Abstract
Agricultural expansion is a major driver of biodiversity loss, especially in the megadiverse tropics. Rice is among the world’s most important food crops, invariably affecting biodiversity worldwide. Although the effects of habitat conversion to rice crops on biodiversity are not completely understood, landscape modification often creates conditions that benefit some species and excludes others. We conducted an integrative evaluation of the effects that habitat conversion to irrigated rice crops has on anuran communities from a Cerrado-Amazon ecotone. We adopted a multidimensional approach to compare anuran communities from agricultural and pristine environments considering (i) taxonomic metrics; (ii) functional and phylogenetic diversity; (iii) selected and excluded traits and (iv) body condition indices. When compared to their pristine counterparts, agricultural waterbodies showed increased functional divergence and decreased species diversity and functional richness. Furthermore, agricultural anuran communities exhibited lower phylogenetic diversity. Nonetheless, taxonomic diversity did not vary significantly, suggesting that it should not be used without complementary metrics. Species with small range, habitat specialization, small clutches and large body size were excluded from rice crops. Furthermore, frogs showed lower body condition in crops than in pristine areas. Understanding how species traits correlate with specific responses to agriculture will allow better predictions of the functional effects of anthropogenic land-use. Maintaining high diversity in anthropogenic environments is important for ecosystem resilience because diverse communities are more likely to hold multiple species capable of contributing to ecological functions. Our results show that converting natural vegetation to irrigated rice crops drives many species to local extinction, and resilient species to exhibit lower body condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deforestation is a major driver of biodiversity loss, especially in the megadiverse Neotropics, where it is mainly caused by agricultural expansion (García-Barrios et al. 2009). Agriculture and cattle farming are known to alter communities and ecosystems (Williams-Guillén and Perfecto 2010; Frishkoff et al. 2014). Several studies have reported negative effects of habitat conversion to irrigated rice crops on biodiversity (e.g. Attademo et al. 2011; Hyne et al. 2009). However, agricultural wetlands have also been considered valuable for birds and amphibians (Czech and Parsons 2002; Elphick and Oring 2003; Bambaradeniya and Amarasinghe 2004; Lepš et al. 2006) and even functional equivalents to natural wetlands.
Rice is an important food crop, occupying 11% of the world’s arable land (Kole et al. 2010). Latin America and the Caribbean produce 4.1% of the world’s rice production, half of which comes solely from Brazil (FAO 2011). Given the extension and location of rice fields worldwide, it is important to determine if these agricultural wetlands can maintain biodiverse and resilient communities. This takes special significance in the Neotropics, where rice production meets important social and economic demands, and biodiversity is especially high and unique.
The effects of anthropogenic landscape modification are complex, often originating new environmental, compositional and configurational conditions for biodiversity. This concept, defined as environmental filtering, suggests that the environment acts as a filter, favoring species with particular traits over others (Lebrija-Trejos et al. 2010). The environmental filters originated by disturbances often favor generalist species, creating communities with species richness similar and even higher than in pristine environments. In the last case, local species richness may be higher, while diversity and species turnover remain unchanged (Dornelas et al. 2014). General patterns of environmental filtering have been identified for multiple organisms (Lebrija-Trejos et al. 2010; Hanspach et al. 2012; Maitner et al. 2012; Kivlin et al. 2014). Traits such as body size and geographic range have been identified as determinant for species resiliency to disturbances (Poff 1997; McIntyre 2008). Going beyond a species richness-based approach, incorporating environmental filtering of communities may hold great promise as a predictive framework to assist conservation planning and management of anthropogenic environments, favoring species with traits associated with decreased resilience (Hanspach et al. 2012). To attain more reliable deductions on the effects human activities have on communities, we must go beyond species number and account for different components of biodiversity.
Wetlands are important breeding habitats for many amphibian species, and irrigated rice fields are used to forage and reproduce. Due to their biphasic life cycle, highly permeable skin and ectothermic physiology, amphibians are very dependent on environmental quality and vulnerable to its change (Niemi and McDonald 2004). They play keystone roles in ecosystem functioning, acting as both predators and prey, enable nutrient transport between aquatic and terrestrial systems, and are among the most diverse and abundant vertebrates (Wells 2010; Blaustein et al. 2011). Amphibians can hence be used as surrogates to evaluate the effects of human disturbances on communities and ecosystems (Davic and Welsh 2004).
Here we conduct an integrative evaluation of the effects of habitat conversion to rice fields on anuran communities in a Cerrado-Amazon ecotone. This region has three large rivers (Araguaia, Formoso and Javaés), hosting the world’s largest continuous area of irrigated rice, with over 500,000 ha. As an ecotone, it has unique biodiversity, resulting from constant and historical contact between species typical of the Cerrado and the Amazon biomes. We adopted a multidimensional approach to assess (i) how taxonomic diversity, species richness and evenness are modified; (ii) how functional and phylogenetic diversity are affected; (iii) which anuran traits are selected and excluded in Neotropical agricultural landscapes; and (iv) use body condition indices to assess population fitness under agricultural pressure. We expect that indices considering functional and phylogenetic diversity will provide better assessments of community alteration than indices based on taxonomy and abundance, since species–environment relationships are assumed to be mediated via functional traits (e.g., Suding et al. 2008). Furthermore, we expect rice fields to pose different environmental filters on anuran communities, resulting in more homogeneous communities, with marked selection for traits that ensure persistence on anthropogenic landscapes. Finally, we hypothesize that, although generalist species are apparently successful in these environments, body condition indices will be lower than in pristine areas, which may suggest that agricultural environments are unable to host healthy generalist anuran communities. This is the first study to simultaneously assess taxonomic, functional and phylogenetic dimensions of anuran communities, along with body condition, within a Neotropical agricultural landscape.
Materials and methods
Study area
This study took place in the states of Goiás and Tocantins, Brazil. Both pristine and agricultural study areas are located in an Amazon-Cerrado ecotone region. This ecotone has a very characteristic vegetation, with species typical of rainforest and savanna biomes coexisting and forming a specific phytophisiognomy.
There are four types of rice production in Brazil, depending on topography, soil type and condition, temperature, pluviometry and available technology. Irrigated and upland rice fields are the most expressive regarding crop area and yield, accounting for 67 and 33% of the annual production, respectively (IBGE 2005). While upland rice fields are distributed on montane areas, irrigated rice is grown in periodically flooded regions, usually close to large rivers. The Cerrado is considered Brazil’s “water tank”. Its large rivers, periodically flooded areas and suitable climatic and territorial extension, hold great agricultural potential for irrigated rice crops (Santos and Rabelo 2004).
Sampled rice fields are located in the municipal district of Lagoa da Confusão (Tocantins). Pristine areas are located in Pium, Ilha do Bananal (Tocantins) and Monte Alto (Goiás). The region is a seasonally flooded area, with two large rivers (Formoso and Javaés) and a very pronounced rainy season from October to April. This highly unique ecotone is characterized by the presence of ipucas, “islands” of closed native vegetation that remain flooded longer than the surrounding areas, and sustain biodiversity adapted to this conditions, as an endemic rodent (Rhipidomys ipukensis) was found within these structures (Rocha et al. 2011). If an ipuca is destroyed to make place for crops, it will result in low crop yields because when the native vegetation is removed, the ipuca area remains flooded for longer, thus becoming inappropriate for agriculture. The ipucas exist only in the state of Tocantins, making the agricultural landscapes of this state quite peculiar, with vast crop areas speckled by many “islands” of native vegetation with variable dimensions.
Irrigated rice fields in the Cerrado-Amazon ecotone present a dynamic hydrologic regime, with variation between aquatic and terrestrial phases. Rice fields hold surface water throughout the rice growing phase (December–April), being completely drained right before harvest (Santos and Rabelo 2004). After rice harvesting, fields may remain with or without other crops, such as soybeans or watermelons, depending on the landowner’s will. Besides rice fields, the region’s landscape is also occupied by pasture areas for cattle, emus or water buffalos.
The area here referred to as pristine and used as a reference in this study, is occupied by natural Cerrado-Amazon ecotone vegetation, with no significant anthropogenic activities.
Sampling design
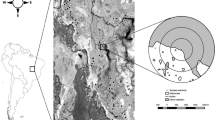
To ensure we represented most of the environmental variability of this agricultural landscape, we randomly chose a total of 10 rice fields along the Rio Formoso irrigation project (Fig. 1b). Chosen rice fields had similar management, but varied in size, ipuca and waterbody abundance and size. We selected 4–5 lentic waterbodies for anuran sampling in each rice field, totaling 35 waterbodies sampled in rice fields (Fig. 1d). All waterbodies were embedded inside rice quadrats, or located less than 50 m from rice fields. Waterbodies were chosen based on type, size and hydroperiod. The pristine area is occupied by typical Cerrado-Amazon ecotone vegetation, and has numerous natural lentic waterbodies. We chose 30 natural lentic waterbodies for anuran sampling, in order to assess the pristine community composition (Fig. 1c). We chose small (25 m2) to large (1200 m2) temporary lentic waterbodies in both agricultural and pristine areas. To ensure spatial independence, all sampled waterbodies (pristine and agricultural) were at least 450 m apart (Dodd 2010).
Study areas. a Map of Brazil with study area location and delimitation of the country’s five biomes: Amazon (AM), Cerrado (CE), Caatinga (CA), Atlantic Forest (AF), Pantanal (PA), and Pampas (PP); b Geographic location of both pristine and agricultural study areas. White dots represent pristine sampling sites and dark dots represent agricultural sampling sites. Lighter areas represent agricultural lands while darker areas represent native vegetation. c Detail of pristine study area (10°7′25.56″S, 49°31′10.66″W), with sampling sites represented by white dots; d Detail of agricultural study area (10°40′4.18″S, 49°49′53.18″W), with sampling sites represented by black dots
Anuran surveys
Agricultural and pristine surveys were conducted during two rainy seasons, from February to May of 2013, and from October to May of 2014. Anuran sampling was performed by two trained observers, using nocturnal visual encounter surveys along a 10 m wide margin of each waterbody, for approximately 30 min. Every individual seen perched on the vegetation, on the ground, under the leaf litter, logs or stones was caught, identified, measured, weighed, photographed and then released near the site of origin. Each waterbody was sampled 10 times.
Trait data
We compiled life history and ecological traits for every species encountered in the agricultural and pristine areas. We compiled information on 12 traits for each species: activity, habits, habitat, fossorial behavior, adult snout-vent length (SVL), breeding site, breeding strategy, clutch size, parental care, breeding season, breeding pattern and geographic range size. Traits were chosen based on perceived importance for determining species resilience to agricultural activities (Table A1) and on data availability. Trait data were compiled from the literature, the AmphibiaWeb and IUCN online databases. If published data were unavailable, we relied on expert opinion.
Data analysis
Sampling efficiency and species richness
To assess the effectiveness of our sampling effort, we fitted species accumulation curves for the agricultural and pristine areas. We calculated observed species richness, estimated species richness (Chao1 estimator), species diversity (Shannon–Weaver’s H) and species evenness (Pielou’sJ) for every site sampled in both agricultural and pristine areas, using the Vegan package (Oksanen et al. 2015) in program R version 3.1.2 (R Core Team 2014).
Taxonomic diversity
We calculated taxonomic diversity and taxonomic distinctness (Clarke and Warwick 2001) based on information derived from a hierarchical taxonomic tree. Mean values of taxonomic distinctness and diversity are considered to be less susceptible to variability in sample size than species richness (Clarke and Warwick 2001). We used taxonomic classification of all species sampled in both agricultural and pristine areas. Taxonomic indices were calculated using package vegan (Oksanen et al. 2010) on program R.
Functional diversity
Functional diversity describes the variability in ecological attributes among species, expressing ecosystem resistance, resilience and functioning (Petchey and Gaston 2006). Mason et al. (2005) divided functional diversity into three independent components: the amount of trait space filled by the community (functional richness), the divergence of abundance distribution in the occupied trait space (functional divergence) and the evenness of distribution of abundance in trait space (functional evenness). We quantified functional richness according to Villéger et al. (2008), which corresponds to the volume inside the hull that contains all trait combinations represented in the community. Functional richness is only influenced by the identity of species and more particularly by the most extreme species (in terms of functional traits), which delimitate the convex hull. We quantified functional evenness and functional divergence according to Villéger et al. (2008). Functional evenness includes both the regularity of species distribution and the regularity of their abundances along the “skeleton” of the functional volume occupied. It decreases when functional distances among species are less even or when abundances are less evenly distributed among species, i.e. when the main abundances belong to functionally close species. Functional divergence, the third facet of functional diversity, describes whether higher abundances are close to the volume borders. It approaches zero when highly abundant species are very close to the center of gravity of the volume occupied and approaches unity when highly abundant species are very distant from the center of gravity. There are other two important components to consider, functional distance and functional group richness. Functional distance is the mean distance in multidimensional trait space of individual species to the centroid of all species being unaffected by species richness by construction (Laliberté and Legendre 2010). Functional group richness represents the number of functional groups per community, as well as the abundance of each group in each community. These facets are complementary and describe the distribution of species and their abundances within the functional space.
All of the above mentioned components of functional diversity were computed for both areas, based on trait values and species relative abundances. To assess differences among indices from agricultural and pristine areas, we used t tests or Wilcoxon–Mann–Whitney tests for non-parametric data. All indices were calculated using packages FD (Laliberté et al. 2015), Ape (Paradis et al. 2004) and Picante (Kembel et al. 2010) in program R.
Null model validation
Most changes in functional diversity components cannot be interpreted directly, since communities differ in species richness. Thus, we must not assess if observed diversity indices are significantly different between the agricultural and pristine areas, but if diversity indices are significantly different between agricultural and pristine areas after randomizing the samples between the areas. We tested the null hypothesis of no change in pristine and agricultural communities, designing an appropriate randomization procedure to test community changes in diversity indices (Swenson 2014). Our null models were independent swap models, which randomize community data, while maintaining species occurrence frequency and sampling species richness for each community. The randomization process was carried out 999 times for each index and both pristine and agricultural areas, and the P value associated with the null hypothesis (no community effect) rejection was estimated (Manly 2006). We tested whether the actual functional diversity for each community was significantly higher or lower than the null FD distribution, at α = 0.05. This approach allowed us to determine if changes in functional diversity simply reflected species richness, or if species composition and trait diversity varied in important ways between agricultural and pristine areas. In addition, a standardized effect size was calculated for each index and area, to measure the statistical amount of deviation of the observed index of community structure from the distribution of simulated indices (Gotelli and McCabe 2002). Null models and respective significance metrics were generated on program R.
Phylogenetic diversity
Phylogenetic diversity describes the evolutionary differences among species based on times since divergence from a common ancestor (Faith 1992), representing an estimate of phylogenetically conserved ecological and phenotypic differences among species (Cavender-Bares et al. 2009). This dimension may characterize the long-term evolutionary potential of specific clades to respond or adapt to current and future environments. We used the phylogenetic tree built by Isaac et al. (2012) from the amphibian ‘tree of life’ described by Frost et al. (2006), with 5713 amphibian species and respective branch lengths. We pruned this supertree to build a smaller phylogenetic tree with every species sampled in the pristine and agricultural areas. All operations related to pruning the original Isaac et al. (2012) supertree were performed using package Ape (Paradis et al. 2004) in program R. We quantified the phylogenetic diversity with three different measures: Faith’s index of phylogenetic diversity (Faith 1992), mean phylogenetic distance and mean nearest phylogenetic taxon distance (Webb et al. 2002). Faith’s index of phylogenetic diversity is defined as the minimum total length of the phylogenetic branches connecting the species together on the phylogenetic tree. The abundance-weighted measures mean phylogenetic distance and mean nearest phylogenetic taxon distance are respectively the average distance between two random individuals in a sample and the average distance to the closest heterospecific individual for all individuals in the sample (Webb et al. 2008). The branch lengths on the phylogram were used as distance measures. Mean phylogenetic distance is a measure of the tree-wide phylogenetic relatedness of the community, while mean nearest phylogenetic taxon distance focuses more on the relationships between the tips of the phylogenetic tree (Kraft et al. 2007). Measures of phylogenetic diversity were calculated with the Picante package (Kembel et al. 2010) in program R.
Anuran trait association with agricultural landscapes
To identify which traits were selected and excluded by land use, we used machine learning techniques to build classification and regression trees. Although these tools have been previously used in ecology and recommended for conservation practice and wildlife management (Oliver et al. 2012), their application is still limited (Olden et al. 2008). Decision and regression trees are designed to identify nonlinear, context-dependent relationships between multiple correlated predictor variables (Olden et al. 2008). They do not assume a specific distribution of predictor variables or data independence, avoiding potential concerns about pseudoreplication and alleviating the need for explicit phylogenetic control (Bielby et al. 2009). Predictor variables may appear repeatedly in the model as necessary, a fundamental difference from the single-predictor variables of linear models. Finally, these analyses produce graphical outputs that quantify and summarize the interactions in easily interpretable formats.
Because traditional tree models can be unstable, we also used random forest models, which combine the predictions of many independent decision-tree models to produce robust results regarding variable importance (Breiman 2001). However, because random forest models do not produce a visual representation of trait selection, we chose to also fit traditional classification and regression tree models. We used packages random forest (Liaw and Wiener 2015) and rpart (Therneau and Atkinson 1997) on program R to determine the main predictors of anuran presence and species relative abundance in rice fields. Trees are built by repeatedly partitioning the dataset into a nested series of mutually exclusive groups, each group as homogenous as possible regarding the response variable. Homogeneity is measured by the mean decrease in accuracy. Branches or split points in the tree are determined by considering all possible splits of all predictor variables and selecting the split that results in the most homogenous subgroups for the data. Lower branches are afterwards pruned by 10-fold cross-validation to produce an optimal tree, balancing complexity (i.e., number of nodes) with prediction accuracy. The smallest tree with an error rate within 1 standard error of the minimum-error tree is selected as the optimal tree.
We quantified overall model accuracy using the percentage of species correctly classified, the percentage of species not present correctly classified (specificity), and the percentage of species present correctly classified (sensitivity). We assessed the accuracy of the relative abundance model using cross-validation from package rpart (Therneau and Atkinson 1997) in program R.
Body condition index
The original method of estimating body condition involved the quantification of fat deposits and was fatal for the studied individuals (Blackwell 2002). A non-destructive alternative to the above-mentioned method is the use of body condition indices based on allometric relationships between length and weight. We extracted body condition indices from the residuals of the regression of body mass logarithm on snout-vent length logarithm (Jakob et al. 1996; Peig and Green 2010). To evaluate if body condition differed between arboreal and terrestrial frogs from pristine and crop areas, we calculated body condition indices for three treefrog genera (Dendropsohpus, Boana and Hypsiboas—D. nanus, D. minutus, D. melanargyreus, D. rubicundulus, B. albopunctata, B. gr. semilineatus, B. raniceps, H. lundii) and one terrestrial genus (Leptodactylus—L. latrans; L. gr. melanonotus, L. fuscus, L. pustulatus, L. labyrinthicus, L. mystaceus; L. mystacinus; L. petersii; L. troglodytes). Hypsiboas represents larger treefrogs while Dendropsophus represents smaller treefrogs. Samples consisted of 264 individuals of Dendropsophus, 179 Boana/Hypsiboas and 701 Leptodactylus. Half of the datasets corresponded to individuals from pristine areas, and the other half to individuals collected from rice fields. Differences in body condition indices from frogs collected in crops and in pristine waterbodies were tested using the nonparametric Kolmogorov–Smirnov test in R.
Results
Sampling efficiency and species richness
Sampling was effective in capturing the vast majority of the species present in rice fields, but sampling effort on pristine areas showed that the intensification of sampling effort could increase richness (Table 1; Fig. 2). Species richness was significantly higher in pristine areas than in rice fields (Table 1, Fig. A.1). The Hylidae family was represented by 7 species in the agricultural area and 17 species in the pristine area, the Leptodactylidae family was represented by 5 species in the agricultural area and 16 species in the pristine area, the Microhylidae family was represented by 1 species in the agricultural area and 3 species in the pristine area, while the Bufonidae family was represented by 1 species in the agricultural area and 3 species in the pristine area.
Taxonomic diversity
Differences between taxonomic metrics in pristine and agricultural areas were not significant (Table 1).
Functional diversity
Functional richness was significantly higher in pristine areas than in rice fields (W = 19, p = 0.02). Functional divergence was significantly lower in pristine areas than in rice fields (t(12.7) = 3.02, p = 0.01). Regarding functional trait clusters, cluster 1 only had 40% of the species present in pristine areas represented in rice fields, cluster 2 had 30%, cluster 3 had 31% and cluster 4 had 25% (Fig. 2). Cluster 3 was significantly more abundant in pristine areas than in rice fields (t(3.923) = 9.04, p = 0.01) (Fig. 2).
Null model validation
Observed functional richness in rice fields was significantly lower than null expectation 73% of the times (Table 2). Faith’s phylogenetic index is strongly affected by species richness. Observed mean phylogenetic distance in rice fields was significantly lower than randomly expected 82% of the times. Observed mean phylogenetic distance to the nearest taxon in rice fields was significantly lower than null expectation 77% of the times (Table 2). Hence, significant differences in functional and phylogenetic diversity metrics are not due to differences in species richness, but largely attributed to trait composition and community phylogenetic structure (Fig. 3).
Functional trait dendrogram (a), with every species observed during this study, considering both pristine and agricultural areas. Species with asterisk are present in agricultural areas. Boxplots representing the relative abundances of the corresponding four functional clusters (b–e) on agricultural (black box) and pristine (grey box) areas
Phylogenetic diversity
Faith’s index of phylogenetic diversity (t(10.35) = 4.378, p = 0.02) and mean phylogenetic distance (t(10.86) = 7.12, p = 0.003) were significantly lower in rice fields than in pristine areas. Mean nearest phylogenetic taxon distance (t(13.89) = 7.90, p = 0.002) was significantly higher in rice fields than in pristine areas (Fig. 4).
Of the seven clades that compose the total phylogeny, two are not represented in rice fields, three have ca. 30% of the clades represented in rice fields and two have approximately 50% of the clade represented in rice fields (Fig. 5).
Anuran trait association to agricultural landscapes
Our random forest models selected range size, SVL, clutch size and habitat as predictors of anuran species presence in rice fields. Our classification tree models identified two optimal trees for trait association with species presence in rice fields. In the first tree, small species ranges (<599 km2) determine species presence in crops, with 83% of the species with ranges smaller than 599 km2 being absent from rice fields. In the second tree, anurans associated with forest and open area habitats have 77% probability of being absent from rice fields. If the described habitat association occurs in synergy with small clutch size (<182 or 432), the probability of that species being absent from rice fields is high (100% or 82%). Anurans associated to forest or open areas, with larger clutches, have a relatively high probability of occurring in rice fields (57%) (Fig. A.2).
Our random forest models selected habitat, SVL, range size and clutch size as predictors of relative abundance of anuran species in rice fields. Our regression tree models identified three optimal trees for trait regression against species relative abundance in rice fields (Fig. A.3). In the first tree, small species ranges (<685 km2) results in low relative abundance in crops and, if in synergy with larger body size (≥18 mm), corresponds to a very low relative abundance in rice fields. On the contrary, anurans with small ranges but smaller body size (<18 mm) are associated with relatively higher abundances in rice fields (Fig. A.3A). The second optimal tree identifies forest or open area habitat associations as determinant for low relative abundance in crops, which, if in synergy with larger body size, results in absence from crops (Fig. A.3B). The third optimal tree identifies larger body size and smaller clutches as determinant for low or null abundances in rice fields (Fig. A.3C).
Body condition index
Body condition indices for the genera Dendropsophus (D = 0.8541, p = 8.32e−16), Hypsiboas (D = 0.8756, p = 3.695e−15) and Leptodactylus (D = 0.6356, p < 3.2e−17) were significantly higher in pristine areas than in rice fields (Fig. 6).
Discussion
Our study is the first to integratively assess the effects of natural habitat conversion to rice fields on anuran communities. Our results strongly suggest that habitat conversion and agricultural management have caused profound alterations to the anuran community, including dramatic loss of species diversity and functional richness and increased functional divergence. Community composition suffered severe alterations, resulting in dominance of generalist hylids and leptodactylids and low representation of specialist microhylids and bufonids. Anuran communities in rice fields exhibited decreased phylogenetic diversity and mean phylogenetic distance, and increased mean nearest phylogenetic distance. This suggests that anuran communities in agricultural areas are composed of phylogenetically related species and, therefore, are not as diverse as their pristine counterparts.
Taxonomic indices often show increased species richness for intermediate disturbance levels, failing to accurately describe how disturbances affect biodiversity, unless other metrics are also considered. Functional diversity indices are expected to decrease under high disturbance levels due to environmental filtering (Cornwell et al. 2006; Flynn et al. 2009), and are thus perceived as better translators of disturbance effects on communities and ecosystems. According to the performance filter hypothesis, the environment determines the performance of species according to their trait syndromes, posing environmental filters to communities and selecting trait combinations associated with increased fitness in the occurring conditions (Mouillot et al. 2013). Our study corroborates such conceptions because, although anuran species richness, functional richness and phylogenetic diversity were significantly lower in rice fields, taxonomic diversity did not vary significantly. This means that even mild disturbances may deplete populations of species with vulnerable combinations of traits (e.g., habitat specialists, with larger body size, smaller clutch size and geographic range, as our results indicate) without modifying species composition and thus, without affecting taxonomic indices. Functional and phylogenetic diversity indices provide deeper insights into the complex effects that disturbances have on biodiversity (Cornwell et al. 2006). Functional richness has been considered a good predictor of ecosystem services (Dĺaz and Cabido 2001), while functional divergence or specialization may act as early-warning indicators of decline (Villéger et al. 2010).
Phylogenetic diversity represents the total evolutionary history or phylogenetic relatedness of all species in a community (Faith 1992), being recognized as intrinsically valuable for conservation (Winter et al. 2013). Despite the known impacts of agriculture on species loss, the effects of habitat conversion on phylogenetic diversity remain largely unknown. Studies on plants and invertebrates suggest that environmental disturbances favor subsets of closely related clades, resulting in phylogenetic diversity loss (Helmus et al. 2010). Global extinction risk assessments of birds and mammals suggest that particular branches are at greater extinction risk than others (Arregoitia et al. 2013). Although anuran species richness was significantly decreased in rice fields, species loss alone did not account for declining phylogenetic diversity. The changes we detected in phylogenetic diversity result from species loss and increased species relatedness. Species in pristine areas were less related to one another than expected by chance, whereas species in rice were more closely related. These patterns indicate that anuran phylogenetic diversity loss in agriculture causes a shift in community composition while taxonomic diversity remains relatively stable.
The decline in functional richness and increase in functional divergence following land conversion to rice fields suggests that the loss of more sensitive species is not counterbalanced by the addition of new disturbance-tolerant species that could fill vacant functional niches or occupy different functional roles. Anuran communities from rice fields were strongly dominated by a few generalist species (e.g., D. nanus, L. latrans), while communities from pristine areas were composed by more species, many specialists, occurring at lower abundances. These community changes provide strong evidence for environmental filtering in agricultural landscapes. Generalists with short life cycles and high reproductive capacities (i.e. r-selected species, Begon et al. 1996) are favored, and K-selected specialists are excluded from the communities due to lower ability to adapt to the fluctuating resources of intensively managed agricultural lands (Begon et al. 1996). Habitat fragmentation impairs dispersal, affects meta-population dynamics and results in increased extinction risk for less vagile species (Schweiger et al. 2005). Consequently, intensively cultivated landscapes will be dominated by generalist and mobile species, while specialized, rare, and less mobile species will incur in increased extinction risks (Schweiger et al. 2007). Ecological resilience, the ecosystem’s ability to maintain key functions after disturbances (Folke et al. 2004), safeguarding services such as seed dispersal or pollination (Foley et al. 2005), is partially determined by functional richness and redundancy (Allen et al. 2005). While functional richness describes the diversity and range of functional traits responsible for ecosystem services (Wright et al. 2006), functional redundancy represents the number of species fulfilling the same function, buffering against trait extinction (Tscharntke et al. 2005). As amphibians play keystone roles in ecosystem functioning, loss of amphibian functional richness and redundancy may affect ecosystem processes and decrease ecosystem resilience (Davic and Welsh 2004).
Small geographic ranges, association with forest and open area habitats, small clutches and large body size are associated with low to null species abundances in rice fields. Indeed, while pristine communities were composed of many, similarly abundant species, with varied degrees of habitat specialization, clutch and body size, agricultural communities were composed of few widespread habitat generalists, with smaller body size and larger clutches, such as D. nanus or L. latrans. Large body size and small range are the most common predictors of high extinction risk for several organisms (Sodhi et al. 2008; Botts et al. 2013; Gilroy et al. 2014). Small-range species are often less abundant, less mobile and poorer competitors being more likely to decline, become threatened and eventually suffer extinction (Cooper et al. 2008; Devictor et al. 2010; Murray et al. 2011; Sodhi et al. 2008). Species with large ranges often tolerate a wide variety of conditions, while species with small ranges are only able to persist under a narrower range of conditions (Sodhi et al. 2008; Cooper et al. 2008; Devictor et al. 2010). Strong association with forest and open area habitats are also associated to increased specialization. In the Cerrado, forest associated anurans have specialized breeding strategies, such as terrestrial clutches and lotic tadpoles, while open area specialists often produce foam nests in subterranean chambers to minimize humidity loss and temperature fluctuation. Anuran body size reflects effects of reduced surface to volume ratios in larger species to control both heat and water balance (Olalla-Tárraga et al. 2011; Sodhi et al. 2008). However, our results indicate that agricultural activities such as pesticide application and ploughing may be significantly more detrimental to larger species, offsetting the physiological advantage that larger body size has in regulating body temperature. Small clutches are correlated with decreased survival probabilities, especially in unstable and adverse conditions, such as those present in agricultural environments.
Although the effects of habitat change on body condition are seldom studied, the few studies available indicate that anurans in agricultural areas have lower body condition than their pristine counterparts (e.g. Brodeur et al. 2011; Karraker and Welsh 2006; Neckel-Oliveira 2007). Body condition indices are surrogates for the animal’s mass associated with energy reserves after correcting for structural body size. As energy stores can be allocated for maintenance, growth or reproduction, animals with larger energy reserves will usually have better fasting endurance, survival and reproductive output than individuals with smaller reserves (e.g., Bachman and Widemo 1999). Consequently, the lower body condition observed in frogs from cultivated sites suggests that these animals have reduced fitness, and impaired reproductive output and survival (Brodeur et al. 2011). Besides increased competition for resources, exposure to fertilizers and pesticides may stimulate the organism’s compensation/detoxification systems, also reducing energy reserves (e.g., Costa and Nomura 2016). Furthermore, food intake may be lower in crops due to habitat quality and insecticide applications. Our results highlight the need for further investigations into the causes and consequences of the low body conditions consistently found in anuran communities from agricultural environments.
Understanding how species traits correlate with their responses to agriculture will allow better predictions of the functional effects of anthropic land-use (Newbold et al. 2013). Diversity itself is an important component of ecosystem resilience because diverse communities are more likely to hold multiple species capable of contributing to a single given function. Our results show that the conversion of natural Cerrado-Amazon vegetation to vast rice fields results in such environmental alterations, that large subsets of species are driven to local extinction irrespective of their specialization. Species commonly perceived as resilient to habitat alteration did not occur in these environments (e.g. Leptodactylus labyrinthicus, Heyer 2005), which suggests that the new environmental filters, arisen as a consequence of replacing native vegetation with rice fields, are more detrimental on Neotropical anurans than was expected. Our results corroborate previous studies (e.g., Cooper et al. 2008; Lips et al. 2003) but also deliver new insights to potential synergies of different drivers of anuran community change in agricultural environments. Creating and/or maintaining networks of abundant and large forest patches and waterbodies may be key to sustaining higher levels of FD and PD in agricultural landscapes (Ribeiro et al. 2017). This study suggests that traditional metrics such as taxonomic diversity can hide important information about the real impacts of anthropogenic land-use on species composition and ecosystem functioning. Integrative approaches provide holistic insights on the impacts and consequences of human-mediated disturbances on biodiversity, potentially contributing to future conservation and agricultural management decisions (Vandewalle et al. 2010; Hidasi-Neto et al. 2012).
References
Allen CR, Gunderson L, Johnson AR (2005) The use of discontinuities and functional groups to assess relative resilience in complex systems. Ecosystems 8:958–966
Arregoitia LD, Blomberg SP, Fisher DO (2013) Phylogenetic correlates of extinction risk in mammals: species in older lineages are not at greater risk. Proc Biol Sci 280:1092
Attademo AM, Cabagna-Zenklusen M, Lajmanovich RC, Peltzer PM, Junges C, Bassó A (2011) B-esterase activities and blood cell morphology in the frog Leptodactylus chaquensis (Amphibia: Leptodactylidae) on rice agroecosystems from Santa Fe Province (Argentina). Ecotoxicology 20:274–282
Bachman G, Widemo F (1999) Relationships between body composition, body size and alternative reproductive tactics in a lekking sandpiper, the Ruff (Philomachus pugnax). Funct Ecol 13(3):411–416
Bambaradeniya, CNB, Amarasinghe, FP (2004) Biodiversity associated with the rice field agro-ecosystem in Asian Countries: a brief review. Working Paper 63. International Water Management Institute, Colombo Sri Lanka
Begon M, Sait SM, Thompson DJ (1996) Predator-prey cycles with period shifts between two-and three-species systems. Nature 381(6580):311
Bielby J, Cardillo M, Cooper N, Purvis A (2009) Modelling extinction risk in multispecies data sets: Phylogenetically independent contrasts versus decision trees. Biodivers Conserv 19:113–127
Blackwell GL (2002) A potential multivariate index of condition for small mammals. New Zeal J Zool 29:195–203
Blaustein AR, Han BA, Relyea RA, Johnson PT, Buck JC, Gervasi SS, Kats LB (2011) The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Ann New York Acad Sci 1223(1):108–119
Botts EA, Erasmus BFN, Alexander GJ (2013) Small range size and narrow niche breadth predict range contractions in South African frogs. Glob Ecol Biogeogr 22:567–576
Breiman L (2001) Random forests. Mach Learn 45:5–32
Brodeur JC, Suarez RP, Natale GS, Ronco AE, Elena Zaccagnini M (2011) Reduced body condition and enzymatic alterations in frogs inhabiting intensive crop production areas. Ecotoxicol Environ Saf 74:1370–1380
Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW (2009) The merging of community ecology and phylogenetic biology. Ecol Lett 12:693–715
Clarke KR, Warwick RM (2001) A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Mar Ecol Prog Ser 216:265–278
Cooper N, Bielby J, Thomas GH, Purvis A (2008) Macroecology and extinction risk correlates of frogs. Glob Ecol Biogeogr 17:211–221
Cornwell WK, Schwilk LDW, Ackerly DD (2006) A trait-based test for habitat filtering: convex hull volume. Ecology 87:1465–1471
Costa RN, Nomura F (2016) Measuring the impacts of roundup Original® on fluctuating asymmetry and mortality in a neotropical tadpole. Hydrobiologia 765(1):85–96
Czech H, Parsons K (2002) Agricultural wetlands and waterbirds: a review. Waterbirds 25:56–65
Davic RD, Welsh HH (2004) On the ecological roles of salamanders. Annu Rev Ecol Evol Syst 35:405–434
Devictor V, Mouillot D, Meynard C, Jiguet F, Thuiller W, Mouquet N (2010) Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: the need for integrative conservation strategies in a changing world. Ecol Lett 13:1030–1040
Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, Sievers C, Magurran AE (2014) Assemblage time series reveal biodiversity change but not systematic loss. Science 344(6181):296–299
Dĺaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16(11):646–655
Dodd CK (2010) Amphibian ecology and conservation: a handbook of techniques. Oxford University Press, Oxford
Elphick CS, Oring LW (2003) Conservation implications of flooding rice fields on winter waterbird communities. Agric Ecosyst Environ 94:17–29
Faith DP (1992) Conservation evaluation and phylogenetic diversity. Biol Conserv 61:1–10
FAO (2011) The state of food and agriculture Women in Agriculture Closing the Gender gap for Development FAO
Flynn DFB, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, Simpson N, Mayfield MM, DeClerck F (2009) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33
Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570–574
Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS (2004) Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst 35:557–581
Frishkoff LO, Karp DS, M’Gonigle LK, Mendenhall CH, Zook J, Kremen C, Hadly E, Daily GC (2014) Loss of avian phylogenetic diversity in neotropical agricultural systems. Science 345:1343–1346
Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, De Sá RO, Channing A, Wilkinson M, Donnellan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC (2006) The amphibian tree of life. Bull Am Museum Nat Hist 297:1–291
García-Barrios L, Galván-Miyoshi YM, Valdivieso-Pérez IA, Masera OR, Bocco G, Vandermeer J (2009) Neotropical forest conservation, agricultural intensification, and rural out-migration: the Mexican experience. Bioscience 59:863–873
Gilroy JJ, Medina Uribe CA, Haugaasen T, Edwards DP (2014) Effect of scale on trait predictors of species responses to agriculture. Conserv Biol 29:463–472
Gotelli NJ, McCabe DJ (2002) Species co-occurrence: A meta-analysis of J M Diamond’s assembly rules model. Ecology 83:2091–2096
Hanspach J, Fischer J, Ikin K, Stott J, Law BS (2012) Using trait-based filtering as a predictive framework for conservation: a case study of bats on farms in southeastern Australia. J Appl Ecol 49(4):842–850
Helmus MR, Keller WB, Paterson MJ, Yan ND, Cannon CH, Rusak JA (2010) Communities contain closely related species during ecosystem disturbance. Ecol Lett 13:162–174
Heyer WR (2005) Variation and taxonomic clarification of the large species of the Leptodactylus pentadactylus species group (Amphibia: Leptodactylidae) from Middle America, Northern South America, and Amazonia. W Arq Zool S Paulo 37:269–348
Hidasi-Neto J, Barlow J, Cianciaruso MV (2012) Bird functional diversity and wildfires in the Amazon: the role of forest structure. Anim Conserv 15:407–415
Hyne RV, Spolyarich N, Wilson SP, Patra RW, Byrne M, Gordon G, Sánchez-Bayo F, Palmer CG (2009) Distribution of frogs in rice bays within an irrigated agricultural area: links to pesticide usage and farm practices. Environ Toxicol Chem 28:1255–1265
IBGE Instituto Brasileiro de Geografia e Estatística Sistema IBGE de recuperação automática—SIDRA. (2005) Available from: http://www.sidra.ibge.gov.br
Isaac NJB, Redding DW, Meredith HM, Safi K (2012) Phylogenetically-informed priorities for amphibian conservation. PLoS ONE 7:e43912
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77:61–67
Karraker NE, Welsh HH (2006) Long-term impacts of even-aged timber management on abundance and body condition of terrestrial amphibians in Northwestern California. Biol Conserv 131:132–140
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464
Kivlin SN, Winston GC, Goulden ML, Treseder KK (2014) Environmental filtering affects soil fungal community composition more than dispersal limitation at regional scales. Fungal Ecol 12:14–25
Kole PC, Chakraborty NR, Bhat JS (2010) Analysis of variability, correlation and path coefficients in induced mutants of aromatic non-basmati rice. Trop Agric Res Ext, 11
Kraft NJB, Cornwell WK, Webb CO, Ackerly DD (2007) Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am Nat 170:271–283
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305
Laliberté E, Legendre P, Shipley B (2015) FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 10–12
Lebrija-Trejos E, Pérez-García EA, Meave JA, Bongers F, Poorter L (2010) Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 91(2):386–398
Lepš J, de Bello F, Lavorel S, Berman S (2006) Quantifying and interpreting functional diversity of natural communities: practical considerations matter. Preslia 78:481–501
Liaw A, Wiener M (2015) Package “randomForest”. http://cran.r-project.org/web/packages/randomForest/randomForest.pdf
Lips KR, Reeve JD, Witters LR (2003) Ecological traits predicting amphibian population declines in Central America. Conserv Biol 17:1078–1088
Maitner BS, Rudgers JA, Dunham AE, Whitney KD (2012) Patterns of bird invasion are consistent with environmental filtering. Ecography 35(7):614–623
Manly BF (2006) Randomization, bootstrap and Monte Carlo methods in biology, vol 70. CRC Press, Boca Raton
Mason NWH, Mouillot D, Lee WG, Wilson JB (2005) Functional richness, functional and functional evenness divergence: the primary of functional components diversity. Oikos 111:112–118
McIntyre S (2008) The role of plant leaf attributes in linking land use to ecosystem function in temperate grassy vegetation. Agr Ecosyst Environ 128(4):251–258
Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR (2013) A functional approach reveals community responses to disturbances. Trends Ecol Evol 28:167–177
Murray KA, Rosauer D, McCallum H, Skerratt LF (2011) Integrating species traits with extrinsic threats: closing the gap between predicting and preventing species declines. Proc Biol Sci 278:1515–1523
Neckel-Oliveira S (2007) Effects of forest disturbance on breeding habitat availability for two species of anurans in the amazon. Phyllomedusa 2007:186–192
Newbold T, Scharlemann JPW, Butchart SHM, Sekercioglu ÇH, Alkemade R, Booth H, Purves DW (2013) Ecological traits affect the response of tropical forest bird species to land-use intensity. Proc R Soc Lond B Biol Sci 280(1750):20122131
Niemi GJ, McDonald ME (2004) Application of ecological indicators. Annu Rev Ecol Evol Syst 35:89–111
Oksanen AJ, Blanchet FG, Kindt R, Legendre P, Hara RBO, Gavin L, Solymos P, Stevens MHH, Wagner H (2010) Package “vegan”. R. Package Version 2.0
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Gavin L, Solymos P, Stevens MHH, Wagner H (2015) Vegan: community ecology package. R. Package Version 2.0 e7
Olalla-Tárraga MÁ, Mcinnes L, Bini LM, Diniz-Filho JAF, Fritz SA, Hawkins BA, Hortal J, Orme CDL, Rahbek C, Rodríguez MÁ, Purvis A (2011) Climatic niche conservatism and the evolutionary dynamics in species range boundaries: Global congruence across mammals and amphibians. J Biogeogr 38(12):2237–2247
Olden JD, Lawler JJ, Poff NL (2008) Machine learning methods without tears: a primer for ecologists. Q Rev Biol 83:171–193
Oliver TH, Smithers RJ, Bailey S, Walmsley CA, Watts K (2012) A decision framework for considering climate change adaptation in biodiversity conservation planning. J Appl Ecol 49:1247–1255
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Peig J, Green AJ (2010) The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct Ecol 24:1323–1332
Petchey OL, Gaston KJ (2006) Functional diversity: back to basics and looking forward. Ecol Letters 9(6):741–758
Poff NL (1997) Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J N Am Benthol Soc 16(2):391–409
R Core Team (2014) Team. R: A language and environment for statistical computing
Ribeiro, J, Colli, G R, Batista, R, & Soares, A (2017) Landscape and local correlates with anuran taxonomic, functional and phylogenetic diversity in rice crops. Landsc Ecol 1–14
Rocha RG, Ferreira E, Costa BMA, Martins ICM, Leite YLR, Costa LP, Fonseca C (2011) Small mammals of the mid-Araguai River in central Brazil, with the description of a new species of climbing rat. Zootaxa 2789:1–34
Santos AB, Rabelo RR (2004) Cultivo do Arroz Irrigado no Estado do Tocantins. Sistemas de produção-EMBRAPA
Schweiger O, Maelfait JP, Wingerden WV, Hendrickx F, Billeter R, Speelmans M, Bukacek R (2005) Quantifying the impact of environmental factors on arthropod communities in agricultural landscapes across organizational levels and spatial scales. J Appl Ecol 42(6):1129–1139
Schweiger O, Musche M, Bailey D, Billeter R, Diekötter T, Hendrickx F, Herzog F, Liira J, Maelfait JP, Speelmans M, Dziock F (2007) Functional richness of local hoverfly communities (Diptera, Syrphidae) in response to land use across temperate Europe. Oikos 116:461–472
Sodhi NS, Bickford D, Diesmos AC, Lee TM, Koh LP, Brook BW, Sekercioglu CH, Bradshaw CJA (2008) Measuring the meltdown: drivers of global amphibian extinction and decline. PLoS ONE 3:1–8
Suding KN, Lavorel S, Chapin FS, Cornelissen JHC, Díaz S, Garnier E, Goldberg D, Hooper DU, Jackson ST, Navas ML (2008) Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob Chang Biol 14:1125–1140
Swenson NG (2014) Functional and phylogenetic ecology in R. Springer, New York
Therneau TM, Atkinson EJ (1997) An Introduction to Recursive Partitioning Using the RPART Routines Program. (Vol. 61, p. 452). Mayo Foundation: Technical report
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol Lett 8:857–874
Vandewalle M, Bello F, Berg MP, Bolger T, Dolédec S, Dubs F, Feld CK, Harrington R, Harrison PA, Lavorel S, Silva PM, Moretti M, Niemelä J, Santos P, Sattler T, Sousa JP, Sykes MT, Vanbergen AJ, Woodcock BA (2010) Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodivers Conserv 19:2921–2947
Villéger S, Mason NWH, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301
Villéger S, Ramos Miranda J, Flores Hernández D, Mouillot D (2010) Contrasting changes in taxonomic vs functional diversity of tropical fish communities after habitat degradation. Ecol Appl 20:1512–1522
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ (2002) Phylogenies and community ecology. Annu Rev Ecol Syst 33:475–505
Webb CO, Ackerly DD, Kembel SW (2008) Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24:2098–2100
Wells KD (2010) The ecology and behavior of amphibians. University of Chicago Press
Williams-Guillén K, Perfecto I (2010) Effects of agricultural intensification on the assemblage of leaf-nosed bats (Phyllostomidae) in a coffee landscape in Chiapas, Mexico. Biotropica 42:605–613
Winter M, Devictor V, Schweiger O (2013) Phylogenetic diversity and nature conservation: where are we? Trends Ecol Evol 28:199–204
Wright JP, Naeem S, Hector A, Lehman C, Reich PB, Schmid B, Tilman D (2006) Conventional functional classification schemes underestimate the relationship with ecosystem functioning. Ecol Lett 9:111–120
Acknowledgements
The authors wish to Fausto Nomura for helping with the sampling design, and Rita Gomes Rocha, for the helping during part of the fieldwork. Joana Ribeiro is a PhD candidate with a fellowship (reference SFRH/BD/51414/2011) awarded by FCT (Foundation for Science and Technology). Guarino R. Colli wishes to thank Coordenação de Apoio à Formação de Pessoal de Nível Superior—CAPES, Conselho Nacional do Desenvolvimento Científico e Tecnológico—CNPq and Fundação de Apoio à Pesquisa do Distrito Federal—FAPDF for financial support. Eduardo Ferreira was supported by a post-doctoral grant from FCT (Program POPHQREN, ref: SFRH/BPD/72895/2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dirk Sven Schmeller.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ribeiro, J., Colli, G.R., Caldwell, J.P. et al. Evidence of neotropical anuran community disruption on rice crops: a multidimensional evaluation. Biodivers Conserv 26, 3363–3383 (2017). https://doi.org/10.1007/s10531-017-1410-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-017-1410-0