Abstract
Application of monoammonium phosphate has been demonstrated to re-immobilize glyphosate sorbed by soil under model laboratory experiment conditions. This effect was most pronounced in the gray forest soil (Haplic Phaeozem), where the concentration of herbicide in the presence of fertilizer was 3.6 times higher than in its absence. For soddy-podzolic soil (Albic Retisol) and leached Chernozem (Luvic Chernozem), this ratio was 1.5 and 2.8, respectively. Thus, the introduction of monoammonium phosphate into soils contaminated with glyphosate may result in an increase of the risk of herbicide migration into the neighboring environments. The estimated number of functional genes of bacteria responsible for glyphosate degradation by means of the C–P bond cleavage did not show statistically significant effect of the fertilizer on the number of copies of the phnJ gene, encoding the C–P lyase of α- and γ-proteobacteria. The release of glyphosate was not accompanied by any adverse effects on the length and biomass of wheat plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Glyphosate [N-(phosphomethyl)glycine] was put into agricultural practice in 1974 as a non-selective herbicide under the Roundup brand name. The patent for exclusive manufacturing of Roundup outside the USA expired in 1991 and in USA in 2000. This resulted in sharp decrease of herbicide prices and increase of the number of manufacturers. Glyphosate price decreased by 40% only in the first year after repeal of the patent, and currently it is manufactured in USA, Europe, Australia, and China [54]. In Russia, glyphosate is manufactured by Kirovo-Chepetsk Chemical Company. Moreover, the Orgsyntez Group companies planned the construction of glyphosate manufacturing plant with the production output up to 30 thousand tons per year in 2020.
Glyphosate has a unique mode of action: it inhibits 5-enolpyruvylshikimate-3-phosphate synthase; an enzyme participated in the synthesis of aromatic amino acids, presented in plants, bacteria, and fungi, but not found in animals [24]. This allowed holding out glyphosate as safe pesticide [33], and application of this pesticide increased year by year: it increased only in the USA from 0.36 thousand tons in 1974 to 12.5 thousand tons in 1995, i.e. almost 35 times [15]. Introduction into agricultural practice in 1996 of genetically modified (GM) crops resistant to glyphosate was added incentive, and glyphosate became recently the most widely used herbicide [24] with annual use (as of 2014) about 826 thousand tons [15]. The glyphosate-based herbicides are certified for use not only in agriculture but also for private users; and this raises the risk of this herbicide abuse. Sum total 86 glyphosate-based herbicides were recommended for use in our country in 2019 (https://www.agroxxi.ru/ goshandbook). Application of glyphosate implies spraying of plants; in the case of such treatment, 70–95% of pesticide enters the soil [7]. Additionally, glyphosate get into soil with plant residues. This causes accumulation of glyphosate residues in the soil, so that its concentration may reach more than 1.5 mg/kg of soil [12, 52].
Glyphosate is quickly inactivated in soil owing to sorption and mineralization [7, 16]. There are two primary ways of biological degradation of glyphosate: with breakdown of C–N bond and aminomethylphosphonic acid (AMPA) formation, or with breakdown of C–P bond and formation of sarcosine (N-methylglycine) and inorganic phosphate \({\text{PO}}_{4}^{{3-}}\) [9, 22]. Subsequently, glyoxylate is a source of carbon, and phosphates are a source of phosphorus. Degradation of glyphosate with AMPA formation is more common among bacteria. It can be carried out by bacteria isolated from the media contaminated by glyphosate as well as from the habitats not treated by the herbicide earlier. The C–N bond is destructed with the help of enzyme glyphosate-oxidoreductase. This metabolic pathway was found in bacteria of Bacteroidetes, Actinobacteria, Firmicutes, and Proteobacteria phyla [27, 60]. Degradation of glyphosate with breakdown of C–P bond was performed with the help of C–P-lyases. Such destruction of glyphosate was found in representatives of the Proteobacteria phylum genera, such as α-proteobacteria Ochrobacterium anthropi, Rhizobium sp. [25], betaproteobacteria Achromobacter sp. [26], Alcaligenes sp., and Comamonas sp. [61], and γ-proteobacteria Pseudomonas sp. [9] and Enterobacter sp. [38], and rarely in actinobacteria [46]. It was found that among microorganisms capable of degrading glyphosate (bacteria, fungi, micromycetes, and actinomycetes) bacteria play a key role [61]. Of 26 described strains degrading glyphosate, 19 ones belong to proteobacteria [61]. So, the input of glyphosate to soil often results in the increase of the abundance of proteobacteria [40], and particularly of α-proteobacteria [19] and γ-proteobacteria [43].
C–P-lyases participate in catabolism of phosphonates; usually one operon (phn) encoding this metabolic pathway is presented in the genome of bacteria. In the case of presence of this enzyme system, glyphosate can be the only source of phosphorus for bacteria in case of its deficiency, but this is rarely found in natural habitats [9]. Many bacteria cannot use glyphosate as the only source of phosphorus, but they perform the breakdown of C–P bond in glyphosate under the conditions of low content of available phosphates [22]. So, microbiological degradation of glyphosate in soils depends directly on the presence of easily available sources of phosphorus, inorganic phosphates.
Additionally to degradation, sorption of glyphosate on soil particles results in inactivation of this substance in the soil, because it is a strong chelating agent due to presence in its molecule of carboxyl, phosphonate, and amino groups, which allow glyphosate binding with clay particles and iron and aluminum in the form of oxides and hydroxides [16, 44]. Despite active sorption of glyphosate by soils, this process is reversible. Among other issues, the assessment of fertilizers effects on glyphosate behavior, and first of all, of phosphorus fertilizer, is one of unsolved challenges. This is caused by the fact, that phosphates often play a role of herbicide antagonists from the sorption viewpoint, because they compete with it for combining sites [16, 41]. It was found for some soils that application of phosphorus fertilizer can result in releasing of glyphosate or its primary metabolite AMPA, which is also phytotoxic [17, 34]. Moreover, it was demonstrated that high content of phosphates in the soil could inhibit degradation of glyphosate to sarcosine, being a not toxic product of glyphosate degradation [39]. Due to expanding world application of phosphorus fertilizers, which accounted for about 48.6 million tons in 2016 (http:// www.fao.org/faostat/), many researches are focused recently on interactions between phosphates and glyphosate in soil [16, 17, 21, 31, 32, 34, 37, 41]. It was found that phosphates in most cases compete with glyphosate for binding sites, but this does not occur in some soils [16, 31]. So, the information about the influence of phosphorus fertilizers on the interaction between glyphosate and soil is often discrepant, and this determines the necessity to assess this interaction under the conditions of particular soil [16, 31]. Such researches for Russian soils are absent. The processes of glyphosate sorption–desorption affect in turn the rate of its microbiological degradation: it was found that absorbed herbicide is mineralized by soil microflora to a lesser extent than the herbicide in free form [16].
Hence, inactivation of glyphosate in soils is determined by a complex of conditions, among which a leading position is occupied by the adsorption capability of soil towards the herbicide and peculiarities of microbial community structure. Both these factors depend significantly on the presence of easily available phosphates. Our research was focused on evaluating the possibility of glyphosate mobilization in soddy-podzolic, gray forest soil, and chernozem under the influence of phosphates, and the influence of this process on the abundance of functional genes of bacteria responsible for glyphosate degradation with C–P bond breakdown.
OBJECTS AND METHODS
Collection and characteristics of soil samples. Soil samples were taken from humus horizons from the depth of 0–5 cm. Composite sample was made of five individual samples taken with ’envelope’ method in sampling plot about 1 m2, from which average sample was obtained. The list of studied soils is presented in Table 1. Sum total three soil samples were taken in different soil-geographical zones: soddy-podzolic (Albic Retisol) soils (Moscow oblast, Solnechnogorsk district), gray forest (Haplic Phaeozem) soils (Tula oblast, Shchekino district), and leached chernozems (Luvic Chernic Phaeozem) (Lipetsk oblast, Danki district).
Primary soil chemical characteristics. Actual soil acidity was determined according to [10]. The measurements were carried out in the unit Hanna Microprocessor pH Meter pH 211, electrode HI 1230 (Hanna Instruments Inc., USA). Concentrations of organic carbon (C) and nitrogen (N) in soil were determined with catalytic combustion at 960°С [35] in oxygen flow in element analyzer Vario Macro Cube (Elementar Analysen Systeme GmbH, Germany). Concentrations of mobile forms of phosphorus in soddy-podzolic (SP) and gray forest (GF) soils were determined with Kirsanov method modified by TsINAO [3] and in leached chernozem (LC) with Chirikov method modified by TsINAO [2]. When determining phosphorus, optical density of solutions was measured in spectrophotometer PortLab512 (Portlab, Great Britain) at wavelength 710 nm. Concentration of mineral nitrogen was calculated as the sum of free ions \({\text{NH}}_{4}^{ + }\) and \({\text{NO}}_{3}^{-},\) determined according to [8] with ion-specific electrodes EKOM-NH4 and EKOM-NO3, respectively (Research and Production Enterprise Ekoniks, RF). Primary agrochemical characteristics of studied soils are presented in Table 1.
Model laboratory experiments. To assess the effects of monoammonium phosphate (MAP) on glyphosate immobilization, an experiment was carried out, which included four variants: 1, control (introduction of distilled water); 2, MAP; 3, glyphosate; 4, glyphosate + MAP. The experiment was carried out in triplicate. Incubation was performed in two stages: at the first stage, herbicide was equilibrated in the soil; at the second stage, MAP was introduced, and the release of glyphosate under the influence of MAP was evaluated. The duration of these stages was 7 and 14 days, respectively.
In order to provide concentrations of herbicide in the soil 8 mg/kg, the glyphosate solutions (Roundup WS 360 g/L, JSC Avgust, RF) were added to weighed portions of air-dry soil of variants glyphosate and glyphosate + MAP, preliminary sieved through the sieve 2 mm mesh. It is understood that the herbicide is fixed mostly in the upper soil layer 0–5 cm, so application of glyphosate in recommended rates 2–8 L/ha provided concentration of herbicide 2.4–9.6 mg/kg of soil [17]. Hence, concentration of glyphosate used in our experiment corresponded to the rate of herbicide application 6.7 L/ha recommended for the control of harmful perennial weeds: field bindweed, Bermuda grass, creeping thistle, etc. (6–8 L/ha). In order to provide uniform distribution of herbicide in the soil sample, the volume of introduced solution was taken in such a way as to moist soil to 70% of maximum water capacity. The mass of air-dry soil in the beaker was 100 g; beaker volume was 100 ml. Equal volumes of distilled water were added into the beakers of variants: control and MAP. The beakers were placed at the first stage of incubation in growth chamber (day/night 12 h/12 h, temperature 24°С, without watering) for 7 days to equilibrate the herbicide in soil with subsequent sampling for glyphosate content, AMPA, and microbiological analyses. The samples were taken in sixfold, because soils before fertilizer application did not differ in variants: control and MAP (introduction of distilled water) and variants: glyphosate and glyphosate + MAP (introduction of herbicide solution).
At the second stage of incubation, solution of MAP (mark А, N : P 12 : 61, JSC Lifosa, Lithuania), water-soluble nitrogen-phosphorus fertilizer recommended for application at early stages of plant development, in the period of root system formation, was added to the beakers of variants: MAP and glyphosate + MAP. Soil concentration of MAP was 0.34 g P2O5/kg of soil, and this approximately corresponded to the rate 120 kg P2O5/ha. The volume of introduced solution was taken in such a way as to moist soil to 70% of maximum water capacity. Similar volumes of distilled water were added into the beakers of variants control and glyphosate.
As there are data indicating soil phytotoxicity of glyphosate in the case of introduction of phosphates [17], we carried out the assessment of the herbicide influence on plant growth under these conditions. For this purpose, we sowed sprouting grains of common wheat Triticum aestivum L., variety L-1, 5 grains/pot. The pots were placed into growth chamber (day/night 12 h/12 h, temperature 24°С) for plant growth, watering was performed on an as-needed basis. After 14 days of growing, the second stage of incubation was completed, plant length and biomass were measured, and soil samples were taken to determine glyphosate, AMPA, microbiological analyses, and concentrations of mobile phosphorus and mineral nitrogen in triplicate.
Determination of concentrations of glyphosate and AMPA in the soil. Water extraction of the herbicide and its primary metabolite was carried out according to [23]; chromatographic separation and mass-spectrometric determination were carried out according to [57]. Weighed 1-g portions of soil were placed into plastic tubes; 4 mL of deionized water, the mixture was intensely agitated and stayed on agitator for extraction for 30 min. Then the extract was separated from sediment via centrifuging at 17 000 g during 10 min. Concentrations of glyphosate and AMPA were determined with high-performance liquid chromatography with tandem mass-spectrometric detecting (HPLC-MS/MS). The HPLC-MS/MS system included liquid chromatograph Ultimate 3000 (Dionex, USA) with diode array detector and hybrid triple quadrupole mass spectrometer Qtrap 3200 (ABSciex, Canada) equipped with ionization source via electrospraying. Chromatographic separation of glyphosate and AMPA was carried out on the column Shodex NH2P-50 2D (150 × 2.1 mm, 3 µm) with a precolumn. Mobile phase A: water solution of 5 mM ammonium acetate, рН 11. Mobile phase B: acetonitrile : water 20 : 80. Separation was carried out in gradient mode at following proportions of mobile phases A : B: 0–3 min 0 : 100, 3–8 min 50 : 50, 8–28 min 0 : 100. Flow rate 0.25 mL/min, the temperature of column thermostat 35°С. Ionization was carried out in negative mode. Detecting was carried out in monitoring of selected reactions (MSR) mode. The m/z 168 → m/z 63 was used as the main transition for glyphosate and m/z 110 → m/z 63 for AMPA. Additional transitions for the herbicide and its metabolite were m/z 168 → m/z 149,9 and m/z 110 → m/z 79 respectively. MSR of transitions and parameters of detecting was carried out in automatic mode with the help of software deliverables (Analyst 1.5.1).
To account for the matrix effect (i.e. effect of sample matrix on ionization efficiency and degree and efficiency of solute extraction), every sample was analyzed in duplicate, and the mixture of glyphosate and AMPA was introduced into soil in the course of one of these determinations. Correction factor k was calculated as the ratio of the areas of peaks of analyzed component after introduction of addition into weighed portion and extracting of the sample without addition to the area of the peak of analyzed component in standard solution with concentration equal to concentration in extract with addition. Concentration of glyphosate and AMPA were calculated taking into account the correction factor. Detection limits of glyphosate in studied soils taking into account matrix effect were for SP, GF, and LC: 0.60, 0.44 and 0.69 mg/kg. Analogous values for AMPA were: 25, 15, and 7 mg/kg. Detection limits for glyphosate and AMPA without accounting for the matrix effect were 0.08 and 0.14 mg/kg respectively.
Molecular-biological analyses were focused on determining the number of copies of 16S rRNA genes of bacteria reflecting the total abundance of bacteria in soil, genes phnJ of α- and γ-proteobacteria encoding C–P-lyases, and genes phoC and phoD encoding acid and alkaline phosphatases, respectively (Table 2). Isolation of DNA was carried out using the SileksMagNA set for soils according to recommendations of manufacturer with modification at the stage of sample homogenization: we used homogenizer Precellys 24 (Bertin Technologies, France), program 5. Polymerase chain reaction (PCR) was carried out in thermocycler C1000 Thermal Cycler with CFX96 Real-Time System (Bio-Rad Laboratories, USA). Oligonucleotide primers used in the work are presented in Table 2. The number of copies of ribosomal bacterial genes was determined in the samples using the method described in [4]. Protocols for quantitative PCR for the other genes were taken from the works presented in Table 2. Reaction volume for PCR was 20 µL, including 10 µL of BioMaster HS-qPCRSYBR Blue (Biolabmix, Russia), 1 µL of preparation of soil DNA and primers in concentration 0.5–0.8 µM. Melting profiles were analyzed to evaluate reaction specificity. The number of gene copies was estimated in the program CFX Manager (Bio-Rad, CIF). Standards in concentrations 103 to 109 gene copies/µL were used. The standards for all genes excluding bacterial 16S rRNA were obtained by the way of purification of PCR products and quantitative determination of concentration with the help of fluorimeter Qubit3 (Thermo Fisher Scientific, USA).
Statistical treatment of data included calculation of mean value and standard deviation. Significance of difference between mean values was evaluated with two-way ANOVA with calculating the least significant difference (LSD). Confidence limit р = 0.05 was selected.
RESULTS AND DISCUSSION
The performed experiments demonstrated that glyphosate was most strongly inactivated during the first 7 days of incubation in LC: 1.3% of introduced herbicide was found in soil. This value was 4.4% for SP soil and 4.9% for GF soil. Observed inactivation was caused by the process of herbicide sorption as well as by its degradation. When рН increased in the range 4–8, negative charge of glyphosate increased owing to dissociation of carboxyl group (pKa 5.44), and consequently the pH increase was accompanied by the decrease of sorption capacity of soil to the herbicide [16]. On the other hand, the decrease of glyphosate fixation by soils was observed also in the case of increased mobile phosphorus content in soil [21]. So, we can assume that in this case, high degree of inactivation in LC, characterized by maximal pH and concentration of mobile phosphorus among studied soils, was caused by glyphosate degradation. This possibility is suggested by maximal abundance of bacteria indicated by the number of copies of genes 16S rRNA (Table 3).
AMPA, the main metabolite of glyphosate, was found in no variant. This could be connected with high fixing capacity of soils to this compound, which exceeded in most cases the fixing capacity of soils towards glyphosate [51]. It was found, when studying sorption of glyphosate and AMPA on 17 soils, that average value of Freundlich constant for glyphosate equaled 144, and for AMPA 164 mg/kg (L/mg)–nf, and Freundlich constant for AMPA was 1.2–2.3 higher than for glyphosate in 11 cases. On the other hand, detection limit of AMPA in soil, determined by disturbing action of soil matrix, was also much greater than that for glyphosate and 10–40 times exceeded it. Finally, the absence of AMPA can be explained by predomination of sarcosine way of the herbicide degradation under selected conditions.
Introduction of glyphosate resulted in multidirectional changes in the number of copies of functional genes under selected conditions. Treatment with the herbicide did not affect the number of studied genes (Table 1) in SP soil with minimal pH value and the lowest content of mobile phosphorus (Table 1). At the same time, the decrease of the number of some studied target genes was observed in GF soil and LC, which had higher values of actual acidity and greater concentration of mobile phosphorus. The most pronounced effect of glyphosate was in GF soil, where the decrease was observed in the presence of the herbicide in concentrations of all studied target genes, excluding phoC, for which the decrease of the number of copies from 2.0 × 105 to 1.5 × 105 was statistically insignificant. Obtained results are in agreement with the data of other researchers reporting the decrease in the number of bacteria after single application of glyphosate in the soil with high content of mobile phosphorus and the absence of similar effect in the soil with low value of this index [14]. It is interesting to note that the number of copies of the gene encoding the C–P lyase of γ-proteobacteria decreased in variants with glyphosate in GF soil and in LC. It can be assumed that the abundance of γ-proteobacteria in variant with glyphosate relative to control variant was due to less active development of prokaryotic community. The increase of the content of phnJ gene of α-proteobacteria encoding C–P lyase, responsible for glyphosate degradation via the sarcosine way, was found in LC in variants with glyphosate. This result agrees well with observed earlier maximum degradation of glyphosate in LC.
The comparison of concentration of 16S rRNA with residual concentration of glyphosate in soils after first week of incubation demonstrated that there was significant inverse correlation between these parameters (r = –0.97). This apparently indicated the probability of partial suppression of bacteria by glyphosate under selected conditions or the decrease of the rate of glyphosate destruction in the case of low abundance of bacteria. Despite the fact that the absence of pronounced negative effect of glyphosate on microbial community was noted in most researches [60], some researchers reported little decrease of the number of bacteria [13, 50] in the presence of glyphosate, or the increase of the ratio of carbon of fungal biomass to bacterial biomass [47]. Since the degradation of glyphosate in soil in most cases is the co-metabolic process [16], the observed disagreement suggests the necessity to study the successional changes in soil under the influence of this herbicide, rather than obtain single estimates.
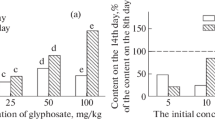
Application of MAP resulted in mobilization of glyphosate in all studied soils: herbicide concentration in variant glyphosate + MAP by the end of incubation exceeded not only the value of variant glyphosate, but also the herbicide content before the beginning of the second stage of incubation (Fig. 1). This indicates that the disappearance of glyphosate from soils in the first week of incubation was caused not only by herbicide degradation, but also by a reversible absorption as the result of phosphates application. This effect was most pronounced in GF soil: concentration of glyphosate in the presence of MAP was 3.6 times higher than in the case of fertilizer absence. These values for SP soil and LC were 1.5 and 2.8 times, respectively.
The obtained results are in agreement with the data of other researchers, who reported the possibility of glyphosate release from soil after application of phosphorus fertilizer [41, 45]. Both glyphosate and phosphates can be fixed on mineral surfaces with variable charge such as clay minerals and Al and Fe oxides and hydroxides, forming the bonds Al–O–P or Fe–O–P [16]. So, the content of phosphates, which can compete for binding sites with glyphosate, is generally regarded as one of the main factors affecting the sorption-desorption behavior of glyphosate.
The decrease was observed of glyphosate content in SP and GF soils in the case of MAP absence in comparison with its content in soil after the first stage of incubation, and this decrease was attributed to herbicide degradation. Unlike SP and GF soils, the change of herbicide content in LC was not observed under these conditions, and this indicates that glyphosate was completely inactivated in LC owing to binding and degradation during a week. This fact well agreed with previously found fact that the maximal degree of herbicide disappearance was observed in the chernozem. This was attested by the absence of increase in bacteria abundance estimated on the basis of the number of copies of genes 16S rRNA during further incubation both in the presence and in the absence of MAP (Table 4). Significant increase of bacteria abundance was observed in SP and GF soils, where further incubation was accompanied by glyphosate degradation. Obtained results agreed with the data of researchers who reported the probability of short-time increase of the number of bacteria in soil under the influence of glyphosate [43, 60]. Taking into consideration that the decrease of the number of copies of gene 16S rRNA in GF soil was recorded after first 7 days of incubation after glyphosate introduction, the increase of this index during further incubation can attest to the development of bacteria resulting of the decrease of glyphosate concentration and the decrease of its suppressive effect.
Introduction of glyphosate in SP soil in the presence of MAP as well as in its absence promoted the increasing concentration of copies of gene phoC (Table 4), encoding acid phosphatase, despite the recorded increase of concentration of mobile phosphorus in all variants with MAP introduced in 2.4–4.2 times (Table 5). This fact does not agree with formerly obtained data on the decrease of phosphatase activity in the case of glyphosate introduction [55]. The number of copies of phosphatase genes in soil depended on pH and increased in the cases of available phosphorus deficiency and organic fertilizer application [18]. Application of mineral phosphorus fertilizer can cause the decrease as well as increase of abundance of genes phoC and phoD [48]. An increase of abundance of gene phoC in the presence of glyphosate recorded in our experiment could be caused by nonspecific activity of phoC with using glyphosate or products of herbicide destruction as a substrate. We did not find any changes in the abundance of genes phnJ of α- and γ-proteobacteria after introduction of MA at the second stage of experiment. It should be noted that the increased abundance of bacteria in all microcosms in comparison with control in SP and GF soils with low content of mobile phosphorus, and this can attest to a more intense development of community in the presence of phosphorus-containing substrate. This fact agreed with earlier obtained data on the increase of respiratory activity of microbial community as a result of glyphosate introduction into some soils [5, 42].
Along with the increasing content of available phosphorus, MAP is the source of mineral nitrogen in ammonium form, the factor, which also could affect soil microflora. So, the content of mineral nitrogen was determined in soil samples after the second stage of incubation. It was found that the application of MAP to SP and GF soils resulted in the increase of ammonium nitrogen concentration, whereas the changes in LC were statistically insignificant. This apparently attested to low nitrifying capacity under selected conditions of SP and GF soils and high capacity of LC. This assumption was confirmed by the fact that the increase of nitrate nitrogen content was observed in variants MAP and glyphosate + MAP in LC (Table 5).
Correlation analysis demonstrated direct correlation (r = 0.83) between the number of copies of 16S rRNA and concentration of mineral nitrogen in ammonium form, and this could indicate the contribution of nitrifiers into the total bacteria population under selected conditions and be explained by the introduction of available nitrogen in ammonium form.
Evaluation of the content of mineral nitrogen demonstrated that introduction of glyphosate in SP soil resulted in the decrease of available nitrogen content in ammonium form. Some researchers reported earlier similar effect after soil treatment by the herbicide [20, 49, 53]. Glyphosate at the rate 4 kg/ha caused significant decrease of nitrogen-fixing capability of Azotobacter chroococcum and A. vinelandii, and the rates 20–28 kg/ha caused practically complete inhibiting of this process [49]. Moreover, the observed decrease of ammonium nitrogen content could be connected with intensification of nitrification process, reported in some works [11, 53]. It was found that introduction of glyphosate could initiate the increase of the fraction of ammonium-oxidizing bacteria in the case of single application of the herbicide [11].
Despite a significant increase of glyphosate concentration in soils after the introduction of MAP, the herbicide did not produce any adverse effect on the length and biomass of plants, (Fig. 2), and this well agreed with commonly-known fact that glyphosate is not a soil herbicide.
Obtained results attested to a high rate of glyphosate inactivation under selected conditions. Additionally, a positive effect was recorded in LC of MAP, it was observed in the absence of herbicide as well as in its presence: application of fertilizer promoted the increase of the length (by 10–15%) and mass (16–28%) of wheat seedlings. The observed positive effect of MAP on plant growth in chernozem could be apparently explained by low content of mineral nitrogen. Soils fall into the group with very low supply of plants with nitrogen and very high fertilizer requirement, when nitrogen content is less than 15 (5.3 ± 0.2 mg/kg for LC) mg/kg [1].
CONCLUSIONS
Glyphosate applied in recommended rates was inactivated under the conditions of laboratory experiment in soddy-podzolic and gray forest soils and in leached chernozem by 95–99% during a week. Maximal inactivation was observed in chernozem. A temporary decrease of bacteria abundance estimated by the concentration of the number of gene copies of 16S rRNA was observed, when concentration of mobile phosphorus was above 6 mg/kg and actual acidity was above 5. Significant inverse correlation (r = –0.97) was demonstrated between residual concentration of glyphosate and the content of 16S rRNA.
Application of MAP caused mobilization of glyphosate in all studied soils. This effect was most pronounced in gray forest soil, where concentration of glyphosate in the presence of MAP was 3.6 times higher than in the absence of this fertilizer. This value was 1.5 and 2.8 for soddy-podzolic soil and chernozems, respectively. Despite the 2.4–4.2 times increase of available phosphorus content in the case of MAP application, there was no statistically significant change in the number of copies of genes phnJ, encoding C–P-lyase of α- and γ-proteobacteria. The decrease of ammonium nitrogen content and increase of nitrate nitrogen content in the presence of glyphosate was observed in soddy-podzolic soil, and this fact can suggest intensification of nitrification process. Release of glyphosate was not accompanied by adverse effects on the length and biomass of wheat plants. The results obtained suggest that application of phosphorus fertilizers in soils contaminated by glyphosate can be accompanied by the increase of the risk of herbicide migration into adjacent environments.
REFERENCES
G. P. Gamzikov, “Forecast of nitrogen abundance in soils and demand of field crops n nitrogen fertilizers,” Innovatsii Prod. Bezop., No. 3, 11–20 (2015).
GOST (State Standard) 26204-91: Soils. Determination of Mobile Compounds of Phosphorus and Potassium by Chirikov’s Method Modified by TsINAO (Izd. Standartov, Moscow, 1992) [in Russian].
GOST (State Standard) 26207-91: Soils. Determination of Mobile Compounds of Phosphorus and Potassium by Kirsanov’s Method Modified by TsINAO (Izd. Standartov, Moscow, 1992) [in Russian].
A. D. Zhelezova, A. K. Tkhakakhova, N. V. Yaroslavtseva, S. A. Garbuz, V. I. Lazarev, B. M. Kogut, O. V. Kutovaya, and V. A. Kholodov, “Microbiological parameters of aggregates in typical chernozems of long-term field experiments,” Eurasian Soil Sci. 50, 701–707 (2017).https://doi.org/10.1134/S1064229317060126
A. D. Zhelezova, N. A. Manucharova, and M. V. Gorlenko, “Structural and functional characteristics of the prokaryotic community of soddy-podzolic soil influenced by the herbicide glyphosate,” Moscow Univ. Soil Sci. Bull. 73, 89–94 (2018).
Classification and Diagnostics of Soils of the Soviet Union (Kolos, Moscow, 1977) [in Russian].
N. A. Kulikova and G. F. Lebedeva, Herbicides and Ecological Aspects in Their Application (Librokom, Moscow, 2015) [in Russian].
Practical Manual on Agrochemistry, Ed. by V. G. Mineev (Moscow State Univ., Moscow, 2001) [in Russian].
A. V. Sviridov, T. V. Shushkova, I. T. Ermakova, E. V. Ivanova, D. O. Epiktetov, and A. A. Leontievsky, “Microbial degradation of glyphosate herbicides (review),” Appl. Biochem. Microbiol. 51, 188–195 (2015).
Theory and Practice of the Chemical Analysis of Soils, Ed. by L. A. Vorob’eva (GEOS, Moscow, 2006) [in Russian].
M. Allegrini, E. del V. Gomez, and M. C. Zabaloy, “Repeated glyphosate exposure induces shifts in nitrifying communities and metabolism of phenylpropanoids,” Soil Biol. Biochem. 105, 206–215 (2017). https://doi.org/10.1016/j.soilbio.2016.11.024
V. C. Aparicio, E. De Geronimo, D. Marino, J. Primost, P. Carriquiriborde, and J. L. Costa, “Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins,” Chemosphere 93, 1866–1873 (2013). https://doi.org/10.1016/j.chemosphere.2013.06.041
A. S. F. Araujo, R. T. R. Monteiro, and R. B. Abarkeli, “Effect of glyphosate on the microbial activity of two Brazilian soils,” Chemosphere 52, 799–804 (2003). https://doi.org/10.1016/S0045-6535(03)00266-2
M. L. Banks, A. C. Kennedy, R. J. Kremer, and F. Eivazi, “Soil microbial community response to surfactants and herbicides in two soils,” Appl. Soil Ecol. 74, 12–20 (2014). https://doi.org/10.1016/j.apsoil.2013.08.018
Ch. M. Benbrook, “Trends in glyphosate herbicide use in the United States and globally,” Environ. Sci. Eur. 28 (3), (2016). https://doi.org/10.1186/s12302-016-0070-0
O. K. Borggaard and L. Gimsing, “Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review,” Pest Manage. Sci. 64, 441–456 (2008). https://doi.org/10.1002/ps.1512
S. Bott, T. Tesfamariam, A. Kania, B. Eman, N. Aslan, V. Römheld, and G. Neumann, “Phytotoxicity of glyphosate soil residues re-mobilised by phosphate fertilization,” Plant Soil 342, 249–263 (2011). https://doi.org/10.1007/s11104-010-0689-3
X. Chen, N. Jiang, Z. Chen, J. Tian, N. Sun, M. Xu, and L. Chen, “Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials,” App. Soil Ecol. 119, 197–204 (2017). https://doi.org/10.1016/j.apsoil.2017.06.019
A. E. Cherni, D. Trabelsi, S. Chebil, F. Barhoumi, I. D. Rodríguez-Llorente, and K. Zribi, “Effect of glyphosate on enzymatic activities, Rhizobiaceae and total bacterial communities in an agricultural Tunisian soil,” Water, Air Soil Pollut. 226, 145 (2015). https://doi.org/10.1007/s11270-014-2263-8
V. Damin, H. C. J. Franco, M. F. Moraes, A. Franco, and P. C. O. Trivelin, “Nitrogen loss in Brachiaria decumbens after application of glyphosate or glufosinate-ammonium,” Sci. Agricola 65, 402–407 (2008).
H. de Jonge, L. W. de Jonge, O. H. Jacobsen, T. Yamaguchi, and P. Moldrup, “Glyphosate sorption in soils of different pH and phosphorus content,” Soil Sci. 166, 230–238 (2001).
R. E. Dick and J. P. Quinn, “Glyphosate-degrading isolates from environmental samples: occurrence and pathways of degradation,” Appl. Microbiol. Biotechnol. 43, 545–550 (1995). https://doi.org/10.1007/BF00218464
C. Druart, O. Delhomme, A. de Vaufleury, E. Ntcho, and M. Millet, “Optimization of extraction procedure and chromatographic separation of glyphosate, glufosinate and aminomethylphosphonic acid in soil,” Anal. Bioanal. Chem. 399, 1725–1732 (2011). https://doi.org/10.1007/s00216-010-4468-z
T. Erban, V. Stehlik, B. Sopko, M. Markovic, M. Seifrtova, T. Halesova, and P. Kovaricek, “The different behaviors of glyphosate and AMPA in compost-amended soil,” Chemosphere 207, 78–83 (2018). https://doi.org/10.1016/j.chemosphere.2018.05.004
I. T. Ermakova, T. Shushkova, A. A. Leontievsky, N. I. Kiseleva, M. Zharikov, and G. A. Zharikov, “Bioremediation of glyphosate-contaminated soils,” Appl. Microbiol. Biotechnol. 88 (2), 585–594 (2010). https://doi.org/10.1007/s00253-010-2775-0
I. T. Ermakova, T. V. Shushkova, A. V. Sviridov, N. F. Zelenkova, N. G. Vinokurova, B. P. Baskunov, and A. A. Leontievsky, “Organophosphonates utilization by soil strains of Ochrobactrum anthropi and Achromobacter sp.,” Arch. Microbiol. 199, 665–675 (2017). https://doi.org/10.1007/s00203-017-1343-8
J. Fan, G. Yang, H. Zhao, G. Shi, Y. Geng, T. Hou, and K. Yao, “Isolation, identification and characterization of a glyphosate-degrading bacterium, Bacillus cereus CB4, from soil,” J. Gen. Appl. Microbiol. 58, 263–271 (2012). https://doi.org/10.2323/jgam.58.263
N. Fierer, J. A. Jackson, R. Vilgalys, and R. B. Jackson, “Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays,” Appl. Environ. Microbiol. 71, 4117–4120 (2005). https://doi.org/10.1128/AEM.71.7.4117
T. D. Fraser, D. H. Lynch, J. Gaiero, K. Khosla, and K. E. Dunfield, “Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields,” Appl. Soil Ecol. 111, 48–56 (2017). https://doi.org/10.1016/j.apsoil.2016.11.013
A. L. Gimsing and O. K. Borggaard, “Effect of phosphate on the adsorption of glyphosate on soils, clay minerals and oxides,” Int. J. Environ. Anal. Chem. 82, 545–552 (2002).
A. L. Gimsing, O. K. Borggaard, and M. Bang, “Influence of soil composition on adsorption of glyphosate and phosphate by contrasting Danish surface soils,” Eur. J. Soil Sci. 55, 183–191 (2004). https://doi.org/10.1046/j.1365-2389.2003.00585.x
A. L. Gimsing, C. Szilas, and O. K. Borggaard, “Sorption of glyphosate and phosphate by variable-charge tropical soils from Tanzania,” Geoderma 138, 127–132 (2007). https://doi.org/10.1016/j.geoderma.2006.11.001
Glyphosate Issue Paper: Evaluation of Carcinogenic Potential (US Environmental Protection Agency, Washington, 2016).
M. P. Gomes, S. Maccario, M. Lucotte, M. Labrecque, and Ph. Juneau, “Consequences of phosphate application on glyphosate uptake by roots: impacts for environmental management practices,” Sci. Total Environ. 537, 115–119 (2015). https://doi.org/10.1016/j.scitotenv.2015.07.054
ISO 10694:1995: Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis) (International Organization for Standardization, Geneva, 1995).
IUSS Working Group WRB, World Reference Base for Soil Resources 2014, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, World Soil Resources Reports No. 106 (UN Food and Agriculture Organization, Rome, 2014).
R. G. Kanissery, A. Welsh, and G. K. Sims, “Effect of soil aeration and phosphate addition on the microbial bioavailability of carbon-14-glyphosate,” J. Environ. Qual. 44, 137–144 (2015). https://doi.org/10.2134/jeq2014.08.0331
Y. V. Kryuchkova, G. L. Burygin, N. E. Gogoleva, Y. V. Gogolev, M. P. Chernyshova, O. E. Makarov, E. E. Fedorov, and O. V. Turkovskaya, “Isolation and characterization of a glyphosate-degrading rhizosphere strain, Enterobacter cloacae K7,” Microbiol. Res. 169, 99–105 (2014). https://doi.org/10.1016/j.micres.2013.03.002
D. la Cecilia and F. Maggi, “Analysis of glyphosate degradation in a soil microcosm,” Environ. Pollut. 233, 201–207 (2018). https://doi.org/10.1016/j.envpol.2017.10.017
S. H. Lancaster, E. B. Hollister, S. A. Senseman, and T. J. Gentry, “Effects of repeated glyphosate applications on soil microbial community composition and the mineralization of glyphosate,” Pest Manage. Sci. 66, 59–64 (2010). https://doi.org/10.1002/ps.1831
S. Munira, A. Farenhorst, D. Flaten, and C. Grant, “Phosphate fertilizer impacts on glyphosate sorption by soil,” Chemosphere 153, 471–477 (2016). https://doi.org/10.1016/j.chemosphere.2016.03.028
D. B. Nguyen, M. T. Rose, T. J. Rose, S. G. Morris, and L. van Zwieten, “Impact of glyphosate on soil microbial biomass and respiration: a meta-analysis,” Soil Biol. Biochem. 92, 50–57 (2016). https://doi.org/10.1016/j.soilbio.2015.09.014
M. M. Newman, N. Hoilett, N. Lorenz, R. P. Dick, M. R. Liles, C. Ramsier, and J. W. Kloepper, “Glyphosate effects on soil rhizosphere-associated bacterial communities,” Sci. Total Environ. 543, 155–160 (2016). https://doi.org/10.1016/j.scitotenv.2015.11.008
E. Okada, J. L. Costa, and F. Bedmar, “Glyphosate dissipation in different soils under no-till and conventional tillage,” Pedosphere, (2017). https://doi.org/10.1016/S1002-0160(17)60430-2
I. A. Ololade, N. A. Oladoja, F. F. Oloye, F. Alomaja, D. D. Akerele, J. Iwaye, and P. Aikpokpodion, “Sorption of glyphosate on soil components: the roles of metal oxides and organic materials,” Soil Sediment Contam. 23, 571–585 (2014). https://doi.org/10.1080/15320383.2014846900
R. Pipke and N. Amrhein, “Carbon-phosphorus lyase activity in permeabilized cells of Arthrobacter sp. GLP-1,” FEBS Lett. 236, 135–138 (1988). https://doi.org/10.1016/0014-5793(88)80301-6
J. R. Powell, D. J. Levy-Booth, R. H. Gulden, W. L. Asbil, R. G. Campbell, K. E. Dunfield, A. S. Hamill, M. M. Hart, S. Lerat, R. E. Nurse, K. P. Pauls, P. H. Sikkema, C. J. Swanton, J. T. Trevors, and J. N. Klironomos, “Effects of genetically modified, herbicide-tolerant crops and their management on soil food web properties and crop litter decomposition,” J. Appl. Ecol. 46, 388–396 (2009). https://doi.org/10.1111/j.1365-2664.2009.01617.x
S. A. Ragot, M. A. Kertesz, and E. K. Bünemann, “phoD alkaline phosphatase gene diversity in soil,” Appl. Environ. Microbiol. 81, 7281–7289 (2015). https://doi.org/10.1128/aem.01823-15
A. Santos and M. Flores, “Effects of glyphosate on nitrogen fixation of free-living heterotrophic bacteria,” Lett. Appl. Microbiol. 20, 349–352 (1995). https://doi.org/10.1111/j.1472-765X.1995.tb01318.x
A. Shehata, M. Kühnert, S. Haufe, and M. Krüger, “Neutralization of the antimicrobial effect of glyphosate by humic acid in vitro,” Chemosphere 104, 258–261 (2014). https://doi.org/10.1016/j.chemosphere.2013.10.064
P. Sidoli, N. Baran, and R. Angulo-Jaramillo, “Glyphosate and AMPA adsorption in soils: laboratory experiments and pedotransfer rules,” Environ. Sci. Pollut. Res. 23, 5733–5742 (2016). https://doi.org/10.1007/s11356-015-5796-5
V. Silva, L. Montanarella, A. Jones, O. Fernández-Ugalde, H. G. J. Mol, C. J. Ritsema, and V. Geissen, “Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union,” Sci. Total Environ. 621, 1352–1359 (2018). https://doi.org/10.1016/j.scitotenv.2017.10.09
G. W. Stratton and K. E. Stewart, “Effects of the herbicide glyphosate on nitrogen cycling in an acid forest soil,” Water, Air, Soil Pollut. 60, 231–247 (1991). https://doi.org/10.1007/BF00282625
A. Székács and B. Darvas, “Forty years with glyphosate,” in Herbicides—Properties, Synthesis and Control of Weeds (InTech, Rijeka, 2012), pp. 247–284. https://doi.org/10.5772/32491
M. Tejada, “Evolution of soil biological properties after addition of glyphosate, diflufenican and glyphosate + diflufenican herbicides,” Chemosphere 76, 365–373 (2009). https://doi.org/10.1016/j.chemosphere.2009.03.040
C. Wang, X. Lin, L. Li, L. Lin, and S. Lin, “Glyphosate shapes a dinoflagellate-associated bacterial community while supporting algal growth as sole phosphorus source,” Front. Microbiol. 8, (2017). https://doi.org/10.3389/fmicb.2017.02530
M. X. Chen, Z. Y. Cao, Y. Jiang, and Z. W. Zhu, “Direct determination of glyphosate and its major metabolite, aminomethylphosphonic acid, in fruits and vegetables by mixed-mode hydrophilic interaction/weak anion-exchange liquid chromatography coupled with electrospray tandem mass spectrometry,” J. Chromatogr. A 1272, 90–99 (2013). https://doi.org/10.1016/j.chroma.2012.11.069
M. Yao, C. Henny, and J. A. Maresca, “Freshwater bacteria release methane as a by-product of phosphorus acquisition,” Appl. Environ. Microbiol. 82, 6994–7003 (2016). https://doi.org/10.1128/AEM.02399-16
M. C. Zabaloy, J. L. Garland, and M. A. Gomez, “An integrated approach to evaluate the impacts of the herbicides glyphosate, 2,4-D and metsulfuron-methyl on soil microbial communities in the Pampas region, Argentina,” Appl. Soil Ecol. 40, 1–12 (2012). https://doi.org/10.1016/j.apsoil.2008.02.004
M. C. Zabaloy, G. P. Zanini, V. Bianchinotti, M. A. Gomez, and J. L. Garland, “Herbicides in the soil environment: linkage between bioavailability and microbial ecology,” in Herbicides, Theory and Applications (InTech, Rijeka, 2011), pp. 161–192. https://doi.org/10.5772/12880
H. Zhan, Y. Feng, X. Fan, and S. Chen, “Recent advances in glyphosate biodegradation,” Appl. Microbiol. Biotechnol. 102, 5033–5043 (2018). https://doi.org/10.1007/s00253-018-9035-0
Funding
This study was supported by the Russian Science Foundation, project no. 19-16-00053 (refinement of the methods of molecular-biological analyses); soil sampling and soil analyses were performed within the framework of the federal budget theme (CITIS no. 116020110002-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Translated by T. Chicheva
Rights and permissions
About this article
Cite this article
Kulikova, N.A., Zhelezova, A.D., Voropanov, M.G. et al. Monoammonium Phosphate Effects on Glyphosate in Soils: Mobilization, Phytotoxicity, and Alteration of the Microbial Community. Eurasian Soil Sc. 53, 787–797 (2020). https://doi.org/10.1134/S106422932006006X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106422932006006X