Abstract
Biodegradation of glyphosate in sod-podzol soil by both the indigenous micro flora and the introduced strain Ochrobactrum anthropi GPK 3 was studied with respect to its sorption and mobility. The experiments were carried out in columns simulating the vertical soil profile. Soil samples studied were taken from soil horizons 0–10, 10–20, and 20–30 cm deep. It was found out that the most of the herbicide (up to 84%) was adsorbed by soil during the first 24 h; the rest (16%) remained in the soluble fraction. The adsorbed glyphosate was completely extractable by alkali. No irreversible binding of glyphosate was observed. By the end of the experiment (21st day), glyphosate was only found in extractable fractions. The comparison of the effect of the introduced O. anthropi GPK 3 and indigenous microbial community on the total toxicant content (both soluble and absorbed) in the upper 10 cm soil layer showed its reduction by 42% (21 mg/kg soil) and 10–12% (5 mg/kg soil), respectively. Simultaneously, 14–18% glyphosate moved to a lower 10–20 cm layer. Watering (that simulated rainfall) resulted in a 20% increase of its content at this depth; 6–8% of herbicide was further washed down to the 20–30 cm layer. The glyphosate mobility down the soil profile reduced its density in the upper layer, where it was available for biodegradation, and resulted in its concentration in lower horizons characterized by the absence (or low level) of biodegradative processes. It was shown for the first time how the herbicide biodegradation in soil can be increased manifold by introduction of the selected strain O. anthropi GPK 3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glyphosate (GP) [N-phosphonomethyl glycine] is an active ingredient of herbicides applied to annual and perennial weeds. GP inhibits 5-enolpyruvilshikimate-3-phosphate synthase, the enzyme of shikimic acid synthesis that participates in the synthesis of aromatic amino acids (Jaworski 1972).
GP belongs to the class of organophosphonates (OP) that have a direct carbon-phosphorus (C–P) bond resistant to physicochemical impacts. GP can accumulate in the arable soil layer, through the roots it can get into leaves, berries and fruit (Roy et al. 1989), and finally to mammalia via food chains. An extensive application of GP-based herbicides, therefore, needs a constant control of their actual and residual quantity.
The specific enzyme systems of soil microorganisms promote the GP degradation by splitting its C–P bond, the phosphorus released is utilized further by microbial cells as a biogenic element. Chemical decomposition or photolysis is here of secondary importance (Torstensson 1985).
The most active GP-degrading microorganisms were isolated from soils polluted by organophosphonates. All of them utilized GP as a sole phosphorus source rather than a source of nitrogen or carbon (Balthazor and Hallas 1986; Dick and Quinn 1995; Ermakova et al. 2008). They have been quite well characterized at a bench level, though there are no reports on their possible application for cleaning up the GP-contaminated soils. Literature only contains data on GP degradation by indigenous microbial communities (Rueppel et al. 1977; Getenga and Kengara 2004; Sorensen et al. 2006).
The ability to adsorb on soil particles and mobility through the soil profile contributes to the accumulation of herbicides in soil. In case of GP, it adsorbs on soil particles mainly through interactions with soil minerals, aluminum hydroxides, ferric oxides, organic matter (Morillo et al. 2000; Gimsing et al. 2004; Piccolo et al. 1996). The GP distribution coefficient K d, that characterizes its sorption capacity (concentration in liquid and solid phases ratio), depends on a soil type and can vary from 8 to 410 l/kg (Gimsing and Borggaard 2002; Strange-Hansen et al. 2004). Such a wide dispersion is determined mostly by differences in the cation-exchange capacity of soil types (Glass 1987). A better sorption capacity is peculiar of soils with a high content of clay compared to e.g. its sandy types (Sprankle et al. 1975). Organic matter can contribute to GP sorption by formation of mineral-organic complexes (Piccolo et al. 1996). The GP sorption on soil matrixes increases its persistency: it becomes less bioavailable, its biodegradation is retarded (Rueppel et al. 1977; Sorensen et al. 2006).
Irrespective of its high sorption abilities, GP can easily move through a soil profile down to deeper layers: its residues were found in groundwater in the areas of a wide application of pesticides (Vereecken 2005). GP mobility rate increased in soils containing more gravel and less organic matter (Dousset et al. 2004). The column leaching experiments with Roundup showed that GP was held by coarse-textured soils. However, in the course of continued applications, the herbicide began accumulating in such soil due to the reduction of binding sites and its increased mobility to deeper layers. Of the entire GP, added at concentrations of 7.4 and 14.8 mg/kg, from 85 to 95% was held in soil. The same procedure repeated 5 days later, yielded data of 63 and 73%, respectively. It was reported that abundant precipitations could favor the GP mobility from the upper layers of loam soil (Barrett and McBride 2007).
The efficiency of GP biodegradation in soil may change due to its ability of a vertical mobility. GP degradation in subsurface horizons (20–35 cm) of loam soil was much slower than in the upper ones (0–20 cm) due to a low activity of indigenous GP-degrading microorganisms (Veiga et al. 2001).
Such GP features as the level of sorption, distribution throughout the soil vertical profile, and biodegradability form a kind of a parameter that characterizes a GP bioavailability. This parameter should be obligatory taken into account in bioremediation technologies.
The present paper describes the studies of the GP degradation in the vertical profile of sod-podzol soil by indigenous soil microorganisms and selected bacterial strain O. anthropi GPK 3 able of GP destruction, as well as its dependence on the GP abilities of sorption and mobility.
Materials and methods
Soil column model
The experiments were carried out using samples of sod-podzol soil (SP) composed of (%): Corg (1), clay (39.8), sand (16.1), silt (25.4); total exchange bases made up 9.9 mg-eqv/100 g; exchange phosphorus was 4.5 mg P2O5/100 g; pH 7.1. The soil was taken from the sites that had never been subjected to a GP treatment.
The soil was grinded, shaken through a 2-mm sieve, and placed into plastic columns (d = 8.5 cm, h = 40 cm) up to the height of 30 cm. Each column represented a vertical soil profile conventionally divided into three levels: 0–10, 10–20, and 20–30 cm. The first two and the deepest level simulated arable and sub-arable horizons, respectively. Incubation was carried out at room temperature (22–24°C), the uppermost layer was regularly loosened, its humidity was maintained at 24–27% (w/w).
To control the cell density and GP content, soil was taken from the columns, each layer (0.8 kg per one) separately and stirred; three samples were taken from each horizon and used in further investigations. All values were calculated for air-dried soil (a.d.s.).
Ten days later, some columns were subjected to watering with distilled water (simulating rainfall). The efflux was collected and analyzed for the GP content.
Nutrient sources
Herbicide Ground Bio (Tekhnoexport, Russia), containing GP as its isopropylamine salt, and similar to Roundup (Monsanto Chemical Co, USA) served as a source of phosphorus. The soil surface was proportionally treated with 5 ml of the herbicide solution containing 18 mg GP (at the rate of 100 l herbicide/ha, 50 mg GP/kg a.d.s.) and thoroughly mixed throughout the 0–10 cm layer. In our experiments, we applied GP in quantities that were ten times higher than standards, recommended for one-time application in field, taking into account its possible accumulation in the course of repeated treatments.
The earlier obtained results showed that in the soil studied, microorganisms used the available organic substrates (carbohydrates and organic acids), and no additional carbon sources were required.
Mineral salts were added to each column at the start of the experiment in 25 ml of mineral salt medium (MS1). The MS1 medium contained (g/l): 2.0 NH4Cl; 0.2 MgSO4 × 7H2O; and 0.5 K2SO4; trace elements (mg/l): 2.5 FeSO4 × 7H2O; 10.0 CaCl2 × 6H2O; 2.0 CuSO4 × 5H2O; 0.06 H3BO3; 20.0 ZnSO4 × 7H2O; 1.0 MnSO4 × H2O; 0.05 NiCl2 × 6H2O; 0.3 Na2MoO4 × 2H2O; 1 l of distilled water. All salts were analytically extra pure without phosphorus traces.
Microorganisms and cultivation
The GP degradation was studied both with: (1) only microorganisms indigenous for SP soil under study (control) and (2) mixed culture consisting of indigenous microbial community and introduced strain O. anthropi GPK 3 (VKM B-2554 D).
This strain was isolated by the method of enrichment cultures from the soil contaminated by GP as a result of the regular and long term applications of Roundup. O. anthropi GPK 3 is resistant to high GP concentrations and preserves its high destructive activity in the course of a long shelf life on solid MS1 medium supplied with 10.0 g/l sodium glutamate (Difco, USA) as a carbon source and 0.5 g/l GP in the composition of herbicide GroundBio as a phosphorus source. The taxonomy was identified by analyzing primary nucleotide sequences of 16S rRNA genes using BLAST software (Ermakova et al. 2008).
O. anthropi GPK 3 cells, to be introduced to soil columns, were grown as batch culture in liquid MS1 medium, supplied with glutamate and GP, in shaking flasks (200 rpm) at 29°C. The pH value of the medium was maintained at 6.5–7.5 by adding sterile 20% H2SO4 solution. Cells were sampled in the mid exponential growth phase, centrifuged (at 4,200g for 15 min), washed twice with a mineral medium lacking a phosphorus source, suspended in the same, but supplied now with 10 g/l glutamate, medium (OD560 2–2.5), and incubated under the above conditions for 48 h to provide phosphorus starvation. Than, the cells were again centrifuged, washed with the mineral medium lacking phosphorus and carbon sources, and suspended in the same medium. Glutamate was mostly utilized during a starvation period; the residual carbon source was washed out.
The day (24 h) after the herbicide addition, 25 ml of microbial suspension in MS1 medium (4 ± 0.5 × 108 cells/ml) was proportionally distributed over the soil surface, the upper layer was stirred to a 0–10 cm depth. In the control experiment with indigenous soil microorganisms, the suspension was substituted by an equal volume of mineral MS1 medium without glutamate.
The growth of microorganisms in liquid medium was controlled by OD at 560 nm using a Specol 210 spectrophotometer (Carl Zeiss Jena, Germany). The OD data were converted to dry biomass weight using a ratio 0.5 g/OD560 unit determined experimentally. GP-degrading bacteria propagation in soil was determined by the CFU changes when inoculating serial dilutions of soil suspension on solid MS1 medium supplied with GP and glutamate. Unlike indigenous microorganisms, O. anthropi GPK 3 developed on MS1 medium with GP in the form of slimy colonies. It allowed an easy examination of its survival rate and interactions with indigenous micro flora.
At the end of experiment, the survived cells, both from the control and experimental columns, were isolated on solid MS1 medium with GP and glutamate, suspended, and used to inoculate the same but liquid medium. Both isolates, the same as the collection strain O. anthropi GPK 3, were characterized physiologically under conditions of batch cultivation.
GP analysis
GP was determined in soil extracts by HPLC (LKB 2150, Sweden) with UV detection at 204 nm, Repro-Gel H column, 9 µm, 250 × 8 mm, Dr. Maisch (Germany) at 65°C. Mobile phase was 0.01 N H2SO4. The extracts were pre-purified from humic acids by precipitation with 20% H2SO4 (0.2 ml per 1 ml extract), the pellet was separated by centrifugation and filtration through a MF-Millipore filter (d = 0.22 µm) (USA).
To study sorption processes, the quantity of GP solved and sorbed (extractable and bound) in soil was determined. To analyze soluble GP, 25 g soil was shaken with 25 ml water for 30 min; solid and liquid phases were divided by centrifugation at 4,200g for 15 min. The GP concentration in the supernatant (C s, mg/l) was determined; its quantity in soil was calculated according to the formula m s (mg/kg) = C s V 1/M a.d.s., where V 1 was a supernatant volume, M a.d.s. was an air-dried soil sample weight. To evaluate the quantity of GP extracted in the solid phase, the residue was shaken with 60 ml 1 N NaOH for 30 min and centrifuged at 4,200g for 15 min. Its concentration was determined in the supernatant (C e), and the content in soil was calculated according to the formula m e (mg/kg) = C e V 2/Ma.d.s., where V 2 was the supernatant volume. A share of the bound (non-extractable) toxicant was calculated by the formula m b = m 0 − (m s + m e), where m 0 (mg/kg) was the amount of GP added.
The extent of the GP mobility throughout the soil profile was calculated by the change in the total GP in alkaline soil extracts. To do this, 25 g soil was shaken with 60 ml 1 N NaOH for 30 min and centrifuged at 4,200g for 15 min; a GP concentration in the supernatant was determined (C, mg/l), its quantity in soil (m, mg/kg) was calculated as described above.
Results and discussion
Growth dynamics and physiological properties of GP-degrading microorganisms in soil columns
A profound clean-up of GP-polluted soils is possible mostly due to the ability of soil microorganisms to cleave C–P bond. The efficiency of this process depends on many factors: soil structure, climate, depth of herbicide penetration and its adsorption on soil matrixes, composition of microbial populations and their ability to degrade such toxicant (Torstensson 1985; Veiga et al. 2001; Araujio et al. 2003; Strange-Hansen et al. 2004; Sorensen et al. 2006). There are no publications hitherto describing the attempts to develop biotechnological methods for GP disposal from polluted soils by introduction of selected strains capable of its degradation.
To achieve this goal, it was decided to start from studying the behavior of GP-degrading microorganisms in the soil profile. It was found out that throughout the experiment, the introduced cells of O. anthropi GPK 3 remained viable in the upper 0–10 cm layer, and were absent in deeper horizons. The initial content of indigenous microorganisms-destructors was 3.5 ± 0.6 × 105 cells/g a.d.s. throughout the soil column. After 5 days incubation, their density was slightly higher (4 ± 0.5 × 105) in the uppermost layer, but decreased to 1 ± 0.2 × 102 in the next 10–20 cm layer, while the deepest 20–30 cm layer was a sparsely populated level at all.
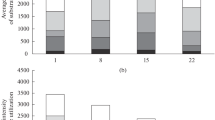
The comparison of growth dynamics of indigenous GP-degrading microorganisms cultivated alone (control) and together with O. anthropi GPK 3 showed that the introduced strain was predominating in the second variant (mixed culture). Its biomass could reach maximum by the 7th day in the presence of sufficient carbon and phosphorus sources, then their cell density began gradually decreasing probably due to depletion of the sources of carbon or other biogenic nutrients source on the background of residual phosphorus. The density of indigenous microorganisms increased successively throughout the experiment due to their gradual adaptation to the phosphorus source (Fig. 1).
Changes in the CFU of GP-degrading microorganisms in soil: filled square mixed culture (strain Ochrobactrum anthropi GPK 3 + indigenous microorganisms), filled triangle strain Ochrobactrum anthropi GPK, open square control (indigenous microorganisms). The vertical bars indicate the standard error of the mean of triplicate
By its physiological characteristics, the mixed culture isolated at the end of the experiment in the soil column and grown as batch culture in GP-containing medium, was quite similar to the collection strain O. anthropi GPK 3 that proved the predominance of this strain in the population structure. In case of indigenous microorganisms, their lag phase was 4–5 times longer, and other parameters such as biomass, specific growth rate, destruction efficiency, were significantly lower (Table 1). Even a prolonged exposure to toxicant in the soil column was evidently insufficient for activation of their destructive potential.
The results obtained showed that in GP-polluted soil, the introduced strain O. anthropi GPK 3 preserved its ability of active propagation in case there are enough nutrients (carbon, phosphorus, and other biogenic elements). In their absence, its cells began dieing thus making possible a regulation of their density in soil.
GP sorption
GP degradation is to a great extent conditioned by degree of its biding with soil matrix. A part of the adsorbed herbicide can be desorbed in the liquid soil pool, whereas the other part remains strong bound and, consequently, low-available biologically. In such form, GP can be preserved in soil for at least 2 years (Eberbach 1998, 1999).
In studies of the GP availability for both indigenous and introduced microbial cells in SP soil, its soluble and adsorbed forms were analyzed at the start of the experiment, before introduction of O. anthropi GPK 3 and 21 days later.
It was shown that during the first 24 h after GP application, up to 84% GP was adsorbed and 16% remained in the soluble phase. On the basis of analytical data, the entire GP adsorbed represented an extractable fraction, no bound GP was found. No evidences of the GP destruction by the indigenous microorganisms were observed during this time, probably because of their poor adaption to the toxicant.
In the course of the experiment, GP failed to bind rigidly (non-extractable fraction) throughout the soil column. Its residues were only found in extractable fractions, soluble GP was absent. These findings proved a biological availability of GP in SP soil.
Vertical mobility of GP
The GP mobility, specifically mobility down the soil profile, determines its bioavailability in soil. Its biodegradability can vary significantly or even cease depending on the depth of penetration (Veiga et al. 2001).
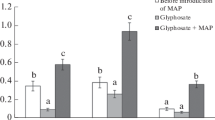
In our experiments, the analysis of the total GP (both solved and adsorbed) in different soil horizons showed that after 7 days incubation of mixed culture, up to 57% of total GP still remained in the upper 0–10 cm layer, 12% GP moved down to a 10–20 cm layer. In the control variant with indigenous microbial community, these values were 82 and 16%, respectively (Fig. 2).
Variations of the GP content in soil depending on depth by the 7th (a), 21st (b) days, and by the 21st day with rainfall (c) (% of initial value): light columns control (indigenous microorganisms), shaded columns mixed culture Data are mean of triplicates and error bars indicate the standard deviations
By the end of the experiment, 44 and 71% GP remained in the uppermost layer with O. anthropi GPK 3 and control, and slightly increased in the 10–20 cm layer to 14 and 18%, respectively. GP was not found in the lowest 20–30 cm layer.
The intense watering of soil column, simulating rainfall, enhanced the GP mobility with water flow resulting in its final decrease to 34% in the upper 0–10 cm layer in the experiment with mixed culture, and to 62% in the control variant with indigenous destructors. Due to wash out, in deeper 10–20 cm layer, the GP quantity increased to 20%, its appearance was registered also at a depth of 20–30 cm (6 and 8%, respectively) (Fig. 2). GP was not found in soil column effluents.
GP biodegradation
Since the physicochemical properties of SP soil samples under study were similar in all the columns, and the incubation conditions were also made equal, a decrease of GP quantity in the upper 0–10 cm layer was explained by its biodegradation by the introduced and indigenous microorganisms.
The data on the GP quantity in soil obtained taking into account its mobility down the soil profile allowed for calculating the extent of its biodegradation. GP was mostly (31% of the total introduced) utilized during 7 days by actively growing O. anthropi GPK 3. During the next 2 weeks, the viability of introduced cells deteriorated and GP biodegradation became much less active: only a 11% decrease registered (compare with total 42% during the entire experiment, 21 mg GP/kg a.d.s.). In case with solely indigenous microorganisms, the GP utilization was very low (2–4%) first, then, in the course of biomass growth, it increased and reached 10–12% at the end of experiment. The activity of O. anthropi GPK 3 against GP in the uppermost soil layer reduced its flux down; however, in deeper 10–30 cm layers, the herbicide accumulated due to the absence of this strain and a low density of indigenous GP-degrading microorganisms.
The intense watering, that brought GP down to a depth of 10–30 cm, consequently reduced the amount of bioavailable toxicant in upper soil horizon. Similar results were obtained with other types of loamy soils: intense irrigation or precipitation washed GP down to the sub-arable layer (30 cm deep) where it could remain not attacked for quite a long time (Veiga et al. 2001; Barrett and McBride 2007).
The efficiency of GP degradation by the introduced strain O. anthropi GPK 3 depends on the character of cell/substrate interactions: 20–33 μg GP/108 cells at batch cultivation in liquid medium (Ermakova et al. 2008), and significantly lower when the herbicide was mostly adsorbed on soil particles, namely, in SP soil water suspension of 14–15 μg GP/108 cells (the data not shown) and directly in soil: 11–13 μg GP/108 cells (the present work). These results pointed out to a significant decrease of the bioavailability of adsorbed herbicide that agreed with the conclusions of other researchers (Rueppel et al. 1977; Sorensen et al. 2006).
It is believed that utilization of soluble GP in soil favors desorption and thus maintains its balance in soil system.
Conclusion
A high ability of GP for sorption and mobility throughout the soil profile facilitates its accumulation in SP soil under study. Biodegradation of GP is the main factor that can prevent, on the one hand, its build-up in soil and, on the other, to promote the disposal of GP residues.
In the experiments described, a concentration of GP added in soil was ten times higher than the recommended dose for an agricultural application in attempts to follow the ways of its accumulation in the course of repeated applications.
During 21 days, 10–12% (5 mg/kg of soil) of GP was degraded in the uppermost horizon in experiments with indigenous microorganisms and 42% (21 mg/kg) in case using mixed culture containing strain O. anthropi GPK 3. Of the total introduced GP, 18 and 14%, respectively, moved down to a 10–20 cm horizon. A more intense water percolation through the soil increased these values to 21 and 20%, respectively, and 6–8% GP was found in the deepest 20–30 cm layer.
GP was only degraded in the uppermost layer that contained viable O. anthropi GPK 3 cells and indigenous microorganisms able of GP destruction. Down the soil profile, at a depth of 10–30 cm, the GP quantity remained almost the same or even increased a bit in places where these microorganisms were absent.
To prevent the GP accumulation in soil due to its sorption and mobility, it is advised, therefore, after extermination of weeds, to immediately remove the GP residues from the arable layer. The indigenous micro flora needs quite a long time for adaptation to the toxicant, hence its low activity in the SP soil under study. However, this problem can be solved by introduction of GP-degrading strains.
The strain O. anthropi GPK 3 degrades GP in the upper arable layer of SP soil many times more effectively than the indigenous microorganisms. Such its features as a high destructive activity, resistance to high concentrations of the herbicide, no negative effects when interacting with indigenous micro flora, rapid lysis of cells in the absence of any of biogenic nutrients, make this culture highly attractive for bioremediation of GP-polluted soils. The efficiency of the process can be considerably increased by providing favorable conditions for reproduction of introduced microorganisms, among them plowing, humidification, and introduction of organic additives.
References
Araujio ASF, Monteiro RTR, Abarceli RB (2003) Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 52:799–804
Balthazor TM, Hallas LE (1986) Glyphosate-degrading microorganisms from industrial activated sludge. Appl Environ Microbiol 51:432–434
Barrett KA, McBride MB (2007) Phosphate and glyphosate mobility in soil columns amended with Roundup. Soil Sci 172:17–26
Dick RE, Quinn JP (1995) Glyphosate-degrading isolates from environmental samples: occurrence and pathways of degradation. Appl Microbiol Biotecnol 43:545–550
Dousset S, Chauvin C, Durlet P, Thevenot M (2004) Transfer of hexazinone and glyphosate through undisturbed soil columns in soils under Christmas tree cultivation. Chemosphere 57:265–272
Eberbach P (1998) Applying non-steady-state compartmental analysis to investigate the simultaneous degradation of soluble and sorbed glyphosate (N-(phosphonomethyl)glycine) in four soils. Pestic Sci 52:229–240
Eberbach P (1999) Influence of incubation temperature on the behavior of triethylamine-extractable glyphosate (N-phosphonomethylglycine) in four soils. J Agric Food Chem 47:2459–2467
Ermakova IT, Shushkova TV, Leontievsky AA (2008) Microbial degradation of organophosphonates by soil bacteria. Microbiology 77:615–620 (translated from Mikrobiologiya)
Getenga M, Kengara FO (2004) Mineralization of glyphosate in compost-amended soil under controlled condition. Bull Environ Contam Toxicol 72:266–275
Gimsing AL, Borggaard OK (2002) Effect of phosphate on the adsorption of glyphosate on soils, clay minerals and oxides. Int J Environ Anal Chem 82:545–552
Gimsing AL, Borggaard OK, Bang M (2004) Influence of soil composition on adsorption of glyphosate and phosphate by contrasting Danish surface soils. Eur J Soil Sci 55:183–191
Glass R (1987) Adsorption of glyphosate in soil and clay minerals. J Agric Food Chem 35:497–500
Jaworski EG (1972) Mode of action of N-phosphonomethylglycine: inhibition of aromatic amino acid biosynthesis. J Agric Food Chem 20:1195–1198
Morillo E, Undabeytia T, Maqueda C, Ramos A (2000) Glyphosate adsorption on soil of different characteristics. Influence of copper addition. Chemosphere 40:103–107
Piccolo A, Celano G, Conte P (1996) Adsorption of glyphosate by humic substances. J Agric Food Chem 44:2442–2446
Roy DN, Konar SK, Banerjee S, Charles DA, Thompson DG, Prasad RP (1989) Uptake and persistence of the herbiside in fruit of wild blue-berry and raspberry. Can J For Res 19:842–847
Rueppel M, Brightwell B, Schaefer J, Marcel J (1977) Metabolism and degradation of glyphosate in soil and water. J Agric Food Chem 25:517–528
Sorensen SR, Schultz A, Jacobsen OS, Aamand J (2006) Sorption, desorption and mineralization of the herbicides glyphosate and MCPA in samples from two Danish soil and subsurface profiles. Environ Pollut 141:184–194
Sprankle P, Meggitt WF, Penner D (1975) Adsorption, mobility, and microbial degradation of glyphosate in the soil. Weed Sci 23:229–234
Strange-Hansen R, Holm PE, Jacobsen OS, Jacobsen CS (2004) Sorption, mineralization and mobility of N-(phosphonomethyl)glycine (glyphosate) in five different types of gravel. Pest Manag Sci 60:570–578
Torstensson L (1985) Behavior of glyphosate in soil and its degradation. In: Grossbard E, Atkinson D (eds) The herbicide glyphosate. Butterworths, London, pp 137–149
Veiga F, Zapata JM, Marcos MLF, Alvarez E (2001) Dynamics of glyphosate and aminomethylphosphonic acid in a forest soil in Galicia, north-west Spain. Sci Total Environ 271:135–144
Vereecken H (2005) Mobility and leaching of glyphosate: a review. Pest Manag Sci 61:1139–1151
Acknowledgments
The work was supported by the International Science & Technology Center, project no. 1892.2, and Ministry of Education and Science of Russian Federation, project “Development of Scientific Potential of the Higher School” (2.1.1.9227).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shushkova, T., Ermakova, I. & Leontievsky, A. Glyphosate bioavailability in soil. Biodegradation 21, 403–410 (2010). https://doi.org/10.1007/s10532-009-9310-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-009-9310-y