isotopes. Org Geochem 39:689–710
Wakeham SG, Canuel EA (2006) Degradation and preservation of organic matter in sediments. In: Volkman JK (ed) Handbook of environmental chemistry, volume 2: reactions and processes 2 (N). Springer, Berlin, pp 295–321
Waterson EJ, Canuel EA (2008) Sources of sedimentary organic matter in the Mississippi River and adjacent Gulf of Mexico as revealed by lipid biomarker and d13C-TOC analyses. Org Geochem 39(4):422–439
Yoshinaga MY, Sumida PYG, Wakeham SG (2008) Lipid biomarkers in surface sediments from an unusual coastal upwelling area from the SW Atlantic Ocean. Org Geochem 39(10):1385–1399
Yoshinaga MY, Sumida PYG, Silveira ICA, Ciotti AM, Gaeta SA, Pacheco LFCM, Koettker AG (2010) Vertical distribution of benthic invertebrate larvae during an upwelling event along a transect off the tropical Brazilian continental margin. J Mar Syst 79:124–133
Acknowledgments
The authors thank to PETROBRAS (Brazilian energy company) for providing samples and support the chemical analyses. D. R. P. Oliveira thanks a master’s fellowship from CAPES. R. S. Carreira was supported by a research fellowship from CNPq (process no. 303399/2010-4). We acknowledge the comments provided by the reviewers that improved the final version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira, D.R.P., Cordeiro, L.G.M.S. & Carreira, R.S. Characterization of organic matter in cross-margin sediment transects of an upwelling region in the Campos Basin (SW Atlantic, Brazil) using lipid biomarkers. Biogeochemistry 112, 311–327 (2013). https://doi.org/10.1007/s10533-012-9726-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-012-9726-z