Abstract—
A model laboratory experiment on arable soil with low organic content and low biological activity showed short-term changes in the intensity of the main microbiological processes of the nitrogen transformation in soil after the application of glyphosate. The soil incubation with glyphosate at the maximum recommended dose of 8 L/ha for 22 days resulted in an increase in nitrogen-fixing and denitrifying activities by 30–80 and 300%, respectively, and in a decrease in nitrification by 20–40%. These effects were of a short-term nature and did not reflect the entire complex of ongoing microbiological processes. Glyphosate had no effect on the CO2 emission, an integral indicator of biological activity. At the end of incubation period, the soil with glyphosate was characterized by an increase in the number of bacteria by 40% and a decrease in the number of micromycetes by 70%. In general, under the selected conditions, the application of glyphosate led to a well-pronounced deterioration in the biological activity of the soil. The multisubstrate test showed that the application of glyphosate leads to an increase in the value of the coefficient of rank distribution of substrate utilization spectra (d) accompanied by a decrease in the specific metabolic activity (W) and the integral vitality index (G). It was shown for the first time that application of the glyphosate for the soil with a low biological activity and phosphorus availability, and the herbicide degradation with a break in the C–P bond excluding the formation of toxic metabolites have a pronounced negative effect on soil microorganisms, which leads to inhibition of wheat plant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, the herbicide glyphosate (N-(phosphonomethyl)-glycine) is widely applied throughout the world. In Russia, the share of cultivated areas treated with glyphosate-based preparations varies from 10 to 30%. According to the conclusion of experts, there are no legislative and market prerequisites for reducing the application of glyphosate in Russia. In the short term, even an increase in its use is possible due to the involvement of new areas into crop growing [10]. It is generally believed that glyphosate is rapidly inactivated in soil because of its binding by clay particles, as well as by iron and aluminum oxides and hydroxides [14]. In addition, microorganisms can utilize glyphosate as a source of nitrogen (biodegradation with C–N bond cleavage) or phosphorus (biodegradation with C–P bond cleavage) [42]. However, recent studies indicate that there is a risk of negative aftereffects of glyphosate on crop growth [21, 23, 42].
Among the most often considered aftereffects of glyphosate application is the accumulation of residual amounts of the herbicide or its most common metabolite, aminomethylphosphonic acid (AMPA), in soil. AMPA, which is formed during the degradation of glyphosate with cleavage of the C–N bond, is a structural analogue of antibiotics from the group of phosphonic acid derivatives; it inhibits the development of bacteria by disrupting cell wall synthesis [13] and is phytotoxic [22]. The predominant degradation of glyphosate with the formation of AMPA is widespread in arable soils due to the presence of readily available phosphorus introduced with fertilizers, while the microbiological destruction of the C–P bond is available only in low concentrations of available phosphates [19]. An assessment of the content of glyphosate and AMPA in the plow horizon of soils in the European Union showed their occurrence in soil in almost 50% of cases. Out of 317 tested samples, 21% contained glyphosate and 42% contained AMPA. The maximum contents of glyphosate and AMPA reached 2.05 and 1.92 mg/kg soil, respectively [39]. Both compounds can have a negative impact on the growth and development of crops [21, 22, 24] and may be released from their complexes with soil minerals upon the application of phosphorus fertilizers [32].
Previously, field experiments showed a delay in germination of beans, oats, and rapeseed sown into the soil 14 days after the application of glyphosate at a rate of 3 kg/ha (maximum recommended dose) [23]. Residual amounts of glyphosate applied at the rate of 3 kg/ha reduce the level of phytohormones in oat plants sown 24 days after the herbicide application [21]; the biosynthesis of these phytohormones involves the use of chorismate (indole-3-acetic acid, phenylacetic acid) as a precursor. The observed effect is explained by the mechanism of glyphosate action—inhibition of 5'-enolpyruvylshikimate-3-phosphate synthase (EPSP synthase, EC 2.5.1.19), a component of the enzyme system of the shikimate pathway for the biosynthesis of aromatic amino acids, which converts shikimate to chorismate, a precursor of phenylalanine, tyrosine, and tryptophan. Phytotoxicity of AMPA has also been repeatedly shown; this acid inhibits chlorophyll biosynthesis and impairs stomata conductance [22]. Thus, both glyphosate in residual amounts and AMPA are toxic to plants.
At the same time, numerous studies have shown that the introduction of glyphosate into soil can lead to disturbances in the functioning of soil microbial communities, such as a decrease in the abundance of rhizospheric bacteria and fungi [34]. This is due, first of all, to the presence of EPSP synthases in some microorganisms, which are sensitive to the glyphosate. In particular, the presence of EPSP synthase, which is sensitive to inhibition of the shikimate pathway for the biosynthesis of aromatic amino acids by glyphosate, has experimentally been shown for soil bacteria Bradyrhizobium japonicum [43] and for some bacterial species of the genus Pseudomonas [12]. According to bioinformatics estimates, 82% of archaea species and 57% of bacterial species have glyphosate-sensitive EPSP synthases, which means that most prokaryotes can be inhibited by glyphosate [30]. Changes in the composition of the microbial community subsequently affect the ecological functions of microorganisms in the soil, including the cycling of biophilic elements, the formation of soil aggregates, and the biodegradation of organic compounds. Consequently, the impact on microorganisms is reflected in soil fertility and in crop yield [24]. This is especially relevant for soils with a low content of biophilic elements and organic matter and characterized by a low level of biological activity.
The aim of this study is to evaluate the impact of glyphosate on the activity of soil microorganisms and soil phytotoxicity in arable soil with a low content of nutrients and organic matter, including available phosphates. According to existing data, the most sensitive to the toxic effect of glyphosate are microorganisms involved in the transformation of nitrogen compounds [26]. Therefore, we carried out an assessment of the influence of glyphosate on the nitrogen-fixing, nitrifying, and denitrifying activities in the soil. To obtain integrated estimates of the impact of glyphosate on soil microorganisms, we used the determination of carbon dioxide emission and the total number of microorganisms; to determine the functional biodiversity and stability of soil microbial communities, the method of multisubstrate testing (MST) was applied. Phytotoxicity was assessed by biotesting using wheat plants as a test culture. To determine the pathway of preferential biodegradation of glyphosate in the soil, the contents of glyphosate and AMPA were determined.
OBJECTS AND METHODS
Soddy-podzolic soil (Albic Retisol) samples were taken in Noginsk district of Moscow oblast (55°48.173′ N, 38°14.908′ E) from the plow horizon (0–20 cm). Five individual samples taken from a plot of 1 m2 using an envelope sampling pattern were mixed together to obtain an average sample [1]. The actual acidity and the contents of available potassium and phosphorus (by Kirsanov’s method in the modification by TsINAO (Central Research Institute for Agrochemical Service), extraction by 0.2 N HCl) were determined according to [1]; the contents of C and N were determined by catalytic combustion at 960°C in an oxygen flow [25] on a Vario Macro Cube elemental analyzer (Elementar Analysensysteme GmbH, Germany); the contents of available forms of nitrogen were determined according to [27]. General characteristics of the soil are the following: \({\text{p}}{{{\text{H}}}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}\), 5.3; organic carbon content C, 1.83%; total nitrogen content N, 0.12%; exchangeable potassium content K2O, 46 mg/kg soil; available phosphorus content P2O5, 75 mg/kg soil; nitrate content, 2.9 mg/kg soil; and ammonium content, 40.6 mg/kg. Thus, this soil is slightly acidic, with a low content of available phosphorus and potassium, a very low content of nitrates, and a moderate, of the total available nitrogen (nitrates and ammonium).
The effect of glyphosate on microbiological processes was assessed using a model laboratory experiment. Soil samples were dried and sieved through a 2-mm sieve after removing the remains of the roots. Next, soil samples (100.00 ± 0.02 g) were placed into 100-cm3 plastic vessels. Glyphosate (Roundup VR 360 g/L, JSC Avgust, Russia) was applied as an aqueous solution up to the soil water content corresponding to 70% of the field water capacity (FWC). The initial content of the herbicide in the soil was 9.6 mg/kg, which corresponds to the herbicide application rate of 8 L/ha (2.88 kg/ha for the active substance). The choice of glyphosate concentration was based on the maximum recommended application rate of 8 L/ha and the assumption that all herbicide is retained in the topsoil layer of 2–5 cm [15]. The same volume of distilled water was added to control vessels with soil. Incubation was carried out in a thermostat at a temperature of 24 ± 2°C. The water content of 70% FWC water was maintained via periodical weighing of the vessels and adding the required amount of water. The experiment was performed in triplicate. After 1, 8, 15, and 22 days, some of the vessels were used to determine the content of glyphosate and its main metabolite, AMPA. The effect on the microbial community was assessed on days 1, 8, 15, and 22 of the experiment based on the determination of nitrogen-fixing, nitrifying, and denitrifying activities, carbon dioxide emission, the total number of microorganisms, and the functional diversity of microorganisms by the MST method. In addition, the phytotoxicity of the soil in relation to wheat plants was evaluated.
Determination of nitrogen-fixing activity was carried out by the acetylene method according to [7]; the method is based on the ability of the nitrogenase of nitrogen-fixing microorganisms to reduce acetylene C2H2 to ethylene C2H4. To determine the actual nitrogen fixation, soil samples (5 g) were placed into 10-mL penicillin flasks with rubber stoppers and 1 mL of acetylene was introduced into each flask. After 24-h incubation of the samples in the thermostat with the temperature of 28°C, the produced ethylene was measured. To determine potential nitrogen fixation, 5 g of soil were placed in 10-mL penicillin flasks, and a glucose solution was added at the rate of 1% glucose per the mass of air-dry soil. Next, the soil was well-stirred with a glass rod to evenly distribute glucose, the flasks were closed with cotton plugs and placed in a thermostat at a temperature of 28°C for 1 day. Then the flasks were sealed with rubber stoppers, 1 mL of acetylene was added into them, and they were incubated in a thermostat at 28° С for 1 h. The determination of produced ethylene was performed on a Kristall-2000 gas chromatograph (Khromatek, Russia) equipped with a Porapak N 80/100 (1 m × 3 mm) column and a flame ionization detector (FID). Determination conditions: column temperature 60°С, detector temperature 160°С, evaporator temperature 100°С, carrier gas (N2) at the flow rate of 50 mL/min, air flow 280 mL/min, hydrogen flow 28 mL/min. The determination was carried out in triplicate.
The determination of nitrifying activity according to Kravkov was carried out following the procedure described in [27]. Soil samples (5 g) were incubated (60% FWC, 28°C) for 8 days, after which free \({\text{NO}}_{3}^{ - }\) ions were determined according to [9]. In a test tube with 5 g of soil after incubation, 12.5 mL of a 0.25 M solution of Mg(CH3COO)2 was added and shaken by stirring at 25 rpm for 1 h on an Intelli-Mixer (ELMI, Latvia), then centrifuged at 2000 rpm (760 g) for 15 min in a CM-6 M centrifuge (ELMI, Latvia). The content of nitrogen in the nitrate form was measured in triplicate in the centrifugate using an Ekom-NO3 ion-selective electrode and an Ecotest-120 ionometer (NPP Ekoniks, Russia). For determination of the potential nitrifying activity, (NH4)2SO4 (0.2 g/kg) was introduced into the soil before incubation.
Denitrification activity was determined according to Fedorova’s method based on the ability of acetylene to inhibit nitrous oxide (N2O) reductase, thus enabling the evaluation the activity of the denitrification process by N2O accumulation in the gas phase [7]. To determine the actual denitrification, a 5-g sample of soil was placed into 10-mL penicillin flasks, hermetically sealed with rubber stoppers, and purged with argon for 1 min. Next, 1 mL of acetylene was added, and the flasks were incubated at 28°C for 5 days. The N2O concentration was measured on a Kristall-2000 gas chromatograph (Khromatek, Russia) equipped with a Porapak N 80/100 column (1 m × 3 mm) and an electron capture detector (ECD). Determination conditions: carrier gas (nitrogen) flow rate 90 mL/min, detector temperature 240°C, column temperature 50°C, evaporator temperature 100°C. Potential denitrification was determined in a similar way, but with glucose (2.5 mg/g) and potassium nitrate KNO3 (0.3 mg/g) added into the soil before incubation, and the incubation time was reduced to 1 day. The experiment was performed in triplicate.
Determination of carbon dioxide emission was carried out according to [7]. To determine the actual CO2 emission, a 2-g sample of soil was placed into penicillin flask, sealed with rubber stopper, and incubated for 1 day at 28°C. Next, the CO2 content was determined on a 3700 gas chromatograph (JSC Khromatograf, Russia) equipped with a Polysorb 1 column (3 m × 3 mm) and a thermal conductivity detector. Determination conditions: evaporator temperature 30°С, temperature of the katharometer 100°С, temperature of measuring elements 150°С, current strength 148 mA, carrier gas (helium) flow rate 30 mL/min. To estimate the potential emission of CO2, glucose was introduced into the soil before incubation at a rate of 2.5 mg/g. The experiment was also performed in triplicate.
Determination of the total number of microorganisms was carried out by luminescence microscopy in incident light with staining by acridine orange [5]. Soil water suspensions (1 : 10) were treated with an ultrasonic disperser Sonopuls (Bandelin, Germany) (22 kHz, 0.44 A, 2 min). Next, 0.01 mL of the suspension was applied with a micropipette onto defatted glass slides and evenly distributed with a microbiological loop over an area of 4 cm2. After complete drying of the drop, the preparation was fixed by light heating on a burner flame. Preparations were stained with a solution of acridine orange (1 : 10 000) for 2–3 min. Direct microscopy of preparations was performed using an Axioskop 2+ microscope (Carl Zeiss, Switzerland). For each preparation, 50–100 visual fields were examined. Based on the obtained data, the total number of bacteria and the length of fungal mycelium were estimated. The experiment was performed in triplicate.
Determination of the functional diversity of microorganisms by the multisubstrate testing (MST) method was carried out in accordance with MM no. 13-06 Methodology for Measuring the Intensity of Consumption of Test Substrates by the Microbial Community of Soils and Soil-Like Objects by the Photometric Method [4]. It is based on the analysis of the utilization of 47 organic monosubstrates of different groups (sugars, alcohols, salts of organic acids, and nitrogen-containing organic compounds and polymers) by the studied microbial community. To extract microorganisms, 35 mL of distilled water was added to 0.7 g of a mixed sample obtained by mixing 3 individual samples (from each soil sample). The resulting suspensions were shaken for 1.5 min at 3000 rpm on a Vortex-type orbital shaker and then centrifuged for 2 min at 2.2g. The obtained supernatant with the introduced microbial growth indicator (tetrazolium violet, which is reduced during the growth of microorganisms to formazan) was added to a 96-well Eco-Log test plate containing a set of test substrates. The filled plate was incubated for 3 days at 28°C. After that, the intensity of substrate consumption was estimated by determining the concentration of formazan by absorption at a wavelength of 510 nm on a Uniplan multiband photometer (ZAO Pikon, Russia). The data on the optical density in cells with different monosubstrates was used to calculate the average intensity of consumption of substrates of different nominal groups. In addition, the data were processed using the original ECOLOG software, and a three-parameter model of the rank distribution of substrate consumption by the soil microbial community was obtained [4]. As output parameters, we used the coefficient of the rank distribution of substrate utilization spectra (d), which reflects the stability of the community. In addition, biodiversity indices of the microbial community were assessed, such as the amount of consumed substrates N; specific metabolic work of the community W corresponding to the average intensity of substrate utilization, and the integral index of vitality G.
The contents of glyphosate and AMPA were determined by high-performance liquid chromatography with tandem mass spectrometric detection on an Ultimate 3000 liquid chromatograph (Dionex, USA) with a diode array detector and a Qtrap 3200 hybrid tandem triple quadrupole mass spectrometer (ABSciex, Canada) equipped with an ionization source electrospray. Aqueous extraction of the herbicide and its main metabolite was carried out according to [20]; chromatographic separation and mass spectrometric determination, according to [17].
Soil phytotoxicity was evaluated using a pot experiment. Preliminarily calibrated sterilized and soaked (24 h, 24°C) seeds of the common wheat Triticum aestivum L., variety L-1 were placed into pots with soil; only the hatched seeds were planted. For surface sterilization, the seeds were soaked for 30 min in 8% H2O2 solution and then washed 5–6 times with sterile distilled water [33]. Planting was carried out at a depth of ~1 cm, 5 seeds per pot. For cultivation, the pots with seeds were placed in a growing chamber for 21 days (photoperiod 12 h, 24°C). Water was added when needed. At the end of the experiment, the length of the shoots and air-dry aboveground biomass were determined. The experiment was performed in triplicate.
Statistical processing of the data consisted in calculation of the mean and standard deviation values. Comparison of the means for the variants without glyphosate (control) and with glyphosate for each day of sampling was carried out on the basis of the one-way ANOVA at the confidence level of 95%.
RESULTS
The content of glyphosate in the soil (Table 1) decreased from 9.6 to 60 μg/kg already a day after glyphosate application, however its most common metabolite, AMPA, was not detected. The results obtained agree with the data of previous researchers, who also noted the rapid disappearance of glyphosate in the soil due to its binding by the ligand exchange mechanism with soil oxides and clay minerals [14, 38]. The absence of AMPA in the studied samples may be explained by the binding of this metabolite with aluminum/iron oxides or soil organic matter, or it may indicate the predominant degradation of the herbicide with C–P bond cleavage (sarcosine pathway), which is usually observed in soils with a low content of available phosphorus [6]. This is consistent with the low availability of phosphates in the studied soil (175 mg/kg). The first stage of glyphosate degradation by the sarcosine pathway is the formation of sarcosine (N-methylglycine) and inorganic phosphate \({\text{PO}}_{4}^{{3 - }}.\) It is believed that the resulting metabolites are not toxic and are quickly utilized by soil microorganisms: phosphates are used as a source of phosphorus, and sarcosine is oxidized to glycine (which decomposes to NH3 and CO2) and formaldehyde, which is used in a complex formation with tetrahydrofolate, a coenzyme for the metabolism of amino and nucleic acids [14].
The observed subsequent further decrease in glyphosate concentration is in good agreement with the earlier assumptions on its utilization by various groups of soil microorganisms [29, 31]. A number of studies have shown that glyphosate is used as a substrate, in particular, by α-proteobacteria [18] and γ-proteobacteria [34], which results in an increase in their abundance in the soil.
The impact of glyphosate on the intensity of nitrogen fixation, nitrification, and denitrification is represented in Table 2. It can be seen that the values of all studied processes of nitrogen transformation are extremely low. This indicates the low biological activity of the soil, apparently associated with the low content of organic matter and the main biophilic elements. This is supported by the detected pronounced excess of potential activity of the processes over the actual one. For nitrogen fixation, the observed excess was 8 times or more, and for denitrification, 26 times or more. However, the only exception is the activity of nitrification: the maximum excess of potential activity over actual activity was low (1.3 times). Most likely, the observed differences are related to the addition of ammonium salt to initiate nitrification, while in the cases of nitrogen fixation and denitrification, it was glucose. This indicates that the low soil biological activity is associated mainly with the low content of readily available carbon sources.
A day after the application of glyphosate, there was an increase in the actual nitrogen fixation by 1.4 times; and after 8 days, by 1.8 times compared with the control. The possibility of a positive effect of glyphosate on the growth of nitrogen-fixing bacteria at the doses corresponding to the recommended ones was previously demonstrated on an example of a 24-hour cultivation of Azotobacter sp. on the Lowry–Bertrand medium in the presence of glyphosate [11]. In all other periods of sampling, no significant effect of glyphosate on either the actual or potential nitrogen-fixing activities in the soil was noted. At the same time, the dynamics patterns of nitrogen-fixing activity in the control soil and in the soil with the herbicide were similar: an increase of activity on the first day of incubation and a subsequent decrease. The absence of the effect of the long-term exposure of glyphosate on the activity of nitrogen fixation was previously shown in a similar experiment on soddy-podzolic soil at application doses of 0.5–50.0 mg/kg and 45-day exposure [2]. Thus, the results obtained indicate that the effect of glyphosate on nitrogen-fixing microorganisms manifests itself in the intensification of nitrogen fixation in the first days after application. Most likely, this is due to the rapid utilization of ammonium formed during the decay of glyphosate along the sarcosine pathway, which did not promote possibilities to a possible inhibition of nitrogenase activity by excessive ammonium ions in the soil. The inhibition of the growth of nitrogen-fixing bacteria in cases of over-application of glyphosate (in doses significantly higher than recommended ones) is known [11].
In contrast to nitrogen fixation, the effect of the herbicide on nitrifying activity is delayed. On the 8th day, the soil with glyphosate had a lower nitrifying activity, both actual and potential, amounting to only 59% of the control values. For potential nitrification, statistically significant inhibition (up to 84% of the control) persisted for 15 days. The negative effect of glyphosate on nitrification in agricultural soils was previously demonstrated [16, 45], although often researchers also noted the stimulating effect of the herbicide [37], or the absence of any effect [40]. Apparently, the factors determining the direction of glyphosate action on nitrifying activity are the characteristics of the microbial community of the soil, as well as the history of the application of glyphosate and fertilizers [36]. Soils continuously contaminated with glyphosate are known to develop a microbiome that is resistant to the toxic effects of the herbicide. It has been shown that treatment with glyphosate can lead to the selection of specific microbial communities capable to efficiently metabolize amine substrates, which explains the stimulating effect of the herbicide [37]. However, it has been previously shown that in the studied soil, on the contrary, the application of glyphosate leads to a decrease in the activity of utilization of nitrogen-containing organic compounds (Fig. 1), which may indirectly indicate the inhibition of heterotrophic nitrifiers, the predominance of which is usually observed in acidic soils [8]. According to [45], the addition of glyphosate to acidic soil (\({\text{p}}{{{\text{H}}}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}\) 5.0) led to a decrease in the number of copies of the amoA genes in it, which reflects the abundance of ammonium-oxidizing archaea and bacteria involved in the process of ammonia oxidation, the limiting stage of nitrification. Thus, a more complete assessment of the effect of glyphosate on the nitrifying capacity of the soil needs separate studies on autotrophic and heterotrophic nitrification, as well as the impact of the herbicide on particular stages of nitrification.
The most remote effect glyphosate application has on denitrification: actual denitrification in the presence of glyphosate was significantly (by three times) higher than in the control only 22 days after the herbicide application. Similar effects of stimulating denitrification are often noted by researchers [40, 41]. Apparently, the observed effect of increased denitrification is explained by the inhibition of nitrification noted on the 8th and 15th days after the application of glyphosate and, as a result, an increase in the content of available nitrates in the soil. Despite the fact that the potential enhancement of denitrification can be assessed as a negative impact of glyphosate on the environment, since N2O is a greenhouse gas, it can be expected that its effect is short-term, because no inhibition of nitrification was noted 21 days after the herbicide application. This assumption is confirmed by reports that the application of glyphosate does not lead to significant changes in N2O emission from soils or reduces it [28].
In general, it can be said that the inhibitory effect of glyphosate applied at the recommended doses on the processes of nitrogen transformation in the soil was noted only for nitrification, while the processes of nitrogen fixation and denitrification were stimulated by the herbicide. At the same time, the observed effects were of a short-term nature, probably associated with the rapid mineralization of glyphosate along the sarcosine pathway, which is not accompanied by the formation of toxic metabolites, such as AMPA. The absence of a pronounced unidirectional trend in the glyphosate effect on the processes of biological transformation of nitrogen is apparently due to the variety of possible herbicide-associated physiological and ecological responses of soil microorganisms, which were previously shown by a number of researchers [2, 3, 31].
The utilization of organic monosubstrates of various nominal groups by the studied microbial community, determined by the MST method showed a multidirectional response to the glyphosate application (Table 3). The dynamics of sugar utilization was similar in the control soil and the soil with glyphosate: by the 8th day of incubation, there was an increase in this indicator, and then a decrease. Apparently, the observed trend in this case reflects the usually observed dynamics of the microbial communities under laboratory conditions: the initial growth during “soil revival” followed by a decrease in the number of microorganisms, associated with the depletion of available substrates. The dynamics of utilization of polymers, although different from the dynamics of sugar utilization, had similar patterns: after an initial gradual decrease in the intensity of their consumption up to the 15th day of incubation, significant intensification of this process was observed.
In the case of nitrogen-containing organic compounds, on the contrary, the introduction of glyphosate led to a fundamental change in the dynamics of utilization of these substrates. In the control variant, similarly to the consumption of sugars, an increase in this indicator was followed by a subsequent decrease. However, in the presence of the herbicide, a constant decrease was observed. The revealed dynamics of the average intensity of utilization of nitrogen-containing substrates coincides with the dynamics of the abundance of fungi in the presence of glyphosate (Table 4) and, possibly, indicates their significant role in the nitrification process. It is known that organic nitrogen is the most preferred substrate for nitrification by micromycetes isolated from soddy-podzolic soils [8].
In general, microorganisms of the studied soil were characterized by uneven consumption of substrates of different nominal groups. The lowest utilization intensity was found for sugars and, on some days of incubation, for alcohols (Fig. 1a). At the same time, microorganisms of the studied soil were characterized by a high activity of the average consumption of nitrogen-containing organic compounds and polymers. The amount of consumed N substrates in the soil of the control variant did not exceed 34 out of 47, which indicates the instability of the community and is reflected in the high value of the calculated coefficient d (characterizing the steepness of the tail of the rank distribution) and in the low value of the integral index of vitality G. During the incubation, an initial increase in the specific metabolic work of the community W and its subsequent decrease were observed. This pattern coincides with the dynamics of nitrification and denitrification in the studied soil and corresponds to the revealed predominance of the consumption of nitrogen-containing organic compounds.
The introduction of glyphosate led to a slight decrease in the coefficient d in the first week of incubation. However, later its values sharply increased up to 2.13 with a simultaneous decrease in the specific metabolic work (W) by 40% in comparison to the control. This indicates a pronounced negative effect of glyphosate on soil microorganisms 21 days after application.
The determination of the total number of microorganisms showed that soil fungi turned out to be more sensitive to the action of the herbicide: 8 days after the application of glyphosate, their number was significantly lower than in the control variant and amounted to no more than 30–40% of the control values. On the contrary, the number of bacteria decreased under the action of glyphosate only in the first days after its application, and after 21 days it exceeded the control values by 40%. The noted trends coincide with the data of other researchers who noted the growth of bacteria, such as proteobacteria, in the presence of glyphosate under conditions of its decomposition mainly due to the disruption of C–P bonds [29]. This is explained by the fact that among microorganisms capable of degrading glyphosate (bacteria, fungi, micromycetes, and actinomycetes), bacteria play the key role in most cases [44].
The multidirectional effect of glyphosate on the abundance of soil bacteria and fungi led to the fact that, in general, the application of the herbicide did not affect the total carbon dioxide emission (Table 5). Our data are consistent with earlier published results of a meta-analysis of 36 studies demonstrating no effect of glyphosate applied at recommended doses on the CO2 emission [35].
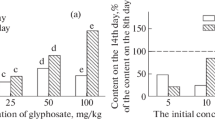
However, the deterioration of soil biological properties detected by the MST method caused the inhibition of the development of wheat plants in the soil in the presence of glyphosate. The growing of plants on the soil incubated with the herbicide for 8–22 days was accompanied by a significant decrease in plant biomass (Fig. 2a). The length of wheat plants grown on this soil was 73–92% of the control values; however, this decrease was not statistically significant (Fig. 2b). It should be emphasized that plant suppression was observed in the absence of AMPA in the soil and at the minimum concentrations of glyphosate in the soil (32–44 µg /kg), while at a higher herbicide content (60 µg/kg), the length and biomass of plants grown on the soil with glyphosate did not differ from the control. This indicates that the deterioration of the microbiological properties of the soil plays a decisive role in this case.
CONCLUSIONS
Glyphosate is one of the most widely applied herbicides. Despite the fact that numerous studies have shown its rapid disappearance from the soil due to binding and biodegradation, a number of studies have demonstrated its negative aftereffect on crop growth. The reason for this aftereffect is not always obvious. On the one hand, there are data on the release of glyphosate from complexes with soil minerals due to its displacement by phosphates entering the soil in the form of phosphate fertilizers. On the other hand, there are suggestions about the possible phytotoxicity of AMPA, the first and most common metabolite of glyphosate appearing during its biodegradation with a C–N bond cleavage. It has been shown for the first time that the introduction of glyphosate into a soil with a low content of available phosphorus and subsequent herbicide degradation by the sarcosine pathway with the C–P bond cleavage, which excludes the formation of toxic metabolites, leads to a pronounced negative effect on soil microorganisms 21 days after herbicide application. This is associated with a decrease in the functional biodiversity of the soil microbial community and is manifested both by an increase in the value of the rank distribution coefficient d and a decrease in the specific metabolic work W of the microbial community and in the integral vitality index G. The decrease in soil biological activity after soil treatment with glyphosate results in the suppression of wheat growth. This allows us to suggest that the deterioration of the microbiological properties of the soil is one of the possible causes of the negative aftereffect of the herbicide. An assessment of the impact of glyphosate on individual processes of the nitrogen biological conversion indicated inhibition of the nitrification (by 20–40%) and stimulation of nitrogen fixation (by 30–80%) and denitrification (by 300%) 21 days after the application of the herbicide. However, the observed effects are of a short-term nature and do not reflect the whole set of the main effects: no effect of glyphosate was found on the key integral indicator of biological activity, the carbon dioxide emission. Under the selected conditions, at the end of the experiment, the number of bacteria increased by 40%, while the number of micromycetes decreased by 70%. The results obtained point the necessity of further research. Along with the study of individual microbiological processes, a comprehensive assessment of the diversity of the soil microbial community and determination of the main pathway of glyphosate degradation under given conditions and the agrochemical characteristics of the soil reflecting the level of nutrient supply should be performed.
REFERENCES
E. V. Arinushkina, Manual on Soil Chemical Analyses (Mosk. Univ., Moscow, 1970) [in Russian].
M. V. Gorlenko, O. S. Yakimenko, M. V. Golichenkov, and N. V. Kostina, “Functional biodiversity of soil microbe colonies affected by organic substrates of different kinds,” Moscow Univ. Soil Sci. Bull. 67 (2), 71–78 (2012).
A. D. Zhelezova, N. A. Manucharova, and M. V. Gorlenko, “Structural and functional characteristics of the prokaryotic community of soddy-podzolic soil influenced by the herbicide glyphosate,” Moscow Univ. Soil Sci. Bull. 73 (2), 89–94 (2018).
Method for Measuring the Intensity of Consumption of Test Substrates by Microbial Communities of Soils and Soil-Like Objects by the Photometric Method: FR.1.37.2010.08619; PND FT 16.1.17-10 (Moscow, 2010).
Methods of Soil Microbiology and Biochemistry, Ed. by D. G. Zvyagintsev (Mosk. Univ., Moscow, 1991) [in Russian].
A. V. Sviridov, T. V. Shushkova, I. T. Ermakova, E. V. Ivanova, D. O. Epiktetov, and A. A. Leontievsky, “Microbial degradation of glyphosate herbicides (review),” Appl. Biochem. Microbiol. 51 (2), 188–195 (2015). https://doi.org/10.1134/S0003683815020209
A. L. Stepanov and L. V. Lysak, Methods of Gas Chromatography in Soil Microbiology (MAKS Press, Moscow, 2002) [in Russian].
M. M. Umarov, A. V. Kurakov, and A. L. Stepanov, Microbiological Transformation of Nitrogen in Soil (GEOS, Moscow, 2007) [in Russian].
O. I. Filippova, N. A. Kulikova, Ya. S. Bychkova, A. B. Volikov, and I. V. Perminova, “Delayed release of nitrogen from humic substances modified with aminoorganosilanes,” Probl. Agrokhim. Ekol., No. 1, 42–47 (2015).
L. A. Yuzganinova, Glyphosate: application in the Russian and global markets, Agroxxi.ru: Agro-Industrial Portal (Moscow, 1995–2022). https://www.agroxxi. ru/gazeta-zaschita-rastenii/zrast/glifosat-primenenie-na-rossiiskom-i-globalnom-rynkah.html. Cited July 27, 2022.
V. O. Adero, N. S. Raju, and M. Supreeth, “Effect of glyphosate herbicide on nitrogen fixing bacteria – Azotobacter species,” J. Environ. Chem. Toxicol. 4 (2), 1–7 (2020).
L. Aristilde, M. L. Reed, R. A. Wilkes, T. Youngster, M. A. Kukurugya, V. Katz, and C. R. S. Sasaki, “Glyphosate-induced specific and widespread perturbations in the metabolome of soil Pseudomonas species,” Front. Environ. Sci. 5, 34 (2017). https://doi.org/10.3389/fenvs.2017.00034
F. R. Atherton, M. J. Hall, C. H. Hassall, R. W. Lambert, W. J. Lloyd, P. S. Ringrose, and D. Westmacott, “Antibacterial activity and mechanism of action of phosphonopeptides based on aminomethylphosphonic acid,” Antimicrob. Agents Chemother. 22, 571–578 (1982).
O. K. Borggaard and L. Gimsing, “Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review,” Pest Manage. Sci. 64, 441–456 (2008). https://doi.org/10.1002/ps.1512
S. Bott, T. Tesfamariam, A. Kania, B. Eman, N. Aslan, V. Romheld, and G. Neumann, “Phytotoxicity of glyphosate soil residues re-mobilised by phosphate fertilization,” Plant Soil 342, 249–263 (2011). https://doi.org/10.1007/s11104-010-0689-3
S. M. Carlisle and J. T. Trevors, “Effect of the herbicide glyphosate on nitrification, denitrification, and acetylene reduction in soil,” Water, Air, Soil Pollut. 29, 189–203 (1986). https://doi.org/10.1007/BF00208408
M. X. Chen, Z. Y. Cao, Y. Jiang, and Z. W. Zhu, “Direct determination of glyphosate and its major metabolite, aminomethylphosphonic acid, in fruits and vegetables by mixed-mode hydrophilic interaction/weak anion-exchange liquid chromatography coupled with electrospray tandem mass spectrometry,” J. Chromatogr. A 1272, 90–99 (2013).
A. E. Cherni, D. Trabelsi, S. Chebil, F. Barhoumi, I. D. Rodríguez-Llorente, and K. Zribi, “Effect of glyphosate on enzymatic activities, Rhizobiaceae and total bacterial communities in an agricultural Tunisian soil,” Water, Air, Soil Pollut. 226, 145 (2015). https://doi.org/10.1007/s11270-014-2263-8
R. E. Dick and J. P. Quinn, “Glyphosate-degrading isolates from environmental samples: occurrence and pathways of degradation,” Appl. Microbiol Biotechnol. 43, 545–550 (1995). https://doi.org/10.1007/BF00218464
C. Druart, O. Delhomme, A. de Vaufleury, E. Ntcho, and M. Millet, “Optimization of extraction procedure and chromatographic separation of glyphosate, glufosinate and aminomethylphosphonic acid in soil,” Anal. Bioanal. Chem. 399, 1725–1732 (2011). https://doi.org/10.1007/s00216-010-4468-z
B. Fuchs, M. Laihonen, A. Muola, K. Saikkonen, P. I. Dobrev, R. Vankova, and M. A. Helander, “Glyphosate-based herbicide in soil differentially affects hormonal homeostasis and performance of non-target crop plants,” Front. Plant Sci. 12, 787958 (2022). https://doi.org/10.3389/fpls.2021.787958
M. P. Gomes, E. Smedbol, A. Chalifour, L. Hénault-Ethier, M. Labrecque, L. Lepage, M. Lucotte, and Ph. Juneau, “Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: an overview,” J. Exp. Bot. 65, 4691–4703 (2014). https://doi.org/10.1093/jxb/eru269
M. Helander, A. Pauna, K. Saikkonen, and I. Saloniemi, “Glyphosate residues in soil affect crop plant germination and growth,” Sci. Rep. 9, 19653 (2019). https://doi.org/10.1038/s41598-019-56195-3
M. Helander, I. Saloniemi, and K. Saikkonen, “Glyphosate in northern ecosystems,” Trends Plant Sci. 17, 569e574 (2012).
ISO 10694:1995 Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis).
M. B. Jenkins, M. A. Locke, K. N. Reddy, D. S. McChesney, and R. W. Steinriede, “Impact of glyphosate-resistant corn, glyphosate applications and tillage on soil nutrient ratios, exoenzyme activities and nutrient acquisition ratios,” Pest Manage. Sci. 73, 78–86 (2017).
N. A. Kulikova, O. I. Philippova, Y. S. Bychkova, A. B. Volikov, and I. V. Perminova, “Nitrogen release from natural and aminoorganosilane-modified humic substances,” in Functions of Natural Organic Matter in Changing Environment (Springer, Dordrecht, 2013). https://doi.org/10.1007/978-94-007-5634-2_84
K. M. Kyaw and K. Toyota, “Suppression of nitrous oxide production by the herbicides glyphosate and propanil in soils supplied with organic matter,” Soil Sci. Plant Nutr. 53, 441–447 (2007). https://doi.org/10.1111/j.1747-0765.2007.00151.x
S. H. Lancaster, E. B. Hollister, S. A. Senseman, and T. J. Gentry, “Effects of repeated glyphosate applications on soil microbial community composition and the mineralization of glyphosate,” Pest Manage. Sci. 66, 59–64 (2010). https://doi.org/10.1002/ps.1831
L. Leino, T. Tall, M. Helander, I. Saloniemi, K. Saikkonen, S. Ruuskanen, and P. Puigbò, “Classification of the glyphosate target enzyme (5-enolpyruvylshikimate-3-phosphate synthase) for assessing sensitivity of organisms to the herbicide,” J. Hazard. Mater. 408, 124556 (2020). https://doi.org/10.1016/j.jhazmat.2020.124556
N. Milosevic and M. Govedarica, “Effect of herbicides on microbiological properties of soil,” Matica Srp. J. Nat. Sci. 102, 5–21 (2002).
S. Munira, A. Farenhorst, D. Flaten, and C. Grant, “Phosphate fertilizer impacts on glyphosate sorption by soil,” Chemosphere 153, 471–477 (2016). https://doi.org/10.1016/j.chemosphere.2016.03.028
S. Nardi, D. Pizzeghello, C. Gessa, L. Ferrarese, L. Trainotti, and G. Casadoro, “A low molecular weight humic fraction on nitrate uptake and protein synthesis in maize seedlings,” Soil Biol. Biochem. 32, 415–419 (2000).
M. M. Newman, N. Hoilett, N. Lorenz, R. P. Dick, M. R. Liles, C. Ramsier, and J. W. Kloepper, “Glyphosate effects on soil rhizosphere-associated bacterial communities,” Sci. Total Environ. 543, 155–160 (2016). https://doi.org/10.1016/j.scitotenv.2015.11.008
D. B. Nguyen, M. T. Rose, T. J. Rose, S. G. Morris, and L. van Zwieten, “Impact of glyphosate on soil microbial biomass and respiration: a meta-analysis,” Soil Biol. Biochem. 92, 50–57 (2016). https://doi.org/10.1016/j.soilbio.2015.09.014
E. Nivelle, J. Verzeaux, A. Chabot, D. Roger, Q. Chesnais, A. Ameline, and M. Catterou, “Effects of glyphosate application and nitrogen fertilization on the soil and the consequences on aboveground and belowground interactions,” Geoderma 311, 45–57 (2018). https://doi.org/10.1016/j.geoderma.2017.10.0
E. Nivelle, J. Verzeaux, A. Chabot, D. Roger, F. Spicher, J. Lacoux, J. E. Nava-Saucedo, M. Catterou, and T. Tétu, “Does nitrogen fertilization history affects short-term microbial responses and chemical properties of soils submitted to different glyphosate concentrations,” PLoS One 12, e0178342 (2017).
E. Okada, J. L. Costa, and F. Bedmar, “Glyphosate dissipation in different soils under no-till and conventional tillage,” Pedosphere 29, 773–783 (2019).
V. Silva, L. Montanarella, A. Jones, O. Fernández–Ugalde, H. G. J. Mol, C. J. Ritsema, and V. Geissen, “Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union,” Sci. Total Environ. 621, 1352–1359 (2018). https://doi.org/10.1016/j.scitotenv.2017.10.093
G. W. Stratton and K. E. Stewart, “Effects of the herbicide glyphosate on nitrogen cycling in an acid forest soil,” Water, Air, Soil Pollut. 60, 231–247 (1991). https://doi.org/10.1007/BF00282625
M. Tenuta and E. G. Beauchamp, “Denitrification following herbicide application to a grass sward,” Can. J. Soil Sci. 76, 15–22 (1996).
A. H. C. van Bruggen, M. M. He, K. Shin, V. Mai, K. C. Jeong, M. R. Finckh, and J. G. Morris Jr., “Environmental and health effects of the herbicide glyphosate,” Sci. Total Environ. 616–617, 255–268 (2018). https://doi.org/10.1016/j.scitotenv.2017.10.309
R. M. Zablotowicz and K. N. Reddy, “Impact of glyphosate on the Bradyrhizobium japonicum symbiosis with glyphosate-resistant transgenic soybean: a mini review,” J. Environ. Qual. 33, 825–831 (2004).
H. Zhan, Y. Feng, X. Fan, and S. Chen, “Recent advances in glyphosate biodegradation,” Appl. Microbiol. Biotechnol. 102, 5033–5043 (2018). https://doi.org/10.1007/s00253-018-9035-0
M. Zhang, W. Wang, L. Tang, M. Heenan, and Z. Xu, “Effects of nitrification inhibitor and herbicides on nitrification, nitrite and nitrate consumptions and nitrous oxide emission in an Australian sugarcane soil,” Biol. Fertil. Soils 54, 697–706 (2018). https://doi.org/10.1007/s00374-018-1293-6
Funding
This study was carried out within the framework of the Development Program of the Interdisciplinary Scientific and Educational School of the Lomonosov Moscow State University “The Future of the Planet and Global Environmental Changes.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by D. Konyushkov
Rights and permissions
About this article
Cite this article
Kostina, N.V., Gorlenko, M.V., Mazurov, K.A. et al. Glyphosate Effects on Some Characteristics of Biological Activity and Phytotoxicity of Soddy-Podzolic Soil in a Short-Term Model Experiment. Eurasian Soil Sc. 56, 628–638 (2023). https://doi.org/10.1134/S1064229322602815
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229322602815