Abstract
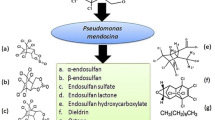
Endosulfan is one of the most widely used wide spectrum cyclodiene organochlorine insecticide. In environment, endosulfan can undergo either oxidation or hydrolysis reaction to form endosulfan sulfate and endosulfan diol respectively. Endosulfan sulfate is as toxic and as persistent as its parent isomers. In the present study, endosulfan degrading bacteria were isolated from soil through selective enrichment technique using sulfur free medium with endosulfan as sole sulfur source. Out of the 8 isolated bacterial strains, strain C8B was found to be the most efficient endosulfan degrader, degrading 94.12% α-endosulfan and 84.52% β-endosulfan. The bacterial strain was identified as Achromobacter xylosoxidans strain C8B on the basis of 16S rDNA sequence similarity. Achromobacter xylosoxidans strain C8B was also found to degrade 80.10% endosulfan sulfate using it as sulfur source. No known metabolites were found to be formed in the culture media during the entire course of degradation. Besides, the bacterial strain was found to degrade all the known endosulfan metabolites. There was marked increase in the quantity of released CO2 from the culture media with endosulfan as sulfur source as compared to MgSO4 suggesting that the bacterial strain, Achromobacter xylosoxidans strain C8B probably degraded endosulfan completely through the formation of endosulfan ether.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endosulfan is a wide spectrum cyclodiene organochlorine insecticide. Technical grade endosulfan contains two isomers, α and β in the ratio of 7:3. Endosulfan is used in number of food as well as non-food crop against many insect pests. In India, it is mainly used in rice, cotton and tea plantation. With various ban and restriction imposed on other highly persistent organochlorine pesticide like dichlorodiphenyltrichloroethane (DDT) and hexachlorocyclohexane (HCH), the use of endosulfan has increased dramatically during the last two decades. The presence of relatively reactive cyclic sulfite diester group in endosulfan makes it less persistent as compared to other organochlorine compounds (Van Woerden 1963).
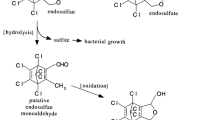
Many microorganisms, both bacteria and fungi had been isolated which have the capability to degrade endosulfan in soil and aqueous medium. These microbes can use endosulfan either as carbon or sulfur source or both (Siddique et al. 2003). Endosulfan can undergo both oxidation and hydrolysis reactions to form endosulfan sulfate and endosulfan diol, respectively. The formation of endosulfan sulfate is exclusively carried out by microbial enzymes while endosulfan diol can be formed due to chemical hydrolysis at alkaline pH (Sutherland et al. 2000; Kwon et al. 2002).
The use of endosulfan as sulfur source and the subsequent removal of sulfur from the compound considerably reduced its mammalian toxicity (Dorough et al. 1978; Goebel et al. 1982). However, oxidation of endosulfan yields endosulfan sulfate which is reported to be as toxic and as persistent as its parent compounds (Kaur et al. 1998). Therefore biodegradation of endosulfan sulfate deserved the same degree of attention as its parent compound endosulfan.
Recently bioremediation of endosulfan is receiving huge attention as an alternative to other methods such as incineration and landfill (Siddique et al. 2003). The first and foremost step in the bioremediation of a particular pollutant is to identify an organism capable of degrading the pollutant most efficiently. Such organism can later be used as source of enzymes for further enzymatic technologies. So far many microorganisms, both bacteria and fungi have been isolated from different sources which have the capability to degrade endosulfan and endosulfan sulfate or both (Kwon et al. 2005; Lee et al. 2006). Weir et al. (2006) reported that in Arthrobacter sp. there is a single monooxygenase Ese gene encoding an enzyme capable of degrading both isomers of endosulfan and endosulfan sulfate. Kumar and Philip (2006) had isolated three bacterial strains, a Staphylococcus sp. and two Bacillus sp. capable of completely mineralizing endosulfan.
In the present study, using the selective enrichment technique in sulfur free medium with endosulfan as the sole sulfur source, we successfully isolated a bacterial strain capable of degrading both endosulfan and endosulfan sulfate. The bacterial strain was able to degrade endosulfan and its toxic metabolite endosulfan sulfate completely without forming any metabolites. The study suggests a complete mineralization of both endosulfan and endosulfan sulfate for the first time.
Materials and methods
Chemicals and medium
Technical grade endosulfan (>99% purity) was obtained from the Institute of Pesticide Formulation Technology, Gurgaon, India. Endosulfan diol, endosulfan lactone, endosulfan sulfate and endosulfan ether were purchased from Sigma-Aldrich, USA. HPLC grade acetonitrile, ethyl acetate and water were purchased from Qualigens. All other chemicals and solvent used in the experiments were of analytical grade.
The composition of different media used in the experiments were, Luria-Bertini medium (LB Medium): 10 g Peptone, 5 g Yeast extract, 5 g NaCl and 1 g Glucose in 1 L of distilled water. Sulfur free medium (SFM): 2 g KH2PO4, 7.5 g K2HPO4, 1 g NH4Cl, 0.5 g NaCl, 1 g Glucose, 0.1 g MgCl2, 0.86 mg p-amino benzoic acid, 0.86 mg Nicotinic acid, 10 ml trace element solution, 0.05% Tween 80 in 1000 ml distilled water. Trace element solution contained 20 mg (NH4)6MO7O24·4H2O, 50 mg H3BO3, 30 mg ZnCl2, 3 mg CoCl2·6H2O, 20 mg FeCl6·6H2O, 10 mg (CH3COO)2Cu·H2O in 1000 ml distilled water (Sutherland et al. 2000). Minimal Carbon Free Medium (CFM): 1 g KH2PO4, 1 g K2HPO4, 1 g NH4NO3, 0.2 g MgSO4·7H2O, 0.02 g CaCl2, 0.01 g Fe(SO4)3 in 1000 ml distilled H2O (Awasthi et al. 1997; Goswami and Singh 2009). Unless mentioned, the pHs of the medium were adjusted at 6.8 using 0.1 N HCl/0.1 N NaOH. For agar plates, 1.6% agar was added.
Enrichment and isolation of microorganism
Soil sample for the isolation of microorganism were collected from a cotton field of Maharashtra, which have previous history of endosulfan application. The sample were ground and passed through 2 mm sieve. Whole soil was mixed completely, air dried and preserved at 4°C until further analysis.
Hundred grams of soils were spiked with endosulfan and incubated at 28°C for 5 weeks. The concentration of endosulfan was increased from 200 ppm initially to 1000 ppm at the end of the 5th week. 10 g of this enriched soil was suspended in 100 ml SFM containing 50 ppm endosulfan as the only sulfur source. After 1 week, 10 ml of this suspension was transferred in another fresh 100 ml SFM containing 50 ppm higher endosulfan concentration from the previous suspension. Thus, the enrichment was carried out for 4 weeks with the concentration of endosulfan increased by 50 ppm each week.
At the end of the 4th week, 1 ml of the culture was pipette out, diluted and spread on SFM agar plates. The plates were then incubated at 28°C for 24 h. Plates showing maximum distinct colonies were chosen for isolation of the bacterial strains. The isolated bacterial strain were repeatedly sub-cultured (>20 times) on fresh agar plates to obtain pure strains.
Screening of endosulfan degrading bacteria
All the isolated strains were grown in SFM containing endosulfan as sole S-source. The growth of bacterial strains was studied by measuring the optical density of the culture media using UV/VIS spectrophotometer (Labomed, Inc.) at 595 nm wavelength. Of all the isolated strains, strain C8B showed maximum growth and degradation of endosulfan in SFM, and hence was chosen for further studies.
Identification of strain C8B
Identification of bacterial strain C8B was carried out using 16S rRNA gene sequence. The isolated genomic DNA was amplified using a thermocycler (2750, Applied Biosystem, USA), using 16S rDNA universal primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGCTACCTTGTTACGACTT-3′). PCR reaction mix of 25 μl final volume contained: 7.5 μl template, 2.5 μl dNTP’s, 2.5 μl reaction buffer, 1.25 μl Primer 8F, 1.25 μl Primer 1492R, 0.33 μl tag polymerase and water to make up the volume. Amplified PCR product was run over an Agarose gel (0.8%) and 1.5 kb band size was eluted using a gel extraction kit (High Yeild Gel/PCR, RBC Taiwan). Amplified product was sent for commercial sequencing at LabIndia Instrument Pvt, Ltd, Gurgaon. The nucleotide sequence of strain C8B sent by LabIndia was then BLAST using NCBI/BLAST program.
Growth kinetics of strain C8B in different media
Growth of strain C8B in different media, LB, SFM and CFM were studied in terms of optical density and biomass. Bacterial strain C8B was grown in 100 ml each of LB, SFM and CFM and incubated at 28°C in a microbial shaker at 150 rpm for 48 h. 2 ml of the culture media was sampled out after every 4 h. The growth of bacterial population was observed by measuring absorbance in a UV/VIS spectrophotometer at 595 nm wavelength. For biomass study, 1 ml sample was taken during each interval and was centrifuged at 8,000 rpm at 4°C in Hermle refrigerated centrifuge for 10 min. The supernatant was decanted from the eppendorf tubes. These tubes were then air dried overnight and weighed to determine the biomass (Goswami and Singh 2009). The specific growth rate of the bacteria, μ was calculated using the formula μ = [3.322 × log (Mt 2/Mt 1)]/Δt where M is biomass at the different timepoints (t 1 and t 2) respectively (Sunlu et al. 2010). Growth kinetics of strain C8B was also studied with different compounds- 0.02 g MgSO4, 50 ppm endosulfan and 50 ppm endosulfan sulfate as sulfur source.
Biodegradation of endosulfan and endosulfan sulfate by strain c8b in broth medium
Degradation of endosulfan and endosulfan sulfate by strain C8B was studied by growing the bacteria in SFM provided with endosulfan and endosulfan sulfate as the sole source of sulfur. For this, 2 ml of the overnight grown culture in SFM (6 ≤ OD ≥ 7) was inoculated in 100 ml sulfur free medium containing 50 ppm endosulfan and endosulfan sulfate as sulfur source. Another same set of flasks without the bacterial cell, was taken as control. The flasks were then incubated at 28°C in a microbial shaker at 150 rpm. 3 ml of the culture was sampled out on 0, 1st, 5th, 10th, 15th, 20th day of the incubation for extraction.
Optimization of pH, temperature and endosulfan concentration for strain C8B
The degradation of endosulfan by strain C8B was studied under different pHs 3, 5, 6.8, 8.5 and 10.5. 100 ml of SFM was prepared in 5 different flasks and their pHs were set at 3, 5, 6.8, 8.5 and 10.5 respectively using 0.1 N HCl/0.1 N NaOH. To this 50 ppm endosulfan was added as S-source. Another set of 5 flasks having the same concentration of endosulfan and different pHs were taken as control. The flasks were incubated at 28°C for 15 days in a microbial shaker (150 rpm).
For temperature, growth of strain C8B was studied at different temperatures 12, 20, 28, 35 and 45°C in two different media (LB and SF medium). Bacterial cells were grown in 100 ml medium and incubated at the different temperatures for 48 h in a microbial shaker at 150 rpm. Sampling was done for every 4 h. Growth was measured as increased in optical density measured through UV/VIS spectrophotometer at 595 nm wavelength.
In order to determined the optimum endosulfan concentration, bacterial strain C8B was grown in SFM containing different concentration of endosulfan 20, 35, 50, 75 and 100 ppm. The same set of flasks without the bacterial inoculants was taken as control. In order to observe the growth, 2 ml of the culture were pipette out after every 4 h and measured the absorbance in UV/VIS spectrophotometer at 595 nm wavelength.
Degradation of endosulfan metabolites by strain C8B
The degradation of all the standard metabolites of α-endosulfan and β-endosulfan viz. endosulfan diol, endosulfan lactone, endosulfan sulfate and endosulfan ether by the bacterial strain C8B were checked by growing the bacterial cells in 100 ml SFM containing 30 ppm each of all the metabolites.
Growth and degradation of endosulfan in carbon free medium
Growth of strain C8B in CFM was studied by growing the bacterial cell in 5 different flasks containing 100 ml CFM supplemented with 50 ppm endosulfan, endosulfan sulfate, endosulfan ether, endosulfan lactone and endosulfan alcohol respectively. In another set of flasks with the same concentration of endosulfan and its metabolites, 0.05% Tween 80 was added as surfactant. One flask contained 0.05% Tween 80 and bacteria were taken as control. The flasks were incubated at 28°C in microbial shaker at 150 rpm.
In order to check the degradation of endosulfan, two flasks containing 100 ml CFM with 50 ppm endosulfan were inoculated with bacterial cells. One flask contains 0.05% tween 80 as surfactant. Another 100 ml medium with 50 ppm endosulfan and without the bacterial cell was taken as control. All the flasks were incubated at 28°C.
CO2 quantification test
The bacterial strain C8B was grown in 100 ml SFM in two biometric flasks. One flask contained 0.02 g MgSO4 while the other flask had 50 ppm endosulfan as S-source. The other tube of the biometric flask was filled with 10 ml of 0.1 N NaOH to absorb CO2 released during respiration and degradation process. The flasks were sealed completely using paraffin film and rubber stoppers and incubated at 28°C in microbial shaker at 150 rpm for 15 days. 10 ml 0.1 N NaOH was pipette out on 0, 3rd, 5th, 7th, 9th, 11th, 13th and 15th day and filled with fresh solution. The amount of CO2 released was quantified by titrating the NaOH sample with 0.2 N HCl solution using phenolphthalein as indicator.
Extraction and analysis of endosulfan and its metabolites
Extraction was carried out using ethyl acetate. 5 ml of ethyl acetate was added in 3 ml of sample, vortex and allowed to settle down for 10 min. The ethyl acetate layer formed above the water layer was pipette out in fresh acid washed glass vial. The process was repeated twice. Glass vials containing the extract were air dried and rinsed with 1 ml HPLC grade acetonitrile for analysis in HPLC and 0.5 ml ethyl acetate for GC-ECD. The operating condition and column speciation of Perkin Elmer Series 200 HPLC used in analysis of endosulfan and its metabolites were: flow rate 1 ml/min duration of cycle 20 min, mobile phase: acetonitrile:water (65:35), detector wavelength 214 nm, column C-18, and injection volume 25 μl.
Gas Liquid Chromatography (Shimadzu chromatograph model GC-17 A) equipped with a 63Ni Electron Capture Detector (ECD) and a SGE column, Code: 30 × 0.25 mm, ID-BP1, 0.25 μm was used for the analysis of endosulfan metabolites. The oven temperature was programmed for an initial temperature of 180°C (hold 2 min) and rose to 250°C (hold 2 min) at 10°C min−1 and then finally rose to 270°C (hold 4 min) at 20°C min−1. The temperature of the injector port and detector was maintained at 250 and 300°C, respectively. For Thin Layer Chromatography, TLC Silica gel 60 F250 (Merck, Germany) was used. Plates were developed with n-hexane–acetone 8:2 as mobile phase in a previously saturated Camag twin-trough chamber (Rajendra et al. 2008). After development, plates were observed under the UV lamp.
Result
Screening and isolation of endosulfan degrading bacteria
Altogether, 8 different bacterial strains were isolated from the soil sample through selective enrichment technique. These strains differed significantly in their growth and ability to degrade endosulfan in SFM during the 15 days of incubation period. Among them, strains C2B, C8A and C8B showed maximum growth in SFM (Fig. 1). Among these three strains, strain C8B shows maximum degradation degrading 85.70% α- and 62.75% β-endosulfan in 15 days followed by strain C8A (51.3 and 37.8%) and C2B (43.91 and 28.33%) respectively (result not shown). Therefore, strain C8B was selected for further studies.
BLAST result of the 16S rRNA gene sequence sent by LabIndia Instrument Pvt, Ltd. showed 100% similarity with Achromobacter xylosoxidans. It was named Achromobacter xylosoxidans strain C8B. The phylogenetic tree of the strain C8B was constructed using neighbor joining method (Fig. 2). The GenBank accession number for the 16S rDNA sequence of strain C8B is HQ426648.
Growth kinetic of achromobacter xylosoxidans strain C8B
Achromobacter xylosoxidans strain C8B showed maximum growth in LB medium followed by the SFM and CFM with Tween 80. No significant growth was observed in CFM (Fig. 3a). The lag phase in LB medium was 4 h while it was 6 and 12 h in SFM and CFM with Tween 80 respectively. The maximum OD recorded in LB medium was 2.578 (40th hour) while the same was 0.679 (32nd hour) and 0.335 (36th hour) in the case of bacterial cells grown in SFM and CFM with Tween 80 respectively. In SFM, Achromobacter xylosoxidans strain C8B showed maximum growth in flask containing MgSO4 as sulfur source followed by the flask containing endosulfan and endosulfan sulfate respectively (Fig. 4a). Maximum OD recorded were 1.128 (16th hour), 0.961 (12th hour) and 0.765 (36th hour) in MgSO4, endosulfan and endosulfan sulfate medium respectively. In the case of CFM supplemented with Tween 80, the growth of strain C8B was greater in the medium provided with either endosulfan or its metabolites than the one with Tween 80 alone. The bacterial strain showed maximum growth in the presence of endosulfan followed by endosulfan alcohol, endosulfan ether, endosulfan sulfate and endosulfan lactone respectively (Fig. 4b).
The study of the biomass also shows similar pattern (Fig. 3b). The biomass after 4th hour of incubation were 1.800 ± 0.529, 1.000 ± 0.100 and 0.867 ± 0.153 mg respectively in LB, SFM and CFM with Tween 80 which increased to 3.300 ± 0.755, 1.967 ± 0.808 and 1.480 ± 0.436 mg by 24th hour. An increased in weight was observed till 44th hour in LB medium (4.233 ± 0.635 mg), 32nd hour (1.967 ± 0.493 mg) in SFM and 40th hour (1.633 ± 0.751 mg) in CFM. The overall specific growth rate was different for different media. LB medium had the highest specific growth rate at 0.054 g g−1 h−1 followed by SFM and CFM 0.029 and 0.019 g g−1 h−1.
Biodegradation of endosulfan and endosulfan sulfate by Achromobacter xylosoxidans strain C8B in broth medium
The degradation of endosulfan and its metabolites was analysed using TLC, HPLC and GC-ECD. The HPLC and GC-ECD chromatograms showing the retention time of both endosulfan isomers and its metabolites is shown in Fig. 5. Achromobacter xylosoxidans strain C8B degrade 94.126% and 84.52% α- and β- endosulfan respectively in 20 days. The degradation in the control flask was 11.189% α- and 4.451% β- endosulfan only (Fig. 6a). No metabolites were detected in TLC, HPLC or GC-ECD, suggesting a possible complete mineralization of endosulfan. The degradation rate was highest during the exponential growth phase of bacteria degrading almost 20.026% α-endosulfan and 11.101% β-endosulfan during the first day of culture. Achromobacter xylosoxidans strain C8B was able to degrade 80.10% endosulfan sulfate during the 15 days of incubation while the degradation of the same concentration in uninoculated flask was 5.37% only (Fig. 7). Again there was no significant accumulation of any metabolites in the culture medium.
In CFM with 0.05% tween 80, Achromobacter xylosoxidans strain C8B was able to degrade 91.80% α-endosulfan and 86.16% β-endosulfan within 25 days of incubation (Fig. 6b). During this period, only 3.73% α- and 1.49% β-endosulfan were found to be degraded in the control flask. Again, no metabolites were detected during the course of degradation.
Optimization of environmental parameters for biodegradation of endosulfan by Achromobacter xylosoxidans strain C8B
Achromobacter xylosoxidans strain C8B shows maximum growth at pH 6.8 followed by pH 5, pH 8.5 and pH 10.5 respectively (Fig. 8). No growth was observed at pH 3. The degradation of endosulfan also corresponded to the growth of the bacterial population with 81.67% α- and 54.17% β- endosulfan degradation within 15 days at pH 6.8. The degradation of the same endosulfan concentration at other pHs i.e. pH 3, pH 5, pH 8.5 and pH 10.5 were 9.524, 18.87, 38.14 and 43.38% α- and 0.034, 9.91, 29.02 and 34.889% β-endosulfan respectively (Fig. 9). At pHs 8.5 and 10.5, there was gradual increased in the concentration of endosulfan diol in the medium. During the same period, degradation of α- and β- endosulfan in the control flask were 7.196 and 6.53% at 3 pH, 12.85 and 6.53% at pH 5, 8.43 and 5.72% at pH 6.8, 34.72 and 28.22% at pH 8.5 and 35.11 and 32.75% at 10.5 pH.
Achromobacter xylosoxidans strain C8B showed optimum growth at 28°C in both LB and SFM. In LB medium the growth was very fast at 35°C initially without any lag phase while it was 4 and 8 h respectively in the case of 28 and 20°C (Fig. 10a). Further increase or decreases of temperature beyond these points discouraged the growth of the bacteria. The bacteria showed slightly faster growth at 45°C with lag period of 12 h as compared to 12°C where the lag phase was 16 h. Unlike the LB medium, the growth of Achromobacter xylosoxidans strain C8B in SFM was comparatively slow. The duration of the lag phases were 4, 8 and 20 h at 28, 35 and 20°C respectively (Fig. 10b). At 12 and 45°C, the growth was much slower with lag phase of 28 and 24 h respectively.
The growth of strain C8B increased with increase in the concentration of endosulfan up to 50 ppm. Thereafter, further increased in the concentration did not affect the growth (Fig. 11). The strain C8B showed optimum growth at 50 ppm. Though the percentage degradation of endosulfan was more at lower concentration, the actual amounts of degradation did not differ significantly. The total endosulfan degradation measures as μg/ml were 17.25, 30.77, 37.72, 38.72 and 37.82 μg/ml at 20, 35, 50, 75 and 100 ppm respectively. The total percentage degradation of α- and β-endosulfan at these concentrations were 86.26, 87.92, 75.44, 51.63, and 37.83% respectively (Fig. 12).
Degradation of endosulfan metabolites by Achromobacter xylosoxidans strain C8B
Achromobacter xylosoxidans strain C8B degraded 39.68% endosulfan diol, 32.93% endosulfan lactone, 80.49% endosulfan sulfate, 83.55% endosulfan ether, 77.93% β-endosulfan and 84.14% α-endosulfan during 15 days of incubation period (Fig. 13). Under control conditions the percentage degradation of endosulfan diol, endosulfan lactone, endosulfan sulfate, endosulfan ether, β-endosulfan and α-endosulfan were 2.79, 4.72, 6.93, 9.68, 4.00 and 13.09% respectively. There was no significant accumulation of any of the metabolites in the culture medium.
Quantification of CO2
In both the bacterial inoculated flask i.e. flask containing MgSO4 and endosulfan as sulfur source CO2 release was highest during the first 2 day of the culture. However the flask with endosulfan as sulfur source showed a distinct biphasic pattern. The amount of CO2 release was highest during the first 2 days and slightly decreased up to the 7th day and started increasing thereafter (Fig. 14).
Discussion
Endosulfan has relatively reactive cyclic sulfite diester group (Van Woerden 1963). Through selective enrichment technique in SFM with endosulfan as sulfur source, those microorganisms which were able to release the sulfite group from endosulfan and use it as a source of sulfur for growth and reproduction will survive. The removal of sulfur moiety from the compound considerably reduced its vertebrate toxicity and hence helped in detoxification of the compound (Goebel et al. 1982; Dorough et al. 1978).
Out of the total eight strains isolated from the soil, strain C8B showed maximum growth and degradation of endosulfan. The difference in degradation capability of various strains may be due to difference in enzyme system and/or difference in their growth rate. The strain C8B identified on the basis of 16S rDNA sequence, showed 100% similarity with Achromobacter xylosoxidans. An Achromobacter xylosoxidans strain CS5 was earlier reported to be able to degrade endosulfan in aqueous medium (Wen et al. 2009).
The degradation of endosulfan without forming endosulfan sulfate has been reported in earlier studies (Kwon et al. 2002). Also, many bacteria capable of degrading endosulfan as well as its toxic metabolites endosulfan sulfate had been reported so far (Kwon et al. 2005; Lee et al. 2006; Weir et al. 2006). In the present study, the bacterial strain Achromobacter xylosoxidans strain C8B was able to utilize both endosulfan and endosulfan sulfate as sulfur as well as carbon source.
Achromobacter xylosoxidan strain C8B has got distinct sigmoidal growth curve in LB and SFM. The growth was almost twice in LB medium as compared to SFM. In CFM the growth of the bacteria was almost negligible. This might be due to the fact that endosulfan is not soluble in water. However, when tween 80 was added in the CFM as surfactant, the bacteria showed distinct growth. The growth of the bacteria in CFM supplemented with endosulfan and tween 80 was much greater than the one with tween 80 alone. This fact confirm that the bacterial strain were capable of using endosulfan as carbon source.
Achromobacter xylosoxidans CS5 (Wen et al. 2009) was capable of utilizing endosulfan as the sole carbon, sulfur and energy source. However, in their study endosulfan diol and endosulfan ether were formed as the major metabolites in the aqueous medium. In the present study, no such metabolites were detected throughout the experiment, suggesting possible complete mineralization of the compound.
The growth of bacteria in SFM containing MgSO4 as sulfur source was higher than the one with endosulfan and endosulfan sulfate. MgSO4 being inorganic salt, easily dissolved in water and therefore has better bio-availability. On the other hand, growth of Achromobacter xylosoxidans strain C8B in endosulfan supplemented medium was greater than the one with endosulfan sulfate. The result was similar to the one shown by Sutherland et al. (2002). The different oxidative state of sulfur in endosulfan and endosulfan sulfate suggested different enzyme systems for the two compounds (Sutherland et al. 2002). The more effective enzyme system for endosulfan might be responsible for better growth in endosulfan supplemented medium.
Endosulfan can readily undergo alkaline hydrolysis (Martens 1976), with approximately 10-fold increases in hydrolysis with an increase in a pH unit. This might be the reason behind higher degradation of endosulfan at pH 8.5 and 10.5 despite lesser growth. This is supported by the fact that there is gradual increased in endosulfan diol concentration at pH 8.5 and 10.5. Under different temperature, strain C8B shows optimum growth at 28°C both in LB and SFM. Variation of endosulfan concentration from 20 to 100 ppm, resulted in increased growth up to 50 ppm, after which the growth was more or less uniform at all the concentration. Though the percent degradation of endosulfan was higher at lower concentration, actual amount of degradation was almost constant with increased in the concentration from 50 to 100 ppm. This might be the reason why growth was almost equal at three different concentrations i.e. 50, 75 and 100 ppm. The higher percentage degradation of endosulfan at lower concentration might be due to better bio-availability, since the concentration of tween-80 was similar in all the concentrations.
Complete mineralization of endosulfan by the bacterial strains—Staphylococcus sp., Bacillus circulans I and II had been studied by Kumar and Philip (2006). Bacillus circulans I and II were found to be excellent degrader of endosulfan lactone and endosulfan ether. The mineralization probably occurs through hydrolysis pathway with formation of carbenium ions and/or ethyl carboxylates which were later converted into simple hydrocarbon (Kumar and Philip 2006). In the present study Achromobacter xylosoxidans strain C8B was able to degrade endosulfan diol, endosulfan lactone, endosulfan sulfate and endosulfan ether. When provided along with tween 80 in CFM, Achromobacter xylosoxidans strain C8B degrades 91.80% α-endosulfan and 86.16% β-endosulfan. The growth of the bacteria was also significantly higher in such medium which contain both tween 80 and endosulfan or its metabolites than the one with tween 80 alone. The findings strongly support the claim that this bacterial strain C8B is capable of using endosulfan and its metabolites as carbon source.
Achromobacter xylosoxidans strain C8B can degrade both endosulfan and endosulfan sulfate without forming any metabolites. Both compounds can undergo hydrolysis reaction to form endosulfan diol which can be further converted to endosulfan ether. The absence of any particular metabolite accumulation in the culture media during the course of degradation could suggest a possible complete mineralization of endosulfan through endosulfan ether in the medium or formation of some polar metabolites unable to extract using ethyl acetate. The possibility for the formation of polar metabolites cannot be ignored as it was reported in earlier studies (Weir et al. 2006).
The increase in respiration rate may be explained partially by organic carbon mineralization (Martens 1976). The carbon and hydrogen content of an organic compound mineralized into simple inorganic compounds like carbon dioxide, CO2 and water, H2O. Therefore, many studies have related the increase in CO2 release to the mineralization of organic compounds (Kate et al. 1986; Haney et al. 2008). The fact that there was an increased in CO2 released quantity when the strain Achromobacter xylosoxidans strain C8B was inoculated in a SFM containing endosulfan as sulfur source support the assumption that endosulfan might have undergone complete mineralization once its sulfur moiety had been removed through hydrolysis.
The release of CO2 from the inoculated biometric flask followed a biphasic pattern. The first peak coincided with the rapid increase in the bacterial cell density in the medium. This peak was observed in the flask containing MgSO4 as sulphur source as well. However the flask with MgSO4 did not show the 2nd peak of CO2, which was probably due to mineralization of endosulfan and its metabolites. Since, increase in biomass is corresponded with increase in the biodegradation of the compound in earlier experiments, the increased in the growth of Achromobacter xylosoxidans strain C8B in CFM (with Tween 80) when either endosulfan or its metabolites were added could support the claim.
From the present study, it can be concluded that the bacterial strain Achromobacter xylosoxidans strain C8B has a potential to be used for bioremediation of endosulfan. It can degrade 85.70% α- and 62.75% β-endosulfan in broth medium without forming any metabolites. The bacterial strain Achromobacter xylosoxidans strain C8B was also able to degrade endosulfan sulfate, degrading 80.10% during the 15 days of incubation period. The absence of any known metabolites in the culture media and the increase in the amount of CO2 release from the biometric flask containing endosulfan as sulfur source suggest a possible complete mineralization of both endosulfan and its toxic metabolites, endosulfan sulfate by the bacterial strain Achromobacter xylosoxidans strain C8B.
References
Awasthi N, Manickam N, Kumar A (1997) Biodegradation of endosulfan by a bacterial coculture. Bull Environ Contam Toxicol 59:928–934. doi:10.1007/S001289900571
Dorough HW, Huhtanen K, Marshall TC, Bryant HE (1978) Fate of endosulfan in rats and toxicological considerations of apolar metabolites. Pestic Biochem Physiol 8:241–252
Goebel H, Gorbanch S, Khauf W (1982) Properties, effects, residues, and analytics of the insecticide endosulfan. Residue Rev 83:1–100
Goswami S, Singh DK (2009) Biodegradation of alpha and beta endosulfan in broth medium and soil microcosm by bacterial strain Bordetella sp. B9. Biodegradation 20:199–207. doi:10.1007/s10532-008-9213-3
Haney RL, Brinton WH, Evans E (2008) Estimating soil carbon, nitrogen, and phosphorus mineralization from short-term carbon dioxide respiration. Commun Soil Sci Plant Anal 39:2706–2720. doi:10.1080/00103620802358862
Kate MS, Stephen ST, Martin A (1986) Kinetics of mineralization of organic compounds at low concentrations in soil. Appl Environ Microbiol 51:1028–1035
Kaur I, Mathur RP, Tandon SN, Dureja P (1998) Persistence of endosulfan (technical) in water and soil. Environ Technol 19:115–119. doi:10.1080/09593331908616663
Kumar M, Philip L (2006) Enrichment and isolation of a mixed bacterial culture for complete mineralization of endosulfan. J Environ Sci Health 41:81–96. doi:10.1080/10889860601021415
Kwon GS, Kim JE, Kim TK, Sohn HY, Koh SC, Shin KS, Kim DG (2002) Klebsiella pneumoniae KE-1 degrades endosulfan without formation of the toxic metabolite, endosulfan sulfate. FEMS Microbiol Lett 215:255–259
Kwon GS, Sohn HY, Shin KS, Kim E, Seo BI (2005) Biodegradation of the organochlorine insecticide, endosulfan, and the toxic metabolite, endosulfan sulfate, by Klebsiella oxytoca KE-8. Appl Microbiol Biotechnol 67:845–850. doi:10.1007/s00253-004-1879-9
Lee JB, Sohn HY, Shin KS, Jo MS, Kim JE, Lee SW, Shin JW, Kum EJ, Kwon GS (2006) Isolation of a soil bacterium capable of biodegradation and detoxification of endosulfan and endosulfan sulphate. J Agric Food Chem 54:8824–8828. doi:10.1021/JF061276E
Martens R (1976) Degradation of [8, 9,-14C]endosulfan by soil microorganisms. Appl Environ Microbiol 31:853–858
Rajendra M, Harishchandra K, Balasaheb D, Madhukar M, Rukmani K (2008) Thin layer chromatographic technique for detection and identification of endosulfan insecticide with m-dinitrobenzene reagent. J Planar Chromatogr 21:197–198. doi:10.1556/JPC.21.2008.3.8
Siddique T, Okeke BC, Arshad M, Frankenberger WT Jr (2003) Enrichment and isolation of endosulfan-degrading microorganisms. J Environ Qual 32:47–54
Sunlu FS, Kutlu B, Buyukisik HB (2010) Comparison of growth kinetics of Chaetoceros gracilis isolated from two different areas in the Aegean Sea (The Bay of Izmir and the Homa Lagoon). J Anim Vet Adv 9:1796–1803
Sutherland TD, Horne I, Lacey MJ, Harcourt RL, Russel RJ, Oakeshott JG (2000) Enrichment of an endosulfan degrading mixed bacterial culture. Appl Environ Microbiol 66:2822–2828. doi:10.1128/AEM.66.7.2822-2828.2000
Sutherland TD, Weir KM, Lacey MJ, Horne I, Russell RJ, Oakeshott JG (2002) Enrichment of a microbial culture capable of degrading endosulphate, the toxic metabolite of endosulfan. J Appl Microbiol 92:541–548
Van Woerden HF (1963) Organic sulfites. Chem Rev 63:557–571
Weir KM, Sutherland TD, Horne I, Russel RJ, Oakeshott JG (2006) A single monooxygenase, Ese, is involved in the metabolism of the organochlorides endosulfan and endosulfate in an Arthrobacter sp. Appl Environ Microbiol 72:3524–3530. doi:10.1128/AEM.72.5.3524-3530.2006
Wen L, Yun D, Beibei X, Yingying L, Xiang P, Zhangg J, Yanchun Y (2009) Biodegradation and detoxification of endosulfan in aqueous medium and soil by Achromobacter xylosoxidans strain CS5. J Hazard Mater 167:209–216. doi:10.1016/J.JHAZMAT.2008.12.111
Acknowledgement
This work was partially supported by DBT research grant, sanction no. BT/PR5755/BCE/08/385/2005, which is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N.S., Singh, D.K. Biodegradation of endosulfan and endosulfan sulfate by Achromobacter xylosoxidans strain C8B in broth medium. Biodegradation 22, 845–857 (2011). https://doi.org/10.1007/s10532-010-9442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-010-9442-0