Abstract
We measured grazing by herbivorous zooplankton (<200 μm fraction) in coastal and slope regions of the South Brazil Bight. Using the dilution technique, we performed nine experiments during the austral summer, when nutrient-rich South Atlantic Central Water is present on the shelf, and five during winter. These experiments provide the first estimates of microzooplankton grazing in the western South Atlantic Ocean. Model II regression showed a strong relationship between phytoplankton intrinsic growth rates and grazing, with a slope of 0.64 (±0.28; 95% confidence interval) indicating that microzooplankton grazing could account for the majority of phytoplankton mortality. Both phytoplankton growth and microzooplankton grazing were higher during the summer upwelling season, compared to winter. For the two experiments that were conducted in oligotrophic slope water, grazing accounted for >80% of phytoplankton production. A comparison of incubations with and without added inorganic nutrients showed no consistent stimulation of phytoplankton growth (slope of enriched versus unenriched treatments not significantly different from 1). Estimates from microscopic counts of heterotrophic organisms >10 μm indicated that copepod nauplii comprised the largest share of the microzooplankton biomass (mean 62.4 ± 5.8% SE). Grazing estimates were not correlated with microzooplankton biomass, whether or not nauplii were included, suggesting that most of the grazing was done by nano-sized zooplankton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The South Brazil Bight extends 1,100 km, from Cabo Frio (23°S) to Cabo de Santa Marta (28°40′ S), in southeastern Brazil (Castro & Miranda, 1998). The shelf in the northern part of this region is narrow (c. 50 km) and productive, and supports important commercial fisheries for sardine (Sardinella brasiliensis), anchovy (Engraulis anchoita), croaker (Micropogonias furnieri), and skipjack tuna (Katsuwonus pelamis), among other species (Bakun & Parrish, 1990; Bakun, 1993; Matsuura, 1996; FAO, 2003). Hydrographic properties of the Bight are determined by mixing between three principal water types: warm, salty tropical water (TW) carried poleward in the upper part of the Brazil Current; cold, less saline South Atlantic Central Water (SACW), which travels along the slope in the lower layer of the Brazil Current; and fresher coastal water (CW) resulting from mixing between shelf water and runoff outwelling from rivers and estuaries (Castro & Miranda, 1998). Variations in the prevailing northeast winds, which are strongest in austral summer, result in pulses of coastal upwelling in the Bight, especially close to the coast off Cabo Frio (“cold cape” in English) in the north (Valentin et al., 1987). SACW is the source water for upwelling. Meanders and cyclonic eddies from the Brazil Current bring this nutrient-rich water onto the shelf. It is usually found at depth over large areas of the shelf in summer, with less extensive intrusions during winter (Campos et al., 1995, Castro & Miranda, 1998).
Although seasonal and shorter-term variations in phytoplankton biomass and productivity have been described for the northern part of the Bight (Gonzalez-Rodriguez et al., 1992; Gonzalez-Rodriguez, 1994), there have been few observations on zooplankton feeding in the area, or on the structure of the pelagic food web. A recent review of microzooplankton grazing found no estimates of this important trophic flow for any area in the western South Atlantic Ocean (Calbet & Landry, 2004).
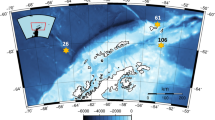
Our goal was to measure microzooplankton abundance and grazing, in relation to phytoplankton growth, across the gradient from productive coastal waters to the oligotrophic waters of the Brazil Current beyond the shelf break. We participated in two cruises in the South Brazil Bight aboard the R.V. Prof. W. Besnard (University of São Paulo), in July 2001 and January 2002. On a third trip, in January 2003, we conducted shore-based experiments at the Instituto de Estudos do Mar Almirante Paulo Moreira (IEAPM), in Arraial do Cabo, just south of Cabo Frio (Fig. 1). Our project was part of a larger study of the physical and biological oceanographic properties of the South Brazil Bight (“Dynamics of the shelf ecosystem of the South Atlantic Western Region”; Portuguese acronym: DEPROAS). The present study focused on the shelf and slope region south of the coastline that stretches from Rio de Janeiro to Cabo Frio (Fig. 1).
Station locations. Station 1 was sampled on both cruises; stations 4–7 were sampled on the winter (2001) cruise; stations 2, 3, 8, and 9 in summer (2002). Station 4 was also sampled four times during the shore-based experiments in summer 2003. Dotted lines show the approximate position of the 100 m and 200 m isobaths
Methods
We used the dilution method of Landry & Hassett (1982) to measure grazing. This method assumes that phytoplankton division rates in an incubated sample are not affected by dilution with filtered ambient water, but that mortality will decline proportional to the degree of dilution, due to decreased likelihood of encounter between phytoplankton and grazers. Thus, plots of phytoplankton realized growth rate (ordinate) versus fractional dilution (abscissa) yield growth (intercept) and grazing (slope) estimates. We conducted 14 dilution experiments, 2 at oceanic stations 2 and 3 (>500 m depth) and the rest in midshelf and coastal areas landward of the 200 m isobath, mostly just south and west of Cabo Frio (Fig. 1).
For each experiment, approximately 40 l of water was collected from the surface mixed layer, using Niskin bottles. When aboard ship, we collected water from the upper 5 m at first light (c. 06:00 h local time) and set up the experiments in dim light in the ship’s wet lab. For the shore-based experiments, water was collected at approximately 09:00 h and held in carboys in a darkened, air-conditioned room during transport to the lab (∼2 h). In the latter experiments, water was collected from slightly deeper (10–15 m) to take advantage of SACW that was evident near the base of the thermocline. For all experiments, water was pooled into a 60 l polyethylene (HDPE) container and gently siphoned through a submerged 200 μm mesh screen to remove mesozooplankton. The dilution series (100, 43, 21, and 8% unfiltered seawater) was prepared in triplicate 2.3 l polycarbonate bottles using as diluent water that had been filtered by gravity through a 0.2 μm Gelman capsule filter (product no. 12112). Bottles were filled completely, without air bubbles, and sealed with Parafilm before caps were screwed on, to avoid potential damage to delicate microzooplankters from bubbles circulating in the bottles during the incubations (Gifford, 1988). In all experiments, we added concentrated Guillard’s F medium (Sigma cat. no. G9903) at a level of F/200 (i.e. 9 μM inorganic nitrogen final concentration) to all treatments, in order to prevent nutrient limitation of phytoplankton growth. In all experiments but one (2 July 2001), we also incubated a separate treatment of undiluted seawater without nutrient addition so that phytoplankton growth rate could be calculated either as the intercept of the Landry–Hassett dilution plot (Landry & Hassett, 1982) or as the net growth in undiluted, unenriched water, plus the grazing rate (Landry, 1993).
On board ship, samples were incubated under one layer of neutral density screen (equivalent to 50% surface light intensity, or the amount of light at approximately 5 m) for 24 h in an insulated, water-cooled, incubator equipped with a rotating wheel to keep particles from settling (Crocker & Gotschalk, 1997). For the shore-based experiments, we incubated the bottles in a temperature-controlled room (20 ± 3°C) under a 12:12 light:dark cycle at 180 μmol photons m−2 s−1 (measured at the back edge of the bottles, away from the light). Based on the measured in situ light profiles on each morning of sampling (data not shown), this is approximately equivalent to the mid-day light intensity at 10 m. Because we were unable to vary the light in the incubators during the daylight period, however, the integrated daily light dose for the shore-based experiments (7.8 mol m−2 d−1) was probably higher than what the phytoplankton would have experienced in situ. The coefficient of variation in light intensity among the bottles for the shore-based experiments was 3.2%, and bottles were rotated by hand periodically to avoid settling.
Five or six replicate samples of the undiluted water were taken for measurement of initial chlorophyll concentrations. At the end of the incubation period, samples were taken from all bottles. To measure chlorophyll, samples were filtered onto 25 mm diameter Whatman GFF glass fiber filters, extracted in 90% acetone overnight at −15°C, and measured by fluorescence, with correction for pheopigments (Parsons et al., 1984).
For all experiments, Model I linear regressions of net phytoplankton growth (ordinate) versus fraction undiluted seawater (abscissa) were used to generate slopes (grazing rate; d−1) and intercepts (phytoplankton growth in the absence of grazing; d−1). Comparisons of growth and grazing among experiments were made using model II regression (reduced major axis method; Sokal & Rohlf, 1995).
For enumeration of microzooplankton, 500 ml samples of undiluted water were preserved with 5% (final concentration) Lugol’s iodine solution. These samples were settled in two stages to 5 ml and counted in tissue-culture well plates on an inverted microscope at 200× magnification. Ciliates, nauplii, and meroplankton were enumerated. Two-dimensional shapes and linear dimensions were recorded for biovolume calculations, except for copepod nauplii, for which length-weight regressions from the literature were used (Uye, 1991; Mauchline, 1998). For non-tintinnid ciliates, a factor of 0.19 pgC μm−3 was used to convert biovolume to carbon mass (Putt & Stoecker, 1989). For tintinnids, we measured lorica volume and used a conversion factor of 0.072 pgC μm−3. This is equal to the conversion factor 0.053 pgC μm−3 for tinntinnids measured for formaldehyde-preserved samples (Verity & Langdon, 1984), increased by 35% to account for the greater shrinkage with Lugol’s preservation (Putt & Stoecker, 1989). The smallest ciliates (10–15 μm) were enumerated and sized, but nanoflagellates were not. Larger dinoflagellates, which may be hetero- or autotrophic, were observed in some samples. With Lugol’s preservation, it is not possible to discriminate between these two trophic modes, so dinoflagellates are not included in our counts. Thus, our microscope counts, which focused on ciliates and copepod nauplii, provide a minimum estimate of microzooplankton abundance.
Vertical casts were made at all stations with a rosette equipped with a CTD and sensors for fluorescence and irradiance. During the summer 2003 sampling, surface and subpycnocline water was sampled with Niskin bottles, and concentrations of inorganic nutrients were measured using standard methods (Strickland & Parsons, 1972).
Satellite-derived sea surface temperature and ocean color data (MODIS Terra instrument) were used to evaluate the extent of upwelling in the Bight during the study period (Ouzounov et al., 2004). Level 2 (1 km resolution) data were obtained for the dates on which sampling occurred. Several days surrounding each cruise were also reviewed. In addition, Level 3 (9 km) data were obtained for the entire study period (mid-June 2001–February 2003) using the 8-day mean MO04MW product for both ocean color and sea surface temperature. The latter data allowed us to look for upwelling events of longer duration or greater spatial extent. Due to clouds or inadequate frequency of satellite overpass, data were not available for every specific sampling day or 8-day mean for the study period.
Results
During the winter cruise (July 2001), we observed typical tropical shelf conditions. At shallow station 1, the water was warm, nearly isothermal, and stratified slightly by salinity (Fig. 2). At midshelf station 6, cooler and fresher mixed CW and TW was layered above TW at mid-depths and much colder, fresher SACW near the bottom. Summer 2002 conditions were quite different. Station 1 had fresher water at all depths and was nearly homogeneous with regard to salinity. Temperature was strongly stratified, and the salinity level and the temperature of the bottom water (<17°C) indicated the presence of SACW, which is normally widespread on the shelf during summer. SACW was also evident at midshelf station 8 during summer (Fig. 2), where cooler water was found as shallow as 20 m, overlain by a warmer, slightly fresher layer. The water column at station 4 was also stratified during the summer 2003 experiments, with SACW present below the thermocline at this nearshore station (Fig. 3), as is typical for the summer. Cold water penetrated 5–10 m into the euphotic zone (mean 1% light depth during this period was 36 ± 2.4 m SE; n = 6), but was never found at the surface. Nutrient concentrations were high in subpycnocline water during these experiments (Fig. 4). The trend of increasing nitrate concentration during the week of sampling suggests a fresh intrusion of SACW from offshore at this coastal site. Inspection of satellite images of temperature and chlorophyll confirmed the limited extent of active upwelling conditions in the region during both sets of summer experiments (images posted as supplemental material).
Microzooplankton biomass ranged from 0.14 to 10.82 μgC l−1 (mean 2.07 ± 0.76 SE, n = 13) over all stations and dates (Table 1). Copepod nauplii usually dominated the biomass (mean 62.4 ± 5.8% SE), followed by non-tintinnid Spirotrich ciliates (oligotrichs and aloricate choreotrichs). Ciliates less than 20 μm in equivalent spherical diameter accounted for an average of 31.7% (±3.8% SE) of the total ciliate biomass. Tintinnids were very low in abundance, especially offshore, with the highest abundance being only 49 individuals l−1. The haptorid ciliate Myrionecta rubra (formerly Mesodinium rubrum) was present in over half of the experiments. This ciliate has often been associated with coastal upwelling (Ryther, 1967). It apparently grazes on phytoplankton (Gustafson et al., 2000), although it has been shown to behave like an autotroph during dilution experiments (Dolan et al., 2000). We counted it separately and include it here in total microzooplankton biomass. During two experiments at station 4 (10 and 13 Jan 2003), this small species comprised over one-third of the total ciliate abundance, but it was never more than 20% of the total microzooplankton biomass.
Results of 14 dilution experiments are summarized in Table 2. Two additional experiments from the oceanic stations are not discussed here because of negative phytoplankton growth estimates (<−0.3 d−1) in all of the treatments; one of the latter also showed negative grazing (higher net growth in less dilute treatments).
Nutrient additions had little effect on phytoplankton growth rates in our experiments (Fig. 5). For the 13 experiments that included unenriched controls, the model II regression slope comparing growth in enriched versus unenriched, undiluted seawater was 1.03 (intercept −0.005), indicating no apparent nutrient stimulation of growth.
(a) Comparison of net growth of phytoplankton in undiluted water with and without addition of nutrients. (b) Comparison of the two alternative methods for calculating intrinsic phytoplankton growth rates. Model II regression slopes (reduced major axis method; (Sokal & Rohlf, 1995)), with 95% confidence intervals, are shown
Phytoplankton in situ growth can be calculated in two ways using dilution experiments. First, growth can be estimated as the intercept of the dilution plot (essentially, net growth when grazers have been completely diluted out); and second, as the sum of net growth in the undiluted, unenriched treatments (growth minus grazing) and grazing rate as estimated from the slope of the dilution plots. The latter is the method usually used when diluted treatments have been enriched with nutrients (Landry, 1993). In the case of our experiments, the two methods produced nearly identical results (Fig. 5). The model II regression slope comparing the two methods was 0.92 (intercept 0.081), and not significantly different from 1 (P > 0.05).
Microzooplankton grazing rates on phytoplankton ranged from <0.1 to >1.3 d−1. In most cases, grazing was a significant fraction of growth (range 0–223%; Table 2). A model II regression of growth versus grazing yielded a slope of 0.64 (intercept −0.004) (Fig. 6).
Discussion
The dilution method of Landry & Hassett (1982) has been widely applied as a means of estimating the grazing impact of microzooplankton on phytoplankton. Through a great many studies using this technique, it is now firmly established that grazers <200 μm in diameter are the principal agents of phytoplankton mortality in the sea (Calbet & Landry, 2004). An exception to this would be bloom situations, when sinking and cell lysis can exceed grazing (Smetacek, 1985; Alldredge & Gotschalk, 1989; Nagasaki et al., 1994a, 1994b; Gifford et al., 1995). A recent review of the microzooplankton grazing literature identified nearly 800 individual estimates of phytoplankton growth and microzooplankton grazing made using this technique (Calbet & Landry, 2004). This review included measurements in the North Atlantic, the North, South, and Equatorial Pacific, the Indian and the Southern Oceans. In the South Atlantic, however, the only published dilution estimates of growth and grazing were from the eastern side of the basin, and mostly at high latitudes (Froneman & Perissinoto, 1996a, b; Froneman & McQuaid, 1997). Our experiments are the first we know of that report on microzooplankton grazing in the western South Atlantic Ocean and among very few in tropical upwelling regions.
Overall, our results are similar to those summarized in Calbet & Landry (2004). For example, they found that microzooplankton consumed 67% of phytoplankton production in the whole data set, slightly higher in oceanic (70%) than in coastal (60%) areas, and also higher in tropical (75%) compared to polar (59%) regions. Our experiments, from the southern edge of the tropical Atlantic, averaged 66% (±0.09 SE, data arctangent transformed, averaged, then tangent back-converted, as in Calbet & Landry (2004)). The 12 shelf stations averaged 63% (±0.10 SE), and the two oceanic stations averaged 87%, suggesting closer coupling between growth and grazing in the offshore oligotrophic waters of the Brazil Current, compared to the shelf.
Phytoplankton growth ranged from 0.38 to 2.13 d−1 in our study, and grazing ranged from 0 to 1.32 d−1. These measurements were highly correlated (Fig. 6), again suggesting close coupling between production and consumption of phytoplankton in this region during our study. When the data are separated by season, the summer experiments show higher growth (1.00 d−1) than the winter experiments (0.53 d−1). Although this difference was not significant statistically (t-test; P = 0.16), it is consistent with the idea that phytoplankton growth is higher during the upwelling season.
We observed no systematic stimulation of phytoplankton growth in the nutrient-added treatments. Among all experiments, only the two at the oligotrophic oceanic stations showed marked stimulation of growth (nutrient-added treatment >1.5 times control; Fig. 5). This result is similar to that of Landry et al. (1998), who found mostly a lack of nutrient-stimulated growth when nitrate concentration was above 1 μmol l−1 in the Arabian Sea. Likewise, in the subarctic Pacific, Liu et al. (2002) found that growth in controls averaged 0.9 times that of nutrient-added treatments. In one of our shore-based experiments from station 4 (13 Jan 2003), controls grew much better than those with nutrient addition (0.48 and −0.03 d−1, respectively). This phenomenon has been observed occasionally in other studies (e.g. Olson & Strom, 2002), although its cause is not understood. It may result from experimental error or toxicity of the nutrient solution to some component of the phytoplankton community. It is worth noting that the nutrient concentration and plankton community was changing at that time in our study (discussed further below).
Grazing mortality of phytoplankton was also higher during the summer (0.62 d−1), compared to winter (0.38 d−1). Surprisingly, grazing mortality was not significantly correlated with microzooplankton biomass in our experiments, whether or not naupliar biomass was included. Probably, this indicates the importance of nanoflagellate grazers, and possibly also dinoflagellates, which we did not count, in this region. We found microzooplankton biomass to be dominated by copepod nauplii in most of our experiments (Fig. 7; nauplii exceeded ciliate biomass on 9 of 13 dates). This contrasts with most published studies of relative biomasses in different microzooplankton groups (Fileman & Burkill, 2001; Fileman et al., 2002; Obayashi & Tanoue, 2002). However, there are also reports of higher naupliar biomass as well. For example, Kamiyama (1995) found nauplii to be approximately equal in abundance to protistan microzooplankton on most sampling dates at a subtropical station in the Seto Inland Sea of Japan. During the North Atlantic spring bloom, Sieracki et al. (1993) found nauplii to be 60 and 90% of microzooplankton biomass during two phases of the bloom (calculated from their Table 1 as nauplii biomass/(nauplii + ciliates + heterotrophic dinoflagellates)).
If we had been able to discriminate phagotrophic dinoflagellates from phototrophic forms, their inclusion might have shifted the biomass distribution to >50% protists in our data. Probably few dinoflagellates are strict autotrophs (Stoecker, 1999), but the relative importance of hetero- and autotrophy in any given species in any particular sample cannot currently be measured. Abundance of total dinoflagellates in the mixed layer (from the phytoplankton counts), varied widely (range 0–9850 cells l−1, median 1749, n = 54 during the 2001 cruise; range 0–4980 cells l−1, median 100, n = 34 during the 2002 cruise), so their feeding impact may have been important at times. The inability to characterize the feeding habits of dinoflagellates in situ is one of the most difficult problems in current studies of microzooplankton ecology.
A conventional view is that nauplii, though they may be similar in aggregate biomass to heterotrophic protists, have lower weight-specific metabolism and hence lower community herbivory. In addition to being herbivores, however, the nauplii may represent an important link between nanograzers and larger zooplankton. Our dilution treatments would thus have had, at least in part, a two-step food chain within the microzooplankton. Landry & Calbet (2004) discussed the idea that multiple trophic steps within the microzooplankton community may actually be compensatory in dilution experiments, with reduced growth (hence also grazing) due to food dilution at one grazer level being equaled by reduced mortality from the level above it. In our experiments, the relatively high ratio of naupliar to protistan herbivorous biomass suggests strong grazing pressure on the latter.
Nutrient-rich, cold SACW is widespread on the shelf in the South Brazil Bight during austral summer. Variations in upwelling-favorable winds can bring it to the surface (Castro & Miranda, 1998), leading to high productivity in pulsed cycles of upwelling and relaxation that last days to weeks (Valentin et al., 1987; Gonzalez-Rodriguez et al., 1992, Gonzalez-Rodriguez, 1994). During both sets of summer experiments in the northern part of the Bight, temperature and salinity indicated that SACW was indeed present in deeper waters, but did not come any closer to the surface than about 20 m. During our summer 2003 experiments, for example (Fig. 3), the photic zone was about 35 m deep. SACW, with its higher nutrient concentrations, penetrated 5–10 m into the photic zone at that time. Also noteworthy were the changes that took place during those shore-based experiments. The base of the thermocline was at 30–35 m for the first two dilution experiments, on 10 and 13 Jan, but it was only 20 m deep during the third and fourth experiments (Fig. 3). This penetration of cold water into the euphotic zone was associated with deeper light penetration, higher nitrate concentrations and phytoplankton growth rates, and lower chlorophyll concentrations and grazing. Ciliate abundance decreased especially sharply, with spirotrichs (choreotrichs plus oligotrichs) declining from 678 individuals l−1 on 10 January to 92 individuals l−1 on 17 January (Fig. 7). Grazing rates on 15 and 17 January were not significantly different from zero. These changes in transparency, nutrients, phytoplankton and grazers were most likely the result of fresh SACW upwelling into the photic zone between 13 and 15 January, but not reaching the surface.
The only time in the entire study that we sampled water with chl-a > 1 mg m−3 was at 10 m at station 4 (10 January 2003), when the in situ temperature was 18.5 °C, indicating perhaps some stimulatory effect of SACW on phytoplankton then. In the days following an upwelling event, however, chlorophyll levels up to 5 or 6 mg m−3 have been routinely observed by other investigators in this area (Valentin et al., 1987; Gonzalez-Rodriguez et al., 1992). It also appeared from the MODIS temperature images that our January 2003 sampling occurred during a non-upwelling interval at Cabo Frio, although satellite-estimated chlorophyll levels were somewhat elevated to the north, off Cabo São Tome, at that time. Subsequent to 10 January, levels of chlorophyll-a and microzooplankton biomass declined off Cabo Frio, and the MODIS 8-day images from 9–16 and 17–24 January confirmed the lack of cold water at the surface during our experiments, as well as the week after. Subsequently, a new cycle of upwelling started, with cold surface temperatures observed in three consecutive 8-day MODIS images, starting on 2 February. Thus, although we sampled a range of conditions, from oligotrophic surface waters of the Brazil Current to coastal areas affected by runoff from land, we did not experience the most productive situations because none of our experiments was conducted during strong upwelling conditions. While upwelling undoubtedly plays a role in the higher productivity of the Bight, relative to other tropical areas, the 8-day chlorophyll images indicated that the spatial extent of the upwelled cold water at the surface and the highest chlorophyll patches are limited, making it difficult to integrate, either temporally or spatially, the overall impact of SACW on summer plankton productivity in the region.
Although 30% of the global continental shelf area is in the tropics, the biological oceanography and especially the plankton ecology of these areas is understudied (Longhurst & Pauly, 1987). For example, although about 1/3 of the 788 microzooplankton grazing estimates compiled by Calbet and Landry (2004) were from tropical or subtropical areas, the great majority of these were from oceanic portions of the Arabian Sea and the Equatorial Pacific, the result of large international programs engaged in studying those areas. Only a few were from tropical and subtropical coastal areas such as the northern Gulf of Mexico and Baja California (Dagg, 1995; Strom & Strom, 1996; Garcia-Pamanes & Lara-Lara, 2001). None was from an upwelling shelf region similar to the South Brazil Bight. Most of the tropical shelf areas that have been studied comprise upwelling regions of eastern boundary currents such as those off Peru and West Africa, where Ekman divergence dominates the circulation and hence nutrient and plankton dynamics. In tropical regions such as the South Brazil Bight, productivity is not as high and plankton communities are dominated by pico- and nanoplanktonic primary producers. Upwelling occurs by both seasonally varying along-coast winds, as off Peru and West Africa, but also by interactions between the tropical western boundary current (Brazil Current) and the shelf and slope. The latter interactions include shed vortices and meanders of the current itself. These intermittent effects give the upwelling an unpredictable pattern of spatial and temporal variability. Such systems thus require much more study before we are confident in the connections between producers, grazers and fisheries. From the preliminary work reported here, the South Brazil Bight shelf is like many other shelf systems in that <200 μm zooplankton are the principal grazers of phytoplankton, and phytoplankton growth is temporally closely coupled to mortality. Further study will be required to verify if the high relative biomass of copepod nauplii and low abundance of tintinnids we observed is a general feature of this area or these kinds of systems.
References
Alldredge, A. L. & C. C. Gotschalk, 1989. Direct observations of the mass flocculation of diatom blooms: characteristics, settling velocities and formation of diatom aggregates. Deep-Sea Res. 36: 159–171.
Bakun, A., 1993. The California Current, Benguela Current, and Southwestern Atlantic Shelf Ecosystems: a comparative approach to identifying factors regulating biomass yields. In Sherman K., L. M. Alexander, B. D. Gold (eds), Large Marine Ecosystems: Stress, Mitigation, and Sustainability. AAAS Press, Washington, DC, 199–221.
Bakun, A. & R. H. Parrish, 1990. Comparative studies of coastal pelagic fish reproductive habitats: the Brazilian sardine (Sardinella aurita). Journal du Conseil 46: 269–283.
Calbet, A. & M. R. Landry, 2004. Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnology and Oceanography 49: 51–57.
Campos, E., J. Goncalves & Y. Ikeda, 1995. Water mass characteristics and geostrophic circulation in the South Brazil Bight: summer of 1991. Journal of Geophysical Research 100: 18537–18550.
Castro, B. M. & L. B. D. Miranda, 1998. Physical oceanography of the western Atlantic continental shelf located between 4° N and 34° S. In Robinson A. R., K. H. Brink (eds), The Sea: the Global Coastal Ocean. John Wiley and Sons, New York, 209–251.
Crocker, K. & C. Gotschalk, 1997. Short communication. A simple seawater-powered plankton wheel. Journal of Plankton Research 19: 155–158.
Dagg, M., 1995. Copepod grazing and the fate of phytoplankton in the northern Gulf of Mexico. Continental Shelf Research 15: 1303–1317.
Dolan, J. R., C. L. Gallegos & A. Moigis, 2000. Dilution effects on microzooplankton in dilution grazing experiments. Marine Ecology-Progress Series 200: 127–139.
FAO, 2003. FAO Yearbook of Fishery Statistics. FAO, Rome.
Fileman, E. & P. Burkill, 2001. The herbivorous impact of microzooplankton during two short-term Lagrangian experiments off the NW coast of Galicia in summer 1998. Progress in Oceanography 51: 361–383.
Fileman, E. S., D. G. Cummings & C. A. Llewllyn, 2002. Microplankton community structure and the impact of microzooplankton grazing during an Emiliania huxleyi bloom, off the Devon coast. Journal of the Marine Biological Association of the United Kingdom 82: 359–368.
Froneman, P. W. & C. D. McQuaid, 1997. Preliminary investigation of the ecological role of microzooplankton in the Kariega Estuary, South Africa. Estuarine Coastal and Shelf Science 45: 689–695.
Froneman, P. W. & R. Perissinoto, 1996a. Microzooplankton grazing and protozooplankton community structure in the South Atlantic sector of the Southern Ocean. Deep-Sea Research Part I 43: 703–721.
Froneman, P. W. & R. Perissinoto, 1996b. Structure and grazing of the microzooplankton communities of the Subtropical Convergence and a warm-core eddy in the Atlantic sector of the Southern Ocean. Marine Ecology-Progress Series 135: 237–245.
Garcia-Pamanes, J. & J. R. Lara-Lara, 2001. Microzooplankton grazing in the Gulf of California. Ciencias Marinas 27: 73–90.
Gifford, D. J., 1988. Impact of grazing by microzooplankton in the Northwest arm of Halifax Harbour, Nova Scotia. Marine Ecology-Progress Series 47: 249–258.
Gifford, D. J., L. M. Fessenden, P. R. Garrahan & E. Martin, 1995. Grazing by Microzooplankton and Mesozooplankton in the High-Latitude North-Atlantic Ocean – Spring Versus Summer Dynamics. Journal of Geophysical Research-Oceans 100: 6665–6675.
Gonzalez-Rodriguez, E., 1994. Yearly variation in primary productivity of marine phytoplankton from Cabo Frio (RJ, Brazil) region. Hydrobiologia 294: 145–156.
Gonzalez-Rodriguez, E., J. L. Valentin, D. L. Andre & S. A. Jacob, 1992. Upwelling and downwelling at Cabo Frio (Brazil): comparison of biomass and primary production responses. Journal of Plankton Research 14: 289–306.
Gustafson, D. E., D. K. Stoecker, M. D. Johnson, W. F. Van Heukelem & K. Sneider, 2000. Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum. Nature 405: 1049–1052.
Kamiyama, T., 1995. Change in the microzooplankton community during decay of a Heterosigma akashiwo bloom. Journal of Oceanography 51: 279–287.
Landry, M. & A. Calbet, 2004. Reality check on microbial food web interactions in dilution experiments: responses to the comments of Dolan and McKeon. Ocean Science Discussions 1: 65–76.
Landry, M. R., 1993. Estimating rates of growth and grazing mortality of phytoplankton by the dilution method. In Kemp P. F., B. F. Sherr, E. B. Sherr, J. J. Cole (eds), Handbook of Methods in Aquatic Microbial Ecology. Lewis Publishers, Ann Arbor: 715–722.
Landry, M. R., S. L. Brown, L. Campbell, J. Constantinou & H. B. Liu, 1998. Spatial patterns in phytoplankton growth and microzooplankton grazing in the Arabian Sea during monsoon forcing. Deep-Sea Research Part II-Topical Studies in Oceanography 45: 2353–2368.
Landry, M. R. & R. P. Hassett, 1982. Estimating the grazing impact of marine microzooplankton. Marine Biology 67: 283–288.
Liu, H. B., K. Suzuki & T. Saino, 2002. Phytoplankton growth, and microzooplankton grazing in the subarctic Pacific Ocean and the Bering Sea during summer 1999. Deep-Sea Research Part I-Oceanographic Research Papers 49: 363–375.
Longhurst, A. & D. Pauly, 1987. Ecology of Tropical Oceans. Academic Press, San Diego.
Matsuura, Y., 1996. A probable cause of recruitment failure of the Brazilian sardine, Sardinella aurita, population during the 1974/75 spawning seasons. South African Journal of Marine Science 17: 29–35.
Mauchline, J., 1998. The Biology of Calanoid Copepods. Academic Press, New York.
Nagasaki, K., M. Ando, I. Imai, S. Itakura & Y. Ishida, 1994a. Virus-like particles in Heterosigma akashiwo (Rhaphidophyceae): a possible red tide disintegration mechanism. Marine Biology 119: 307–312.
Nagasaki, K., M. Ando, S. Itakura, I. Imai & Y. Ishida, 1994b. Viral mortality in the final stage of Heterosigma akashiwo (Raphidophyceae) red tide. Journal of Plankton Research 16: 1595–1599.
Obayashi, Y. & E. Tanoue, 2002. Growth and mortality rates of phytoplankton in the northwestern North Pacific estimated by the dilution method and HPLC pigment analysis. Journal of Experimental Marine Biology and Ecology 280: 33–52.
Olson, M. B. & S. L. Strom, 2002. Phytoplankton growth, microzooplankton herbivory and community structure in the southeast Bering Sea: insight into the formation and temporal persistence of an Emiliania huxleyi bloom. Deep-Sea Research Part II-Topical Studies in Oceanography 49: 5969–5990.
Ouzounov, D., A. Savtchenko, G. Leptoukh, B. Zhou, D. Ostrenga, C. Deroo & L. Gonzalez, 2004. GES DAAC tools for accessing Terra and Aqua MODIS data. Pages 1109–1113 in Climate Change Processes in the Stratosphere, Earth-Atmosphere-Ocean Systems, and Oceanographic Processes from Satellite Data.
Parsons, T. R., Y. Maita & C. M. Lalli, 1984. A manual of chemical and biological methods for seawater analysis. Pergamon, New York.
Putt, M. & D. K. Stoecker, 1989. An experimentally determined carbon:volume ratio for marine “oligotrichous” ciliates from estuarine and coastal waters. Limnology and Oceanography 34: 1097–1103.
Ryther, J. H., 1967. Occurrence of red water off Peru. Nature 214: 1318–1319.
Sieracki, M. E., P. G. Verity & D. K. Stoecker, 1993. Plankton community response to sequential silicate and nitrate depletion during the 1989 North Atlantic spring bloom. Deep-Sea Research Part II 40: 213–225.
Smetacek, V. S., 1985. Role of sinking in diatom life-history cycles: ecological, evolutionary and geological significance. Marine Biology 84: 239–251.
Sokal, R. R. & F. J. Rohlf, 1995. Biometry, 3rd edn. W.H. Freeman and Company, New York.
Stoecker, D. K., 1999. Mixotrophy among dinoflagellates. Journal of Eukaryotic Microbiology 46: 397–401.
Strickland, J. D. & T. R. Parsons, 1972. A Practical Handbook of Seawater Analysis. Fisheries Research Board of Canada.
Strom, S. & M. Strom, 1996. Microzooplankton growth, grazing & community structure in the northern Gulf of Mexico. Marine Ecology-Progress Series 130: 229–240.
Uye, S. I, 1991. Temperature-dependent development and growth of the planktonic copepod Paracalanus sp. in the laboratory. Bulletin of Plankton Society, Japan Special Volume: 627–636.
Valentin, J. L., D. L. Andre & S. A. Jacob, 1987. Hydrobiology in the Cabo Frio (Brazil) upwelling: two-dimensional structure and variability during a wind cycle. Continental Shelf Research 7: 77–88.
Verity, P. G., & C. Langdon, 1984. Relationships between lorica volume, carbon, nitrogen, and ATP content of tintinnids in Narragansett Bay. Journal of Plankton Research 6: 859–868.
Acknowledgements
We thank G. Fernandes de Oliveira, M. Pompeu, M. Oliveira, T. Edison da Silva, S. Colin, A. Smith, S. Haley, W. Carvalho, Dr. E. Gonzalez-Rodriguez, the Brazilian Navy, IEAPM, and the Captain and crew of the RV Prof. W. Besnard. This research was supported by the US National Science Foundation (Office of International Programs: INT 0086659), the National Research Council of Brazil (CNPq; Office of International Cooperation Programs 910092/00-1, and PRONEX-CNPq 66.2368/1996-7, DEPROAS project; grants 477737/03 and 308055/2004-7 to RML), and the University of Connecticut.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S. Wellekens
Electronic supplementary material
Rights and permissions
About this article
Cite this article
McManus, G.B., Costas, B.A., Dam, H.G. et al. Microzooplankton grazing of phytoplankton in a tropical upwelling region. Hydrobiologia 575, 69–81 (2007). https://doi.org/10.1007/s10750-006-0279-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0279-9