Abstract
Sedimentary organic matter (OM) represents the energy supply for the shelf benthos at the Antarctic Ocean, and has yet to be properly characterized in terms of sources and composition for the Potter Cove region, King George/25 de Mayo Island. This energy input occurs mainly during the brief summer and provides the majority of available energy for the year, in a region with high endemism and limited source variety of sedimentary OM. Thus, the aim of this study is to identify the OM origin and degradation degree based on the spatial distribution and type of organic biomarkers. Twelve surficial sediment samples were collected and analyzed for the presence of n-alkanols and sterols. The different spatial patterns between the analyzed compounds indicated distinct OM sources and degradation degrees. First, relatively fresh phytoplankton organic matter and an enhanced bacterial activity were associated with the occurrence of seaweeds detritus and represent the source of n-alkanols. Second, relatively fresh material mainly associated with seaweeds debris were identified as the source of macroalgae sterols. Our results shed some light into the base of the Potter Cove trophic benthic chain and increase our understanding on the region’s biogeochemical processes relating to OM recycling. It also provides a baseline for assessing future changes in the structure of the benthic food web in this environment, which is subject to noticeable glaciers retreat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Antarctic Peninsula is situated almost entirely below 60°S, which gives this region the typical characteristics of polar environments such as low temperatures, being surrounded by the Antarctic Circumpolar Current, short-growing season, high endemism (Aronson et al. 2011) and absence of higher plants (Greene et al. 1967; Teixeira et al. 2013). It results in a limited source variety of sedimentary organic matter (OM), which is provided to the shelf benthos during the brief summer that in most locations accounts for the majority of energy input for the year (Aronson et al. 2011). These features affect the biota abundance and diversity and increase the sensitivity to environmental changes (Tin et al. 2008). Thus, the knowledge on OM composition can provide valuable information to understand biogeochemical processes (Bianchi 2007).
Organic material in aquatic environments is derived from its production in aquatic systems, inputs from terrestrial sources, and bacterial production in sediments. The composition of aquatic and terrestrial biota is not stable over time. Surface temperature changes in the Antarctic Ocean have affected OM production and the structure of ecosystems in the Antarctic Peninsula, which has resulted in an increase in tolerant species and a decline in species that are codependent on ice (Ducklow et al. 2007; Pasotti et al. 2015). This alteration in the Antarctic biota patterns could be evaluated in a more general manner due the analysis of organic biomarkers, among other techniques.
Biomarkers are potential biogeochemical tools because they are involved in specific biosynthesis pathways in different organisms and adaptation of biosynthetic systems to environmental conditions, and are stable in recent depositional environments (Laureillard and Saliot 1993). They have been extensively used to characterize the distribution of sources of sedimentary OM in different environments because they can be linked to a specific source and are preserved after deposition. Several lipid classes are used as biomarkers due to their greater resistance to bacterial degradation, in comparison to other organic compounds classes (Volkman 2006; Costa et al. 2010). n-Alkanols and sterols are organic markers present in the polar fraction of lipid extracts from marine sediments and are directly related to primary production (Hudson et al. 2001). Sterols are among the most specific and diverse of all biological markers and can be used to trace different algae, higher animals, vascular plants and sewage contamination (Volkman 1986; Burns and Brinkman 2011; Faux et al. 2011). n-Alcohols are less studied than sterols but can also be used to distinguish between marine and terrestrial OM inputs (Hu et al. 2009), and identify recent inputs. Because specific groups of organisms synthesize different n-alkanols and sterols, it is possible to use these markers to identify the sources of OM in the Antarctic region.
This study evaluated the lipid composition (n-alkanols and sterols) in surficial sediment samples from Potter Cove, Antarctic Peninsula. The aim was to identify the origin and degradation degree of the organic matter through the characterization of the spatial distribution and type of organic biomarkers. Based on the peculiar geographical characterization, this approach may help to understand the primary sources of organic matter to benthic communities and to serve as a basis for assessing future changes in the structure of the benthic food web of this environment.
Study area
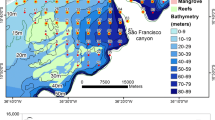
Potter Cove is a fjord-like inlet located in the Maxwell Bay, King George/25 de Mayo Island (62°14′S; 58°40′W), where the Argentine research station Carlini is located (Fig. 1). It is formed by two regions, delimited by moraine deposits, generating a narrowing of the section area due to relative low depths (~30 m) (Klöser et al. 1994). The outer portion is formed by hard substrate shores with dense macroalgae communities (Atencio et al. 2008) and can reach depths of 90 m. The inner cove can be 50 m deep and is formed by muddy soft bottoms (Mayer 2000). The surficial circulation is mainly driven by winds, that bring Maxwell Bay’s waters into the inner portion following a cyclonic pattern (the surficial water enters the cove along the north coast and goes out by the south margin, Mayer 2000). Winds and melting waters create a vertical circulation cell generating upwelling events in the inner portion (Klöser et al. 1994; Roese and Drabble 1998). The inner portion of the cove is surrounded by the Fourcade Glacier, which has retreated almost 1 km in the last 60 years (Pasotti et al. 2015).
The biota in the inner portion of Potter Cove is mainly composed of filter feeders and microphytobenthic algae, mainly diatoms (Mayer 2000; Schloss and Ferreyra 2002; Curtosi et al. 2007). In Potter Cove the planktonic primary production is low, and the phytoplankton blooms, which are characteristic in other Antarctic coastal areas, are almost absent (Schloss and Ferreyra 2002). The benthic secondary production is characterized by high densities and biomass of benthic suspension feeders, especially bivalves [such as Laternula elliptica (King and Broderip 1832)], gastropods [such as Nacella concinna (Strebel 1908)], pennatulids, ascidians and sponges (Schloss and Ferreyra 2002; Graeve et al. 2008; Curtosi et al. 2009). Bivalves and pennatulids are the dominant organisms at 15 m, while ascidians and sponges are more common at deeper waters, because of their lower resistance to the sediment loads from melting runoff (Momo et al. 2008; Sahade et al. 2008).
This local trophic web is primarily supported by algae detritus that are carried to the inner portion of the cove by the cyclonic circulation. The seaweed forest is heavily represented by Phaeophyta and Rhodophyta, particularly Desmarestia anceps (Montagne 1842), D. menziesii (J. Agardh 1848) and Himantothallus grandifolius (Zinova 1959) (Quartino and Zaixso 2008).
Detritus from terrestrial vegetation also contribute to the organic carbon that arrives in the marine environment. The vegetation around Barton Peninsula includes 62 lichens, 33 bryophytes, and two phanerogam species (Kim et al. 2007; Park et al. 2014), while Schulz et al. (1998) observed 33 moss species and the same phanerogam species in Potter Peninsula. Despite the higher abundance of moss and lichens (Schulz et al. 1998; Kim et al. 2007), Park et al. (2014) presented evidences of a significant growth of the phanerogam Deschampsia antarctica Desv. (Poaceae) in the King George Island.
Materials and methods
Sampling
Twelve surficial sediment samples were collected in Potter Cove in the austral summer of 2010/11 (Fig. 1). Samples were collected using a Van-Veen sampler (250 cm²) and the top 2 cm of sediments were wrapped in pre-cleaned aluminum foil. Samples were frozen, freeze-dried, carefully homogenized in a mortar, and stored in cleaned glass bottles until extraction. As the average regional sedimentation rate in Potter Cove is 0.3 cm year−1 (Monien et al. 2014), the samples represent about seven years.
Lipid analysis
Laboratory procedure
The analytical extraction procedure for the free-lipid biomarkers analyzed was described in detail in Wisnieski et al. (2014). Freeze-dried and homogenized sediments (ca. 15 g) were Soxhlet extracted with a mixture of n-hexane and dichloromethane (1:1, v/v; 80 mL) for 8 h. Copper, previously activated by acidification with HCl (1 mol L−1), was added to each extraction flask to remove sulfur. A known amount of 5α-androstanol was added as a surrogate standard before extraction. The extracts were purified and fractionated by column chromatography using silica and alumina (deactivated with 5%wt H2O) in three different fractions (F1 = 10 mL n-hexane; F2 = 15 mL DCM:n-hexane (3:7, v:v); F3, containing sterols and alcohols =20 mL ethanol). The resulted extracts were concentrated using a rotary evaporator. They were silylated with BSTFA/TMCS (N,O-bis(trimethylsilyltrifluoroacetamide)/trimethylchlorosilane) (99:1) at 65 °C for 90 min, evaporated to dryness under nitrogen, and dissolved in n-hexane. Finally, extracts were spiked with an internal standard (5α-cholestane) prior to the gas chromatography (GC) analysis.
Instrumental analysis and quality control
The instrumental analysis procedure was described by Martins et al. (2014a) and, in this study, performed with an Agilent GC (model 7890A, Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and an Agilent 19091J-015 capillary fused silica column coated with 5% diphenyl/dimethylsiloxane (50 m length, 0.32 mm ID and 0.17 µm film thickness) with hydrogen as the carrier gas. The oven temperature was increased from 40 to 240 °C at a 10 °C min−1 rate, followed by 245 °C at 0.25 °C min−1, holding for another 5 min, then to 300 °C at 10 °C min−1, and holding for 5 min. Selected samples were also injected in a gas chromatograph coupled to a mass spectrometer (Agilent 5975C inert MSD with a Triple-Axis Detector), using the same column and injection conditions as used in GC/FID, to confirm the compounds identification.
The compounds were individually identified by matching retention times and quantified using response factors generated from calibration curves in the HP Chemstation (G2070 BA) software. The 5-point calibration curve was created using external standard solutions of n-alkanols and sterols with the range from 0.25 to 10 ng µL−1 (r > 0.995). A total of 19 n-alkanols, from C12 to C30, and 15 sterols were identified.
All chemicals and solvents used were trace analysis or HPLC grade, and the procedural blanks performed for each set of six samples showed no significant level peaks in the analyses of target compounds. The surrogate recovery, calculated based on the comparison between 5α-androstanol and 5α-cholestane concentrations, ranged from 89 to 131% in this polar fraction (mean = 100.7 ± 11.6%). The detection limits (DL) were 0.02 μg g−1 for n-alkanols and phytol, and 0.01 μg g−1 for sterols. The measured concentrations of target sterols in the reference material provided by the International Atomic Energy Agency (IAEA 408) were within 90–110% of the certified values (Martins et al. 2012).
Spatial analysis
The spatial analyses were performed using the QGIS 2.2.0 software (Nanni et al. 2012), and graphics and statistics using R software version 3.0.3 (R Core Team 2013).
Results
The concentrations of n-alkanols and sterols are presented in Tables 1 and 2, respectively. Among n-alkanols, the C14 or C16 homologues were the most abundant among studied sites. Phytol (3,7,11,15-tetramethyl-2-hexadecen-1-ol) accounted for 16 ± 5% of total alcohols, representing the main compound found in this lipid class. Short chain alkanols (SCOH: C12–C19) were predominant in ten samples, whereas long chain alkanols (LCOH: C20–C30) were predominant only in samples from sites #1 and #10. The most abundant sterols were cholesterol (cholest-5-en-3β-ol; 27Δ5) and sitosterol (24-ethylcholest-5-en-3β-ol; 29Δ5). The concentrations of fecal sterols such as coprostanol (5β(H)-colestan-3β-ol) and epicoprostanol (5β(H)-colestan-3α-ol) contributed to a minor fraction of total sterols, followed by dinosterol (4α,23,24-trimethylcholest-22E-en-3β-ol; 30Δ22E).
Discussion
Spatial distribution of polar organic markers
The n-alkanols showed a homogeneous spatial distribution, with higher concentrations found on sites #2, #8 and #10 (Fig. 2; Online Resource 1). Nevertheless, the difference between high and low concentrations is almost negligible. Conversely, concentrations of sterols were higher near the Carlini station and on sites #9 and #10. The high concentrations found on site #10 may be related to local upwelling events (Roese and Drabble 1998), which provide nutrient enriched waters to the surface promoting phytoplankton production. Other studies also observed evidences of this phenomenon. Tatián et al. (2004) observed high amounts of suspended sediment loads in ascidian analyses, and Fuentes et al. (2008) observed some zooplankton species in Potter Cove samples that could only be explained by oceanographic processes like the upwelling of deep sea water masses. High sterol concentrations near the beach suggest the occurrence of a local source of sterols; however Monien et al. (2014) observed higher OM values in the northwestern part of Potter Cove.
Near Carlini Station, the difference between the spatial distribution of n-alkanols and sterols indicates distinct sources. However, samples from northern sites (#7, #8, #10 and #11) presented a similar accumulation trend for n-alkanols and sterols, suggesting a common origin such as marine productivity resulting from upwelling events in this region.
The detected n-alkanols concentrations are below those observed in other regions of the world, such as subtropical estuaries (0.2–1.8 µg g−1: Mater et al. 2004) and continental shelves (0.27–2.02 µg g−1: Jeng and Huh 2004). These areas are characterized by large inputs of terrestrial OM, which is absent or limited in Antarctic environments. The identified sterols concentrations are in the same magnitude as those found in other locations such as subtropical estuaries (2.12–45.0 µg g−1: Martins et al. 2014a, b and 2.16–34.64 µg g−1:; Abreu-Mota et al. 2014), continental shelves (1–9 µg g−1: Méjanelle and Laureillard 2008), and even other Antarctic areas (0.21–10.4 µg g−1: Martins et al. 2002; 0.2–380 µg g−1; Villinski et al. 2008; and 0.9–14.0 µg g−1; Wisnieski et al. 2014). The difference in abundance of n-alkanols and sterols, and the lack of correlation between them (Table 3), can indicate distinct OM sources, increased production from local organisms, or even preferential degradation of n-alkanols over sterols due to a relatively more refractory nature (Yunker et al. 2005).
Distribution of organic markers based on carbon chain length
The short-chain n-alkanols (SCOH) were dominant in the majority of studied samples (Fig. 3), especially n-C14-OH and n-C16-OH. They may derive from marine plankton and/or bacteria (Hu et al. 2009; Holland et al. 2013), indicating strong input from an autochthonous source. A lack of correlation between SCOH and 28Δ sterols (typical phytoplankton markers) (Pearson correlation = 0.026; Table 3) suggest that the primary source of SCOH could be microbial biomass. Graeve et al. (2008) also observed important bacterial activity in surficial sediments of this region by analyzing fatty acids.
Long-chain n-alkanols (LCOH) are usually related to subaerial land plants (Volkman 2006; Andersson and Meyers 2012). One possible source to these compounds, common in polar environments, is the contribution of lichens and mosses to marine sediments (Wang et al. 2007; Andersson and Meyers 2012), especially to n-C28-OH, which was the most abundant compound on site #1, close to the beach. However, the most common source of even LCOH are vascular plants (Yunker et al. 2005; Burns et al. 2008 and references therein; Bechtel and Schubert 2009; Andersson and Meyers 2012). Vascular plant specimens in Antarctica are restricted to two native phanerogams, Deschampsia antarctica (E. Desv.) and Colobanthus quitensis (Kunth Bartl.) (Kim et al. 2006), for which suitable environments have been increasing due to glacier retreats and global warming (Pasotti et al. 2015). This increase could be leading to the exponential growth of these phanerogams abundance in King George Island, especially the D. antártica (Kim et al. 2007; Park et al. 2014), and represents a possible source to the LCOH. This vegetation growth, especially in the Peninsula Barton (north of Potter Cove) (Park et al. 2014), may also explain the abundance on LCOH on sites #9 and #11.
Free phytol in marine sediments is derived mainly from the degradation of chlorophyll-a from marine algae (Shi et al. 2001), but can also be derived from OM processed by bacteria and cyanobacteria (Bechtel and Schubert 2009) and was the most significant constituent in the alcohol fraction in several studies (e.g., Jeng and Huh 2004; Costa et al. 2010). Despite its more labile nature compared with n-alkanols, high concentrations of phytol may reflect an important contribution of fresh OM from marine sources (Costa et al. 2010), corroborated by its relatively high correlation with phytosterols (27Δ and 28Δ; Table 3).
Sedimentary sterols from Potter Cove showed a significant structural diversity and a relative distribution that reflects the multiple organisms living in the region, under different environmental conditions (Fig. 4). As previously discussed by Dauner et al. (2015), the percentage of compounds ascribed to fecal OM was low, representing less than 5% of the total sterols. This indicates that the environment was not impacted by sewage and marine mammal feces and/or the in situ formation (cholest-5-en-3β-ol → 5β-cholestan-3β-ol) of these compounds occurred under anoxic conditions. The abundance of 27Δ5 accounted for the relative high percentage of sterols with 27 carbon atoms on sites #1 and #12. Usually the presence of 27Δ sterols can be attributed to zooplankton (Volkman 1986; Burns et al. 2008; Bechtel and Schubert 2009) and less commonly to mollusks (Jeng and Huh 2004) and Rodophytas (Lopes et al. 2011). In Antarctic regions, they can also be associated with feces from penguins, pinnipeds, and odontocetes (Venkatesan and Santiago 1989; Martins et al. 2002; Huang et al. 2011). Site #12 is a relatively deep region without a significant abundance of mollusks (Momo et al. 2008; Sahade et al. 2008), and Potter Cove does not have colonies of penguin or pinnipeds. Therefore, the concentration of 27Δ sterols in site #12 can be explained by the presence of zooplankton and seaweed detritus. Conversely, due to site #1 proximity to the beach, these compounds, especially 27Δ5 (Jeng and Huh 2004), may be associated with populations of Laternula elliptica and Nacella concinna, which are typical shallow water mollusks in Potter Cove (Curtosi et al. 2009).
Sterols with 28 and 30 carbon atoms are typically related to phytoplankton (diatoms and dinoflagellates, respectively) (Volkman 2006). In this study they showed an almost homogeneous distribution, with slightly higher concentrations in the central part of the cove. Regardless of the identification of these phytoplankton biomarkers, these organisms cannot be considered the major source of organic carbon to sediments as observed by Schloss et al. (2002).
Stigmasterol (29Δ5,22E) and sitosterol (29Δ5) are commonly associated with higher plants (Volkman 2006; Martins et al. 2011; Rontani et al. 2014) though they can also be related to other non-terrestrial primary producers such as diatoms, chrysophytes, and some macroalgae in the Chlorophyta genus (Volkman 2003, 2006). Sitosterol can also be found in some microalgae species, such as diatoms (Volkman 1986, 2003) and in some species of Rhodophyta (Patterson 1971; Lopes et al. 2011) and Phaeophyta (Lopes et al. 2011). However, the main sterol found in brown seaweeds, largely present in Potter Cove, is 24-ethylcholest-5,24(28)E-dien-3β-ol (29Δ5,24(28)E, fucosterol) (Patterson 1971; Andrade et al. 2013). Because fucosterol may coelute with sitosterol in the GC/FID chromatogram (Volkman et al. 1987), the relative high abundance of 29Δ sterols in the study area may include fucosterol from macroalgae detritus, especially from Desmarestia anceps, D. menziesii, and Himantothallus grandifolius (Quartino and Zaixso 2008), which are carried into the inner portion of the cove by currents (Klöser et al. 1994; Tatián et al. 2008). The injection of a selected sample and a calibration curve in GC/MS confirmed that fucosterol and sitosterol are coeluted in the same peak.
Diagnostic ratios
The Carbon Preference Index (CPI; n-C20-OH–n-C28-OH) represents the relative distribution of odd and even carbon numbered n-alkanols on a studied site (Andersson and Meyers 2012) (Table 4; Fig. 5). CPI values higher than 4.0, which are typical of profiles related to fresh OM, were found in all sampled sites (Andersson and Meyers 2012). Low CPI values are attributed to extensive microbial activity over sedimentary OM (Yunker et al. 2005; Bechtel and Schubert 2009; Andersson and Meyers 2012). In this study they can be related to macroalgae detritus and degradation of phytoplankton blooms, especially on sites #2 and #12 in which n-C15OH was found as the main compound. In order to resolve this ambiguity, a Pearson correlation analysis was performed between n-C15OH, brassicasterol (a phytoplankton marker) and sitosterol (and fucosterol in this study, a macroalgae marker). The higher correlation between n-C15OH and sitosterol (0.465) comparing to n-C15OH and brassicasterol (0.324) suggest that bacterial activity related to macroalgae detritus may influence samples with low CPI values.
The Ter/Mar ratio is based on the proportion between the so-called terrestrial (n-C26OH, n-C28OH and n-C30OH) and marine-derived n-alkanols (n-C14OH, n-C16OH and n-C18OH) (Hu et al. 2009). Almost all studied sites presented a predominance of marine-derived compounds, which can be either from phytoplankton or bacterial origin. Only site #9 showed a Ter/Mar ratio higher than 1.0, suggesting a probable input of terrestrial matter that may be related to mosses, lichens and phanerogams. This terrestrial signal in site #9 could be related to the combination between circulation and local production. Surface circulation carries water from Maxwell Bay along the north margin, circumvents Potter Cove and, when the water passes the south margin, becomes enriched in organic matter from terrestrial origin. As site #9 does not present high amounts of alkanols, the marine production in this region is probably not pronounced. Therefore the combination of low marine production and terrestrial organic matter enriched water may produce a predominantly terrigenous signal. The high amounts of 29Δ sterols and abundance of macroalgae detritus in this site may also explain this distinct behavior.
The campesterol (28Δ5): stigmasterol (29Δ5.22E) ratio was calculated to determine the most probable source of these sterols. Values between 0.6 and 0.7 are indicative of terrestrial input, whereas lower values suggest marine planktonic input (Carreira et al. 2009; Martins et al. 2012). Marine-derived campesterol and stigmasterol were clearly detected, which is confirmed by the predominance of SCOH.
Finally, to determine the occurrence of diagenetic processes, the 5α(H)-stanols/Δ5-stenols ratio was calculated for C27 sterols (27Δ0/27Δ5). Values below 0.5 suggest recently deposited OM whereas values above 0.5 may indicate an environment with favorable degradation conditions (Jeng and Han 1994; Wisnieski et al. 2014). All analyzed samples presented values lower than 0.5, which is typical of fresh material input. Since phytol could be related to both phytoplankton and bacterial origin (Bechtel and Schubert 2009), the correlation between these two proxies was performed (Fig. 6). Except for site #1, a trend between both proxies can be observed, which is corroborated by a Pearson correlation of 0.569 (excluding site #1). It suggests that, although the organic matter in all samples is relatively fresh, samples with higher amounts of phytol have a higher degradation rate. Thus, the probable source of phytol is the microbial biomass (bacteria and cyanobacteria), usually associated with the highest concentration of algal biomarkers (phytoplankton blooms).
Conclusion
Based on the results of the composition and spatial distribution of polar lipid biomarkers, the organic matter on Potter Cove surficial sediments is mainly derived from two distinct sources: an autochthonous origin (n-alkanols: phytoplankton and microbial origin) and an allochthonous origin (sterols: seaweed beds). Both lipid classes indicate that upwelling events in the inner portion of the cove play a significant role fertilizing the surface water and promoting blooms.
The predominance of short-chain n-alkanols and phytol confirmed the major contribution of autochthonous materials, especially from the microbial biomass to sedimentary OM. Sterols, on the other hand, were associated to two different sources: 27Δ sterols, such as cholesterol, are probably derived from mollusks near the shoreline and zooplankton from sites away from the shoreline, whereas 29Δ sterols, mainly sitosterol, are derived from macroalgae detritus carried inside the cove by currents. Their relative abundance in comparison to other markers reveals the importance of this allochthonous input to this ecosystem.
The labile nature of n-alkanols in comparison to sterols was helpful to distinguish their two main sources: relatively fresh autochthonous organic matter and enhanced bacterial activity, associated with the occurrence of seaweeds detritus, as the source of n-alkanols; and relatively fresh material, mainly associated with seaweeds debris, as the source of macroalgae sterols. Our results allowed the identification of the sources and degradation degree of the organic matter to this marine environment, shedding some light into the base of the Potter Cove trophic benthic chain. It may serve as a basis for assessing future changes in the structure of the benthic food web in this environment, subject to noticeable glaciers retreat.
References
Abreu-Mota MA, Barboza CA de M, Bícego MC, Martins CC (2014) Sedimentary biomarkers along a contamination gradient in a human-impacted sub-estuary in Southern Brazil: a multi-parameter approach based on spatial and seasonal variability. Chemosphere 103:156–163. doi:10.1016/j.chemosphere.2013.11.052
Andersson RA, Meyers PA (2012) Effect of climate change on delivery and degradation of lipid biomarkers in a Holocene peat sequence in the Eastern European Russian Arctic. Org Geochem 53:63–72. doi:10.1016/j.orggeochem.2012.05.002
Andrade PB, Barbosa M, Matos RP et al (2013) Valuable compounds in macroalgae extracts. Food Chem 138:1819–1828. doi:10.1016/j.foodchem.2012.11.081
Aronson RB, Thatje S, McClintock JB, Hughes KA (2011) Anthropogenic impacts on marine ecosystems in Antarctica. Ann N Y Acad Sci 1223:82–107. doi:10.1111/j.1749-6632.2010.05926.x
Atencio AG, Bertolin ML, Longhi L et al (2008) Spatial and temporal variability of chlorophyll-a and particulate organic matter in the sediments and the water column of Potter Cove (Antarctica). In: Wiencke C, Ferreyra GA, Abele D, Marenssi S (eds) The Antarctic Ecosystem of Potter Cove, King-George Island (1999–2006). Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, pp 154–161
Bechtel A, Schubert CJ (2009) Biogeochemistry of particulate organic matter from lakes of different trophic levels in Switzerland. Org Geochem 40:441–454. doi:10.1016/j.orggeochem.2009.01.011
Bianchi TS (2007) Biogeochemistry of estuaries, 1st edn. Oxford University Press, Inc, New York
Burns KA, Brinkman D (2011) Organic biomarkers to describe the major carbon inputs and cycling of organic matter in the central Great Barrier Reef region. Estuar Coast Shelf Sci 93:132–141. doi:10.1016/j.ecss.2011.04.001
Burns KA, Hernes PJ, Brinkman D et al (2008) Dispersion and cycling of organic matter from the Sepik River outflow to the Papua New Guinea coast as determined from biomarkers. Org Geochem 39:1747–1764. doi:10.1016/j.orggeochem.2008.08.003
Carreira RS, Ribeiro P V., Silva CEM, Farias CO (2009) Hidrocarbonetos e esterois como indicadores de fontes e destino de matéria orgânica em sedimentos da baía de sepetiba, rio de janeiro. Química Nova 32:1805–1811. doi:10.1590/S0100-40422009000700023
Costa TLF, Araújo MP, Knoppers BA, Carreira RS (2010) Sources and distribution of particulate organic matter of a tropical Estuarine-Lagoon System from NE Brazil as indicated by lipid biomarkers. Aquat Geochem 17:1–19. doi:10.1007/s10498-010-9104-1
Curtosi A, Pelletier E, Vodopivez CL, Mac Cormack WP (2007) Polycyclic aromatic hydrocarbons in soil and surface marine sediment near Jubany Station (Antarctica). Role of permafrost as a low-permeability barrier. Sci Total Environ 383:193–204. doi:10.1016/j.scitotenv.2007.04.025
Curtosi A, Pelletier E, Vodopivez CL, Mac Cormack WP (2009) Distribution of PAHs in the water column, sediments and biota of Potter Cove, South Shetland Islands, Antarctica. Antarct Sci 21:329–339. doi:10.1017/S0954102009002004
Dauner ALL, Hernández EA, Mac Cormack WP, Martins CC (2015) Molecular characterisation of anthropogenic sources of sedimentary organic matter from Potter Cove, King George Island, Antarctica. Sci Total Environ 502:408–416. doi:10.1016/j.scitotenv.2014.09.043
Ducklow HW, Baker K, Martinson DG et al (2007) Marine pelagic ecosystems: the West Antarctic Peninsula. Philos Trans R Soc B Biol Sci 362:67–94. doi:10.1098/rstb.2006.1955
Faux JF, Belicka LL, Rodger Harvey H (2011) Organic sources and carbon sequestration in Holocene shelf sediments from the western Arctic Ocean. Cont Shelf Res 31:1169–1179. doi:10.1016/j.csr.2011.04.001
Fuentes VL, Schnack-Schiel SB, Schloss IR, Esnal GG (2008) Mesozooplankton of Potter Cove: community composition and seasonal distribution in 2002 and 2003. In: Wiencke C, Ferreyra GA, Abele D, Marenssi S (eds) The Antarctic Ecosystem of Potter Cove, King-George Island (1999–2006). Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, pp 75–84
Graeve M, Sahade R, Fuentes VL et al (2008) Bentho-pelagic coupling at Potter Cove, Antarctica: a fatty acid approach. In: Wiencke C, Ferreyra GA, Abele D, Marenssi S (eds) The Antarctic Ecosystem of Potter Cove, King-George Island (1999–2006). Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, pp 147–153
Greene SW, Gressitt JL, Koob D et al (1967) Terrestrial life of Antarctic. In: Bushnell VC (ed) Antarctic map folio series. American Geographical Society, New York
Holland AR, Petsch ST, Castañeda IS et al (2013) A biomarker record of Lake El’gygytgyn, Far East Russian Arctic: investigating sources of organic matter and carbon cycling during marine isotope stages 1–3. Clim Past 9:243–260. doi:10.5194/cp-9-243-2013
Hu J, Peng P, Chivas AR (2009) Molecular biomarker evidence of origins and transport of organic matter in sediments of the Pearl River estuary and adjacent South China Sea. Appl Geochem 24:1666–1676. doi:10.1016/j.apgeochem.2009.04.035
Huang J, Sun L, Wang X et al (2011) Ecosystem evolution of seal colony and the influencing factors in the 20th century on Fildes Peninsula, West Antarctica. J Environ Sci 23:1431–1436. doi:10.1016/S1001-0742(10)60601-8
Hudson ED, Parrish CC, Helleur RJ (2001) Biogeochemistry of sterols in plankton, settling particles and recent sediments in a cold ocean ecosystem (Trinity Bay, Newfoundland). Mar Chem 76:253–270
Jeng W-L, Han B (1994) Sedimentary coprostanol in Kaohsiung Harbour and the Tan-Shui Estuary, Taiwan. Mar Pollut Bull 28:494–499
Jeng W-L, Huh C-A (2004) Lipids in suspended matter and sediments from the East China Sea Shelf. Org Geochem 35:647–660. doi:10.1016/j.orggeochem.2003.12.002
Kim JH, Ahn I-Y, Hong SG et al (2006) Lichen flora around the Korean Antarctic Scientific Station, King George Island, Antarctic. J Microbiol 44:480–491
Kim JH, Ahn I-Y, Lee KS et al (2007) Vegetation of Barton Peninsula in the neighbourhood of King Sejong Station (King George Island, maritime Antarctic). Polar Biol 30:903–916. doi:10.1007/s00300-006-0250-2
Klöser H, Ferreyra GA, Schloss IR et al (1994) Hydrography of Potter Cove, a small fjord-like inlet on King George Island (South Shetlands). Estuar Coast Shelf Sci 38:523–537
Laureillard J, Saliot A (1993) Biomarkers in organic matter produced in estuaries: a case study of the Krka estuary (Adriatic Sea) using the sterol marker series. Mar Chem 43:247–261
Lopes G, Sousa C, Bernardo J et al (2011) Sterol profiles in 18 macroalgae of the Portuguese coast. J Phycol 47:1210–1218. doi:10.1111/j.1529-8817.2011.01028.x
Martins CC, Venkatesan MI, Montone RC (2002) Sterols and linear alkylbenzenes in marine sediments from Admiralty Bay, King George Island, South Shetland Islands. Antarct Sci 14:244–252. doi:10.1017/S0954102002000093
Martins CC, Seyffert BH, Braun JAF, Fillmann G (2011) Input of organic matter in a large south american tropical estuary (Paranaguá Estuarine System, Brazil) indicated by sedimentary sterols and multivariate statistical approach. J Braz Chem Soc 22:1585–1594
Martins CC, Bícego MC, Figueira RCL et al (2012) Multi-molecular markers and metals as tracers of organic matter inputs and contamination status from an Environmental Protection Area in the SW Atlantic (Laranjeiras Bay, Brazil). Sci Total Environ 417–418:158–168. doi:10.1016/j.scitotenv.2011.11.086
Martins CC, Aguiar SN, Wisnieski E et al (2014a) Baseline concentrations of faecal sterols and assessment of sewage input into different inlets of Admiralty Bay, King George Island, Antarctica. Mar Pollut Bull 78:218–223. doi:10.1016/j.marpolbul.2013.10.034
Martins CC, Cabral AC, Barbosa-Cintra SCT et al (2014b) An integrated evaluation of molecular marker indices and linear alkylbenzenes (LABs) to measure sewage input in a subtropical estuary (Babitonga Bay, Brazil). Environ Pollut 188:71–80. doi:10.1016/j.envpol.2014.01.022
Mater L, Alexandre MR, Hansel FA, Madureira LAS (2004) Assessment of lipid compounds and phosphorus in mangrove sediments of Santa Catarina Island, SC, Brazil. J Braz Chem Soc 15:725–734
Mayer M (2000) Zur Okologie der Benthos-Foraminiferen der Potter Cove (King George Island, Antarktis). Berichte zur Polar und Meeresforsch 353:1–140
Méjanelle L, Laureillard J (2008) Lipid biomarker record in surface sediments at three sites of contrasting productivity in the tropical North Eastern Atlantic. Mar Chem 108:59–76. doi:10.1016/j.marchem.2007.10.002
Momo FR, Sahade R, Tatián M (2008) Benthic animal communities of Potter Cove (King George Island, Antarctica): Observed patterns and explanatory models. In: Wiencke C, Ferreyra GA, Abele D, Marenssi S (eds) The Antarctic Ecosystem of Potter Cove, King-George Island (1999–2006). Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, pp 162–167
Monien P, Lettmann KA, Monien D et al (2014) Redox conditions and trace metal cycling in coastal sediments from the maritime Antarctic. Geochim Cosmochim Acta 141:26–44. doi:10.1016/j.gca.2014.06.003
Nanni AS, Descovi-Filho L, Virtuoso MA et al (2012) Quantum GIS—Guia do Usuário, Versão 1.7.4 ‘Wroclaw’
Park JS, Ahn I-Y, Lee EJ (2014) Spatial distribution patterns of the antarctic hair grass Deschampsia antarctica in relation to environmental variables on Barton Peninsula, King George Island. Arct Antarct Alp Res 45:563–574. doi:10.1657/1938-4246-45.4.563
Pasotti F, Manini E, Giovannelli D et al (2015) Antarctic shallow water benthos in an area of recent rapid glacier retreat. Mar Ecol 36:716–733. doi:10.1111/maec.12179
Patterson GW (1971) The distribution of sterols in algae. Lipids 6:120–127
Quartino ML, Zaixso ALB (2008) Summer macroalgal biomass in Potter Cove, South Shetland Islands, Antarctica: its production and flux to the ecosystem. Polar Biol 31:281–294. doi:10.1007/s00300-007-0356-1
R Core Team (2013) R: a language and environment for statistical computing
Roese M, Drabble M (1998) Wind-driven circulation in Potter Cove. Berichte zur Polar und Meeresforsch 299:40–46
Rontani J-F, Belt ST, Vaultier F et al (2014) Autoxidative and photooxidative reactivity of highly branched isoprenoid (HBI) alkenes. Lipids. doi:10.1007/s11745-014-3891-x
Sahade R, Tarantelli S, Tatián M, Mercuri G (2008) Benthic community shifts: A possible linkage to climate change? In: Wiencke C, Ferreyra GA, Abele D, Marenssi S (eds) The Antarctic Ecosystem of Potter Cove, King-George Island (1999–2006). Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, pp 331–337
Schloss IR, Ferreyra GA (2002) Primary production, light and vertical mixing in Potter Cove, a shallow bay in the maritime Antarctic. Polar Biol 25:41–48. doi:10.1007/s003000100309
Schloss IR, Ferreyra GA, Ruiz-Pino D (2002) Phytoplankton biomass in Antarctic shelf zones: a conceptual model based on Potter Cove, King George Island. J Mar Syst 36:129–143. doi:10.1016/S0924-7963(02)00183-5
Schulz F, Winkler JB, Kappen L (1998) Components of terrestrial vegetation, pattern and processes. In: Wiencke C, Ferreyra GA, Arntz W, Rinaldi C (eds) The Potter Cove coastal ecosystem, Antarctica. Alfred Wegener Institute for Polar and Marine Research, Bremerhaven, pp 54–58
Shi W, Sun M-Y, Molina M, Hodson RE (2001) Variability in the distribution of lipid biomarkers and their molecular isotopic composition in Altamaha estuarine sediments: implications for the relative contribution of organic matter from various sources. Org Geochem 32:453–467. doi:10.1016/S0146-6380(00)00189-3
Tatián M, Sahade R, Esnal GB (2004) Diet components in the food of Antarctic ascidians living at low levels of primary production. Antarct Sci 16:123–128. doi:10.1017/S0954102004001890
Tatián M, Sahade R, Mercuri G et al (2008) Feeding ecology of benthic filter-feeders at Potter Cove, an Antarctic coastal ecosystem. Polar Biol 31:509–517. doi:10.1007/s00300-007-0379-7
Teixeira LCRS, Peixoto RS, Rosado AS (2013) Bacterial diversity in rhizosphere soil from Antarctic vascular plants of Admiralty Bay in maritime Antarctica. In: Bruijn FJ de (ed) Molecular microbial ecology of the rhizosphere. Wiley, Hoboken
Tin T, Fleming ZL, Hughes KA et al (2008) Impacts of local human activities on the Antarctic environment. Antarct Sci 21:3–33. doi:10.1017/S0954102009001722
Venkatesan MI, Santiago CA (1989) Sterols in ocean sediments: novel tracers to examine habitats of cetaceans, pinnipeds, penguins and humans. Mar Biol 102:431–437
Villinski JC, Hayes JM, Brassell SC et al (2008) Sedimentary sterols as biogeochemical indicators in the Southern Ocean. Org Geochem 39:567–588. doi:10.1016/j.orggeochem.2008.01.009
Volkman JK (1986) A review of sterol markers for marine and terrigenous organic matter. Org Geochem 9:83–99. doi:10.1016/0146-6380(86)90089-6
Volkman JK (2003) Sterols in microorganisms. Appl Microbiol Biotechnol 60:495–506. doi:10.1007/s00253-002-1172-8
Volkman JK (2006) Lipid markers for marine organic matter. The Handbook of Enviromental. Chemistry 2:27–70. doi:10.1007/698
Volkman JK, Farrington JW, Gagosian RB (1987) Marine and terrigenous lipids in coastal sediments from the Peru upwelling region at 15°S: Sterols and triterpene alcohols. Org Geochem 11:463–477. doi:10.1016/0146-6380(87)90003-9
Wang J, Wang Y, Wang X, Sun L (2007) Penguins and vegetations on Ardley Island, Antarctica: evolution in the past 2400 years. Polar Biol 30:1475–1481. doi:10.1007/s00300-007-0308-9
Wisnieski E, Bícego MC, Montone RC et al (2014) Characterization of sources and temporal variation in the organic matter input indicated by n-alkanols and sterols in sediment cores from Admiralty Bay, King George Island, Antarctica. Polar Biol 37:483–496. doi:10.1007/s00300-014-1445-6
Yunker MB, Belicka LL, Harvey HR, Macdonald RW (2005) Tracing the inputs and fate of marine and terrigenous organic matter in Arctic Ocean sediments: a multivariate analysis of lipid biomarkers. Deep Sea Res Part II Top Stud Oceanogr 52:3478–3508. doi:10.1016/j.dsr2.2005.09.008
Acknowledgements
A.L.L. Dauner is thankful to CNPq (121444/2010-4) for the B.Sc. scholarship, to CAPES (Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior) for the M.Sc. scholarship and to Mihael Machado de Souza for the English revision. C.C. Martins is grateful to CNPq (National Council for Scientific and Technological Development) for the Research Grant (305763/2011-3). This study is related to the Brazilian “National Science and Technology Institute on Antarctic Environmental Research” (INCT-APA, FAPERJ E-16/170023/2008). W.P. Mac Cormack and E.A. Hernández thanks the financial support from the European Commission through the Marie Curie Action IRSES, project no 318718, IMCONet (Interdisciplinary Modelling of climate change in coastal Western Antarctica—Network for staff Exchange and Training) also to the grants PICTO 2010-0124 from the ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica) and the UBA (Universidad de Buenos Aires CyT 20020100100378).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dauner, A.L.L., MacCormack, W.P., Hernández, E.A. et al. Sources and distribution of biomarkers in surficial sediments from a polar marine ecosystem (Potter Cove, King George Island, Antarctica). Polar Biol 40, 2015–2025 (2017). https://doi.org/10.1007/s00300-017-2120-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2120-5