Abstract
In this study, two aquatic macrophytes Phragmites australis and Schoenoplectus lacustris and corresponding sediment samples were collected every three months from Lake Sapanca (Turkey) and analysed for their heavy-metal contents (Pb, Cr, Cu, Mn, Ni, Zn and Cd). Accumulation factor ratios of plant parts were calculated for all metals, and the two species were compared in terms of accumulation properties. The highest concentrations were measured in the root systems while relatively low concentrations were found in the rhizome and above-ground parts of the plants. The accumulation ratios of root for P. australis were usually higher than the ratios for S. lacustris. While the accumulation ratios of root were higher in winter than in the other seasons for P. australis, for S. lacustris the highest accumulation ratios were found in the autumn. Both plant species were found to be root accumulators of Pb, Cu, Mn, Ni, Zn and Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals originate from various sources and are added to the aquatic ecosystem. Freshwater ecosystems are believed to be natural sinks for metals (Birch et al. 1996). Because of their acute toxic effects, heavy metals should be removed from the environment using cost-effective and appropriate methods, or should be converted into less-toxic forms. Phytoremediation means the use of plants to reduce, remove, degrade or immobilize environmental toxins (Salt et al. 1995, 1998; Raskin et al. 1997; Terry 2003). The effectiveness of a phytoremediation system depends on the selection of appropriate plants for the particular environment. Knowledge about the accumulation properties of wetland plant species is useful in choosing appropriate plants for wetland phytoremediation systems.
Metal bioaccumulation depends upon numerous biotic and abiotic factors, such as temperature, pH and dissolved ions in water (Lewander et al. 1996; Demirezen and Aksoy 2004). Bioaccumulation of metals varies considerably among species, as well as among morphologically similar species growing in the same area (Brekken and Steinnes 2004). Metal accumulation may also show seasonal variation. Some publications have reported the highest metal contents (Cd, Cu, Ni, Pb, Sn, Zn) during the autumn and relatively low levels during the spring (Brekken and Steinnes 2004; Kim and Fergusson 1994), whereas others have indicated the highest foliar levels during the spring and the lowest during the winter (Martin and Couphtrey 1982; Wilkins 1978).
Phragmites australis (Cav.) Trin. ex Steudel (common reed, Poaceae) is one of the most widely distributed species in the world. P. australis is generally known to accumulate some heavy metals distinctly more than other aquatic plants (Aksoy et al. 2005). This species grows equally well in unpolluted sites (fallow land, meadow) and in polluted sites. In recent decades, P. australis has been widely used in constructed wetlands for the treatment of industrial wastewaters containing metals (Dunbabin and Bowmer 1992). S. lacustris (common club-rush, Cyperacae) is also a widespread perennial aquatic plant and accumulates high levels of heavy metals in its tissues (Szymanowska et al. 1999; Samecka-Cymerman and Kempers 2001).
The aims of this study were to: (1) compare the metal accumulation properties of P. australis and S. lacustris that grow in the same area, seasonally, (2) investigate the low, root and shoot accumulation of metals. P. australis and S. lacustris were selected for their ability to tolerate high concentrations of heavy metals in shoots and as they are widespread species.
Materials and methods
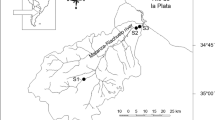
Lake Sapanca is one of the few lakes that provides drinking water in Turkey, but it is exposed to heavy urbanization because of its natural beauty and proximity to the metropolitan İstanbul. The catchment area of Lake Sapanca is 251 km2. The length is 16 km from east to west, and 5 km from north to south. The water surface area is 45 km2, and the maximum depth is 52 m. The lake basin is surrounded by motorways (the trans-European motorway, TEM) and a railway connecting Asia to Europe (Fig. 1).
In this study, nine sampling sites for sediment and plant samples were selected along the shore of Lake Sapanca. Coordinates were determined by global positioning system (GPS). Samplings were performed in May (spring) 2003, July (summer) 2003, November (autumn) 2003 and February (winter) 2004.
The surface sediment samples were collected from the top 5 cm of bottom sediments using a grap sampler. After collection, the sediment samples were air-dried for four days until they reached constant weight. Dried aliquots were ground using a mortar and pestle and sieved through a 2 mm sieve. Then the samples were stored in polyethylene bags in a desiccator with calcium chloride to avoid contamination throughout the analyses. Triplicate subsamples of approximately 1 g of well-homogenized sediment samples were accurately weighed on an analytical balance and then transferred into glass digestion tubes. Aqua regia was used to leach the samples. The extracts were diluted to a final volume of 50 ml using double-distilled water.
Collected plant samples were washed with tap water and then with two rinses of distilled water. After drying at 80°C for 24 h, the component parts of the plants were pulverized with a micro-hammermill, weighed, and then 10 ml of concentrated HNO3 (ultrapure 65%) was added to 1 g plant sample and allowed to stand overnight at room temperature. The sample was then heated for 4 h at 120°C, after which the temperature was increased to 140°C. The digestion was continued at this temperature until about 1 ml of acid remained. After cooling, the suspension was filtered in a 50 ml volumetric flask and diluted to the mark.
Standard stock solutions containing 1,000 μg ml−1 were prepared from nitrate salts of Pb, Cr, Cu, Mn, Ni, Zn and Cd in 1% of HNO3 into calibrated 1 litre flasks. Diluted standard solutions were prepared from the stock standard solutions. In all of the extracts, the concentrations of Pb, Cr, Cu, Mn, Ni, Zn and Cd were determined using a Varian inductively coupled plasma (ICP-OES) machine, while those with low concentrations of metals were quantified with a graphite furnace. All analyses were performed in triplicate. Analysis of variance (ANOVA) was performed with the SPSS version 11.5 software package. Results of testing were considered significant if the calculated P-values were ≤0.05. For comparison of means, the ANOVA and post hoc Duncan tests were used.
Results and discussion
Tables 1 and 2 show the seasonal metal concentrations in sediment and different plant parts. It was found that heavy-metal concentrations in the sediment fell in the order: Mn > Zn > Ni > Cu > Cr > Pb > Cd. The seasonal highest values of heavy metals were observed as Cu in the summer and Cd in the autumn. There was no seasonal difference for Pb and Cd. Mn, Ni and Zn concentrations in the autumn were found to be lower than in the spring and summer. There was no difference between the spring and summer for these metals. The roots, rhizomes and shoots in both species contained different levels of all the studied metals, the highest concentrations being recorded in the root systems while relatively low concentrations were found in the rhizome and above-ground parts of the plants. Cd, Pb, Zn and Cu were primarily concentrated in the roots, but Zn, Cu and Mn also accumulated in the stems and leaves. Beyond differences between species, the concentrations of the metals analysed in the two root systems showed clear seasonal variation. The higher metal concentrations were recorded in the autumn and winter and the lower in the spring. The metal levels in shoots also changed during the year, but their seasonal variation was less clear.
There may be various reasons for the seasonal differences found, including: environmental factors, such as variations in metal concentrations in solution, interactions between metals and other elements, pH, etc.; metabolic factors, such as dilution of metal contents due to growth; or they may be due to interactions between both kinds of factors (Otte 1991; Villares et al. 2002; Nan et al. 2002). Another possible source of seasonal variation is an increase in the levels of metals in solution due to fluvial inputs during winter (Villares et al. 2002).
Brekken and Steinnes (2004) indicated the highest metal contents (Cd, Cu, Ni, Pb, Sn, Zn) during the autumn and relatively low levels during the spring. In pasture herbage from the mining area at Shipham in England a rapid decrease during the spring was observed, increasing again in the autumn to a high level during the winter (Matthews and Thornton 1982). Our findings agree with those of Matthews and Thornton (1982).
Accumulation factor in relation to the sediment concentration [AF] was calculated as the ratio [Element]plant part:[Element]sediment (Figs. 2–4). The [AF] root ratios for P. australis were usually higher than the ratios for S. lacustris. The greatest differences between the two plant species were observed in the autumn and winter. The roots of P. australis had significantly higher [AF] for all metals except for Cd compared with S. lacustris in winter. The highest [AF] root ratio was found to be 7.98 (P. australis, winter), while the highest [AF] shoot ratio was found to be 6.44 (S. lacustris, winter).
For P. australis, the [AF] root values were higher in the spring than the summer except for Cu and Mn. The ratios then fell during the summer, reaching minimum values. Finally, the ratios increased again during the autumn and winter (Fig. 2). The [AF] rhizome ratios of all metals except for Cd fell during the summer, increased during the autumn and then increased again in the winter (Fig. 3). The [AF] shoot ratios of Mn, Zn and Cd fell during the summer, increased during the autumn and then fell again during the winter. The Cr and Cu accumulation ratios increased seasonally and Pb ratios fell during the summer, increased during the autumn and fell again during the winter (Fig. 4).
For S. lacustris the [AF] root ratios of all metals except for Cd fell during the summer, increased during the autumn and then fell again during the winter (Fig. 2). The AF] rhizome ratios of Pb, Cr, Ni and Cd fell during the summer and increased during the autumn and then increased again during the winter. The Cu, Mn and Zn accumulation ratios fell during the summer, increased during the autumn and then increased again in the winter (Fig. 3). The [AF] shoot ratios of Cu Mn and Zn fell during the summer, increased during the autumn and fell again during the winter. The [AF] shoot ratios of Pb, Cr and Cd fell during the summer, increased during the autumn and then increased again in the winter (Fig. 4).
While higher [AF] values were seen in the winter than other seasons for P. australis, the highest [AF] ratios were found in autumn for S. lacustris. This situation is caused by a dilution effect due to growth during favourable periods and in winter with a large decrease in metabolic rates. This pattern was probably accentuated in the levels of heavy metals in autumn and winter because of fluvial inputs. The concentration decrease during spring is generally referred to as a dilution effect (slower uptake than growth), rather than back transport or loss of the element, because corresponding recordings of absolute amounts of the elements in leaves do not seem to decrease similarly. Towards the autumn, changes in plant biomass are normally less pronounced, and differences in metal uptake or translocation, availability or surface contamination may contribute more to the observed concentration changes. The observed seasonal variations of metal concentrations in shoots could be put down to a dilution effect without an increase in translocation. This means that an increase in biomass is not necessarily linked to an increase in bioaccumulation.
Baker (1981) discussed restriction of shoot metal uptake in plants from contaminated soils, and the presence of exclusion mechanisms was suggested. Coughtrey and Martin (1978) showed that the translocation of metals to the shoot of Holcus lanatus is prevented in ecotypes from metal-contaminated sites. The suggested reason was the protection of photosynthesis from toxic levels of trace elements. In P. australis, Pb and Cd were actively transported to the shoot, where they were accumulated, especially in winter ([AF] > 1). Mn and Cd were actively transported by S. lacustris. However, the high shoot concentrations of Cd in P. australis and S. lacustris can be explained by the Cd shoot accumulation ability. Results obtained from studies have indicated that heavy-metal accumulation and transportation strategies differed among the plant species as well as among the metals themselves. The movement of metals into the plants appears to be concentration dependent. The occurrence of lower metal concentrations in sediment samples between the autumn and winter indicates that metal was removed through root uptake or formation of iron plaque in parts of roots that have not been considered for analysis. This results in a decrease of the metal concentration in the sediments between the roots.
Plants with a higher concentration of an element in their tissues are considered accumulators (Baker and Walker 1990). Some species are called root accumulators, since they store metals in their roots. Others are shoot accumulators. Low accumulators are those that can reduce uptake when the substrate has high element concentrations, or have a high net eflux of certain elements. In this study both plant species were found to be root accumulators for Pb, Cu, Mn, Ni, Zn and Cd. P. australis for Pb and Cd and S. lacustris for Mn and Cd were evaluated as shoot accumulators. Chromium was the only metal that was low accumulated by S. lacustris (Table 3).
Conclusions
Metal accumulation shows seasonal variation. For all metals there was a clear concentration decline from the spring to the summer. The highest concentrations were observed in the autumn and winter for both plants. The general picture is that accumulation ratios decline during the growth period and increase towards the autumn and winter. During the growth period the translocation ratio decreases, indicating a restricted transportation of toxic metals from the roots to shoots.
The accumulation ratio of metals in both plants varied from metal to metal, due to differences in solubility and bioavailability. Both plant species were determined to be root accumulators for Pb, Cu, Mn, Ni, Zn and Cd. Cr was the only metal that was low accumulated by S. lacustris. In P. australis, Pb and Cd were actively transported and accumulated in the shoots. Mn and Cd were actively transported and accumulated to the shoots by S. lacustris.
The large variability in metal concentrations found in the aquatic macrophytes in consecutive samples must be taken into account when designing biomonitoring programs, because different conclusions can be reached when using slightly different sampling dates. These findings also showed how complicated it is to compare data from studies carried out at different times of the year.
Our results have also demonstrated that a biomass increase is not necessarily linked to a bioaccumulation increase. For phytoremediation of sediments polluted with multiple metals, both P. australis and S. lacustris could be used for heavy-metal extraction, providing that harvesting occurs at the end of the vegetative cycle.
References
Aksoy A, Duman F, Sezen G (2005) Heavy metal accumulation and distribution in narrow-leaved cattail (Typha angustifolia) and common reed (Phragmites australis). J Freshwater Ecol 20(4):783–785
Baker AJM (1981) Accumulators and excluders—strategies in response of plants to heavy metals. J Plant Nutr 3:643–654
Baker AJM, Walker PL (1990) Ecophysiology of metal uptake by tolerant plants. In: Shaw AJ (ed) Heavy metal tolerance in plants: evolutionary aspects. CRC Press, Boca Raton
Brekken A, Steinnes E (2004) Seasonal concentrations of cadmium and zinc in native pasture plants: consequences for grazing animals. Sci Total Environ 326:181–195
Birch L, Hanselmann KW, Bachofen R (1996) Heavy metal conservation in Lake Cadagno sediments: historical records of anthropogenic emissions in a meromictic alpine lake. Water Res 30:679–687
Coughtrey PJ, Martin MH (1978) Cadmium uptake and distribution in tolerant and nontolerant populations of Holcus lanatus grown in solution culture. Oikos 30:555–560
Demirezen D, Aksoy A (2004) Accumulation of heavy metals in Typha angustifolia (L.) and Potamogeton pectinatus (L.) living in Sultan Marsh (Kayseri, Turkey). Chemosphere 56:685–696
Dunbabin JS, Bowmer KH (1992) Potential use of constructed wetlands for treatment of industrial wastewaters containing metals. Sci Total Environ 111:151–168
Kim ND, Fergusson JE (1994) Seasonal variations in the concentrations of cadmium, copper, lead and zinc in leaves of the horse chestnut (Aesculus hippocastanum L.). Environ Pollut 86:89–97
Lewander M, Greger M, Kautsky I, Szarek E (1996) Macrophytes as indicators of bioavailable Cd, Pb and Zn flow in the river Przemsza, Katowice Region. Appl Geochem 11:169–173
Martin, M, Couphtrey P (1982) Biological monitoring of heavy metal pollution. Applied Sciences Publications, London/New York
Matthews H, Thornton I (1982) Seasonal and species variation in the content of cadmium and associated metals in pasture plants at Shipham. Plant Soil 66:181–193
Nan Z, Li J, Zhang J, Cheng G (2002) Cadmium and zinc interactions and their transfer in soil-crop system under actual field conditions. Sci Total Environ 285:187–195
Otte ML (1991) Contamination of coastal wetlands with heavy metals: factors affecting uptake of heavy metals by salt marsh plants. In: Rozema J, Verkleij JAC (eds) Ecological responses to environmental stresses, Kluwer Academic, Netherlands
Raskin I, Kramer U, Smith RD, Salt DE, Schulman R (1997) Phytoremediation and mechanisms of metal accumulation in plants. Plant Physiol 114:1253–1255
Salt DE, Blaylock M, Kumar N, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13:468–474
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Ann Rev Plant Physiol 49:643–668
Samecka-Cymerman A, Kempers AJ (2001) Concentrations of heavy metals and plant nutrients in water, sediments and aquatic macrophytes of anthropogenic lakes (former open cut brown coal mines) differing in stage of acidification. Sci Total Environ 281:87–98
Szymanowska A, Samecka-Cymerman A, Kempers AJ (1999) Heavy metals in three lakes in west poland. Ecotoxicol Environ Safety 43:21–29
Terry N, Sambukumar SV, LeDuc DL (2003) Biotechnological approaches for enhancing phytoremediation of heavy metals and metalloids. Acta Biotechnol 23:281–288
Wilkins DA (1978) The measurement of tolerance to edaphic factors by means of root growth. New Phytol 80:623–633
Villares R, Puente X, Carballeira A (2002) Seasonal variation and background levels of heavy metals in two green seaweeds. Environ Pollut 119:79–90
Acknowledgement
This study was supported by the Scientific & Technological Research Council of Turkey (TBAG-AY 392 104T298).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duman, F., Cicek, M. & Sezen, G. Seasonal changes of metal accumulation and distribution in common club rush (Schoenoplectus lacustris) and common reed (Phragmites australis). Ecotoxicology 16, 457–463 (2007). https://doi.org/10.1007/s10646-007-0150-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-007-0150-4