Abstract
In the Mediterranean Sea, as well as in other parts of the word, intense bottom trawling threatens deep and mesophotic assemblages, compromising mainly the survivorship of erect organisms and of the habitat complexity they shape. Protection of species able to affect their habitats, by increasing spatial complexity and enhancing interspecific interactions, is crucial for biodiversity conservation. It is urgent to highlight the occurrence of those species which act as ecosystem engineers and/or habitat former to enhance awareness on their ecological role and to develop focused conservation strategies. Lytocarpia myriophyllum is the largest Leptomedusan hydroid of the Mediterranean Sea, with colonies up to 1 m high, and the most abundant Aglaopheniid in the eastern part of the North Atlantic Ocean. This species creates wide forests on soft bottoms stabilizing sediments, providing refuge and food for several other associated organisms and could be defined both a habitat former and an ecosystem engineer. Thanks to trimix diving here we report on new insights on the morphological, biological and ecological features of L. myriophyllum meadows from the Mediterranean Sea furnishing a baseline for protection plans focused on these facies. This work demonstrates that direct studies of mesophotic habitats allow to collect far more detailed information than grabs, ROVs, or towed camera arrays and highlights the urgent need to redefine the vertical extension of several marine protected areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The EC Habitats Directive 1992 aims at protecting biodiversity by taking the habitat as a target of management; surely a wise decision, since it is difficult that, in a reasonable time, we will be able to account for biodiversity by considering species (Fraschetti et al. 2008). Deep benthic communities of the Mediterranean Sea are threatened by several anthropogenic pressures (Claudet and Fraschetti 2010), and bottom trawling is the most used and destructive fishing practice in the Mediterranean basin, causing severe impact both on the living organisms and their habitat (Sacchi 2008; Schejter et al. 2008), with loss of foundation species (Piraino et al. 2002), leading to long-term negative changes in ecosystem structure and functioning (Ellison et al. 2005). The study of benthic fauna is fundamental to plan bottom-fishing activities so as to preserve habitats and their communities. Remote methods (ROV, acoustic swath mapping, dredges…) are increasingly used to characterize deep-sea habitats (Freitas et al. 2003; Tursi et al. 2004; Taviani et al. 2005; Freiwald et al. 2009; Bo et al. 2011) but direct exploration with trimix SCUBA techniques is rapidly enhancing the knowledge of the key role of the mesophotic zone both in tropical (Lesser et al. 2009) and in Mediterranean regions (Cerrano et al. 2010) as key transition zone between deep and shallow environments.
In spite of great interest in deep-sea environments, the distribution and the ecological role of soft bottom habitat formers received little attention. There are very few erect animals able to settle directly on sandy bottoms, such as some sponges (Cerrano et al. 2007), cerianthids, pennatulaceans and hydrozoans (Ammons and Daly 2008), usually creating secondary hard substrata (Pérès and Picard 1964; Morri et al. 1991) exploitable by many species, also of commercial interest (Baillon et al. 2012). Species increasing spatial complexity positively affect biodiversity, enhancing interspecific interactions, a crucial aspect regarding biodiversity conservation.
Hydroid colonies, besides being important benthic filter feeders, represent a substrate for settlement of sessile organisms, provide refuge for a diversified community of eukaryotic and prokaryotic organisms (Hughes 1975; Bavestrello et al. 1996; Genzano 2001, 2002; Di Camillo et al. 2008; Stabili et al. 2008) and represent a food source for many species (Folino 1993; Martin 2003). Most hydroids are not selective feeders, preying upon both on planktonic and benthic species (Gili et al. 1998; Cerrano et al. 2000; Bouillon et al. 2004, 2006). In some areas they can reach high abundances likely affecting the recruitment of larvae of merobenthic species (Standing 1976; Gili and Hughes 1995; Cerrano et al. 2001; Di Camillo et al. 2012).
The taxonomy of deep-sea hydroids received some attention (e.g. Marinopoulos 1981; Ramil et al. 1998; Ansín Agís et al. 2001; Vervoort 2006) but ecological studies on these animals mostly focus on shallow water species (Boero 1984; Gili and Hughes 1995), obviously due to the difficulty to conduct periodic samplings and photographic analyses in deep waters.
Many hydroid species living on soft bottoms are attached on hard substrates present below the sandy layer: some species of Nemertesia are fixed on shells buried in the sand by sponge-like masses of interlacing fibres (Allen 1899). The hydroids living on soft bottoms have modified hydrorhizae that enter the substrate either as a stout, tapering root or as tangles of anchoring filaments. The species with these features are referred to the anthomedusan genera: Balella, Acaulis, Acauloides, Boreohydra, Candelabrum, Fabulosus, Monocoryne, Branchiocerianthus, Corymorpha, Gymnogonos, Euphysa, Lobataria, whereas leptomedusan hydroids are more varied, in terms of substrate preferences, and species dwelling on soft bottoms can be found in the families Aglaopheniidae, Halopterididae, Lafoeidae, Plumulariidae, Sertulariidae, Campanulariidae (see Bouillon et al. 2006).
Lytocarpia myriophyllum is the largest Leptomedusan hydroid of the Mediterranean Sea, growing with colonies up to 1 m high organized in tufts (Fig. 2). Its ecology is unknown and its geographical and bathymetric distribution can be reconstructed by assembling data from numerous reports concerning deep-sea expeditions.
This species, as well as many others in deep soft bottoms, is threatened by trawling in many areas of the Mediterranean Sea (Cattaneo Vietti et al. 2010). The facies with large hydrozoans on infralittoral rocky substrates are considered as remarkable habitats (Priority Habitat code III.6.1.27), while facies with hydroids from Mediterranean deep soft bottoms are not considered at all (Relini and Giaccone 2009).
Lytocarpia myriophyllum is considered a priority species in Ireland and Great Britain (Goodwin et al. 2011); this work underlines the importance of its communities in the Mediterranean Sea and suggests strategies for its conservation. Information about the hydroid distribution are given, with particular attention to the Mediterranean records. Samplings of L. myriophyllum were conducted in different Mediterranean localities in order to supply new records of the species, moreover, from the colonies of Portofino the hydroid morphology was analyzed by electron microscopy in order to evidence the most relevant diagnostic characteristics.
Methods
Sampling sites
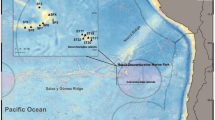
The sampling site (Fig. 1) is a detritic bottom at the base of a vertical cliff of the Marine Protected Area of Portofino (44°18′00.54″N, 09°13′11.25″E; Ligurian Sea, Italy, depth 70 metres) frequently characterized by strong unidirectional currents (Cerrano et al. 2005). A population of L. myriophyllum was sampled six times from February to November 2011 at a depth of 70 m by SCUBA using trimix. Close to this area (about 50 m apart) at the same depth a peculiar sea-fan assemblage was recently described (Cerrano et al. 2010).
Morphological study
Considering the depth of the sampling site ten colonies from Portofino were tagged in April 2011. In order to evaluate the occurrence of a relationship between the size of the colonies and the size of their root-like apparatus, the maximum height and the number of main stems were determined in situ together with the diameter of the base, i.e. the slight uplift made by stolons. The relationship between the size of the colonies (an index obtained considering the max colony height (cm) x number of colony stems) and the diameter (cm) of the burrowed complex root-like apparatus anchoring the colonies was verified using Pearson’s correlation coefficient (Sokal and Rohlf 1981) (Fig. 2a). The mathematical comparison was made using PAST for Windows version 1.91 (Hammer et al. 2001).
Lytocarpia myriophyllum. A Underwater picture (by Portofinodivers) of a large colony on the detrital bottom at Portofino. Vertical line: colony height, horizontal line: diameter of the root-like apparatus, arrows: main stems. B Unbranched hydrocladia (hy) and corbulae (c) alternately arranged along primary tubes (arrow); C Photograph of a living specimen showing hydranths with completely expanded tentacles (arrows); D Mature male gonophore with ramified spadix (s); E Mature female gonophore. Note that the male gonophore is transparent while the female one is darker for the presence of the egg mass. F Complex root-like apparatus; Longitudinal (G) and transversal (H) sections of a polysiphonic branch. Scale bars A vertical line 50 cm; B 10 mm; C, H 500 μm; D, E 200 μm; F 5 cm; G 1 mm
To get new information about the hydroid histology and ultrastructure, small portions of branches, hydrocladia and corbulae were taken with a cutter and directly fixed underwater in 2.5 % glutaraldehyde (buffered in filtered sea water 7.8 pH). Three hours later, samples were washed in filtered sea water and then dehydrated in a graded ethanol series.
For SEM analysis, several of the preserved portions were washed with distilled water, dehydrated in a graded ethanol series and dried with the Critical Point Dryer. Samples were then coated with gold–palladium in a Balzer Union evaporator and examined with a Philips XL20 SEM.
In order to obtain histological sections, other preserved pieces were included in a cold-curing resin (Technovit 8100) and finally mounted on plastic supports. The sections obtained by microtome Histo-Line MRS3500 were coloured with Toluidine blue and then analysed under a compound microscope.
Associated fauna
In order to study the associated fauna, during each sampling date stems from three different colonies were directly put into separate plastic bags to avoid the loss of the associated vagile fauna and one whole colony removed with care from the substrate in order to take the entire colony with hydrorhizae. Samples were preserved in formaldehyde 4 %, then the colonies were observed under the stereomicroscope for the study of the associated fauna.
Hydroid distribution
In order to supply information about the bathymetric and geographical distribution of the studied species, a deep bibliographic study was performed. The records, together with the name of each sampling site and the bathymetric range are summarized in a table (Suppl. mat.), while the areal of distribution of L. myriophyllum in the world, with a detail for the Mediterranean Sea, is represented in a map. We added some new records of the species collected at Imperia (Ligurian Sea, depth 56 m), Giannutri Island (Tyrrhenian Sea, depth 60 metres) and Pantelleria Island (Sicily Channel, depth 78 m).
Results
Morphological study
Lytocarpia myriophyllum was observed at Portofino during each sampling (February, April, May, August, October and November 2011). The studied population showed a core area of about 50 m2 with a maximum density of 1.57 ± 0.75 colonies/m2, surrounded by an area of 300 m2 with a lower density having 0.42 ± 0.51 colonies/m2.
The collected colonies, each made up by several stems, showed both male and female individuals; all the specimens showed corbulae, but these contained gonothecae only from May to November (Table 1).
According to Bouillon et al. (2006), the genus Lytocarpia includes 36 species, occurring in the Pacific Ocean (more than 50 %), Atlantic Ocean (16 %), Indian Ocean (20 %) and South Africa (11 %) (Di Camillo et al. 2011). One species is known from the Antarctic, one from the Arctic, and two from the Mediterranean Sea, namely L. myriophyllum and L. distans (Allman 1877) (Bouillon et al. 2004). The presence of a hydrotheca on the corbula ribs in at least one sex of Lytocarpia allows distinction from the genus Aglaophenia.
Lytocarpia myriophyllum (Linnaeus, 1758)
Lytocarpia myriophyllum was reported for the first time by Ellis (1755) that observed a colony from Dublin (Irish Sea) and described the hydroid as a “Coralline, with sickle-shaped feathered branches, resembling the feathers of a pheasant’s tail”. Later, Linnaeus (1758) named the species Sertularia myriophyllum. There are several descriptions for large colonies of L. myriophyllum (Fig. 2a) summarized in Tab S1. Here we focus on the main diagnostic characters of L. myriophyllum, (Fig. 2, 3). Alternate and unbranched hydrocladia born on primary tubes (Fig. 2b, 3a); hydrocladia with well-marked internal septa and bearing closely spaced hydrothecae (Fig. 2c). Hydrothecal margin characterized by five pairs of shallow lateral teeth and an unpaired median tooth notched in the middle of the theca (Fig. 3b). Hydrothecae with three nematothecae: one median inferior and two laterals (Fig. 3c, d); main cnidocyst type are microbasic mastigophores (Fig. 3e). Corbulae open, with ribs arising from the lower portion of the hydrothecae replacing the median inferior nematotheca (Fig. 3f). Female and male gonothecae similar in shape, male gonophores with ramified spadix (Fig. 2d); female ones with several, large eggs (Fig. 2e). Immature female gonophores containing a mass of small oily drops of irregular size.
Lytocarpia myriophyllum, SEM pictures. A Particular of a primary tube bearing alternate hydrocladia (hy); B Hydrothecal margin with an unpaired median tooth notched in the middle of the theca (white arrow); Hydrothecae on hydrocladia in frontal (C) and lateral (D) view; E Discharged microbasic mastigophores; F Corbulae open, it is possible to see the gonophores (g) between the ribs (r); G–H Accessory tubes showing rows of elliptical holes (arrows); I Nematophore composed of two portions: one, elongated and mobile (sarcostyle) (1) and another C-shaped (cnidostyle) (2); Enlargements of the inner surface of the sarcostyle (J) and the cnidostyle (K) covered with microvillae; L–M Elliptical holes still enclosed by perisarc; N–O The distal portions of newly formed accessory tubes appears wrinkled and soft (arrows). Scale bars A, C, D, F, G 500 μm; B o 50 μm; E, I, L, M 20 μm; H, N 100 μm; J 10 μm; K 2 μm
Stem and branches polysiphonic (Fig. 2g, h); accessory tubes show one or two rows of elliptical holes (Fig. 3g, h), already mentioned by Hincks (1868) and Nutting (1900). These holes contain a peculiar nematophore composed of two portions: one, elongated and mobile (sarcostyle) and another C-shaped, fixed and bearing several packed cnidocysts (cnidostyle) (Fig. 3i). The inner surface of the sarcostyle and cnidostyle is covered with microvillae (Fig. 3j, k). Sometimes the elliptical processes can be totally or partially closed (Fig. 3l, m), suggesting that their formation could requires the enzymatic digestion of the perisarc. However, Kosevich (2005) observed that no chitinase activity occurs during the formation of new branches in colonial hydroids. The perisarc openings in fact, are formed when the new branches emerge and mechanically break the old perisarc. The distal portion of newly formed accessory tubes appears wrinkled and soft (Fig. 3n, o), suggesting that the just secreted perisarc has a different biochemical composition from to the old exoskeleton (Kossevitch et al. 2001).
The colonies of L. myriophyllum are anchored to the sand by a complex root-like apparatus (Fig. 2f). The basal portion of the hydrocaulus is about 20 cm sunk into the sand and gives rise to a spongy web of thin anastomosing stolons. The stolons adhere to small sand grains or organogenous fragments. Even if we measured only ten colonies we highlighted a strong positive relation between the colony size and the diameter of the anastomosed root-like apparatus (R = 0.95; Pearson’s correlation p < 0.001) (Fig. 4). Repeated measures during the studied period on the tagged specimens showed neither growth nor coalescence of the colonies. Morphometric data of the studied specimens are reported in Table 2.
Associated fauna
Three species of epizoic hydroids were observed in various parts of the colonies of L. myriophyllum. Plumularia setacea was found between the hydrocladia, while Campanularia hincksii colonized both the hydrocladia, the hydrocaulus and the hydrorhizae. Finally, Bougainvillia sp. was found on the most superficial and exposed hydrorhizae.
Concerning the vagile fauna, two species of nudibranchs—Dondice banyulensis and Eubranchus confr. exiguus—were associated with L. myriophyllum that represents a food source and a hard substrate to lay eggs. D. banyulensis was frequently observed, especially feeding on young colonies growing from the the hydrorizae of the root-like apparatus; whereas just two specimens of E. confr. exiguus were found, with their eggs. Three species of amphipods were also observed: seven specimens of the caprellid Phtisica marina, three specimens of the gammarid Lysianassa sp. and an unidentified specimen of Lysianassidae.
Discussion
Except for coral reefs or commercially exploited species, people generally feel there is little reason for the conservation of marine invertebrates, owing to the lack of knowledge on their actual value and role. This is why conservation strategies are often focused on few flag species. The European Commission has defined vulnerable marine ecosystems (VMEs) as "any marine ecosystem whose specific structure and function is, according to the best scientific information available and to the principle of precaution, likely to be compromised by stress resulting from physical contact with bottom gears in the course of fishing operations, including inter alia reefs, seamounts, hydrothermal vents, cold water corals or cold water sponge beds" (EC 2007). Mesophotic ecosystems can be considered VMEs and species characterizing these habitats need to be included in conservation policies.
Hydroid distribution
Most of the records of L. myriophyllum (Fig. 5a, b) are localized in the Boreal and the Macaronesian regions, from Greenland to Ghana. L. myriophyllum is the most frequently occurring Aglaopheniid in the eastern part of the North Atlantic Ocean but it has been also found along the American coasts, from Cape Cod to the Gulf of St. Lawrence. The species was reported from several Indo-Pacific sites (Philippines, Papua New Guinea, New Caledonia, Japan and South China Sea) and the South America (Chile and Argentina). L. myriophyllum is also common in the Mediterranean Sea (Fig. 5b). The species was found along the eastern Mediterranean coasts (from the Strait of Gibraltar to the Gulf of Genoa), the Thyrrenian Sea (Corsica, Tuscany and Gulf of Naples), the Levantine coasts (Israele) and the eastern coasts of the Adriatic Sea (Slovenia and Croatia). The records from Indo-Pacific, South Indian Ocean and South America should be considered doubtful: all the specimens described as Thecocarpus myriophyllum var. orientalis Billard, 1908, T. myriophyllum var. elongatus Billard, 1910 and T. myriophyllum var. angulatus Billard, 1913, cannot be considered as synonyms of L. myriophyllum since they show closed corbulae and Schuchert (2003) raised them to full species level (L. orientalis n. comb., n. status). According to Schuchert (2003), the wide geographical separation between the Atlanto-Mediterranean specimens and those from Indo-Pacific and South America suggests that these morphotypes can be ascribed to separate species. Moreover, Ramil and Vervoort (1992) and Ansín Agís et al. (2001) retained that the samples from the Mediterranean Sea classified as L. distans (Allman 1877) should be attributed to L. myriophyllum. In this case, L. myriophyllum would be the only species of Lytocarpia in the Mediterranean waters.
Moreover, the original description of L. distans (Allman, 1877) does not mention the presence of the cauline nematophores composed of sarcostyle and cnidostyle. This feature could be an important diagnostic character exclusive of L. myriophyllum. Preserved Mediterranean specimens referred to L. distans should be re-examined to check the presence of the nematophores in order to establish if the samples belong to L. myriophyllum.
Lytocarpia myriophyllum spreads at a depth ranging between 15 m (West of Scotland, Ritchie 1911) and 1800 m (Canary Islands, Ansín Agís et al. 2001); in the Mediterranean Sea it was found between 40 (Ligurian Sea, Boero and Fresi 1986) and 1,005 m (Alboran Sea, Ramil and Vervoort 1992).
Concerning the local distribution of the species, the population of L. myriophyllum is located at the north-eastern side of the Portofino Promontory exposed to a secondary branch of the main Ligurian current which flows westwards leading to a high sedimentation area (Cerrano et al. 2005), close to a Savalia savaglia population (Cerrano et al. 2010), suggesting that the hydroid biomass is supported by terrigenous sediment supply. Moreover, the population here described and all those found during this research were located at the base of rocky cliffs, where mud and/or silt are mixed with organogenous mineral debris. This is confirmed by another study on the benthic assemblages along the Catalan coasts describing that most of the cnidarian species—including L. myriophyllum—were found especially in areas located near the major river flow (Gili 1986; Gili et al. 1987). Similarly, Manaro et al. (1989) affirmed that L. myriophyllum, together with other species, was more frequent on coastal detrital or muddy bottoms. As well as other cnidarian species, L. myriophyllum is more abundant on the continental shelf where the food availability is enough to guarantee the hydroid development while the competition for the substrate or presence of predators are low (Gili et al. 1987).
Morphological study
When collected, colonies of L. myriophyllum are covered by a mucus layer that may contain molecules discouraging the colonization of other organisms. The numerous holes present along the stem and branches of L. myriophyllum contain a sarcostyle that probably plays a role in cleaning the surrounding perisarc or phagocytizing foreign particles adhered to perisarc (Gravier-Bonnet 2004). This behaviour has been also observed for the sarcostyles of Kirchenpaueriidae, Halopterididae, Plumulariidae and other Aglaopheniidae (Gravier-Bonnet 2004). Moreover, it is possible that the sarcostyles of L. myriophyllum are involved in the production of the conspicuous mucus layer surrounding the colonies. This hypothesis is enforced by the presence of microvillae.
Time series
Results suggest L. myriophyllum could be always fertile except winter. Even if further investigations are necessary to confirm this hypothesis, it is noticeable that the period of presence and fertility of L. myriophyllum is considerably longer than that reported in literature for the Mediterranean specimens (occurrence: June to August; corbulae: August; see Bouillon et al. 2004).
The colony size remains almost constant during the period of observation, suggesting that the species does not undergo seasonal regression, that, on the contrary is observed in shallow water hydroids (Boero and Fresi 1986; Bavestrello et al. 2006; Di Camillo et al. 2012). These features can be a consequence of the higher environmental stability of mesophotic zone respect to shallow habitats.
Associated fauna
During this study we found 8 taxa of epibionts of L. myriophyllum. Other organims were cited in literature, such as hydrozoans (Zygophylax cf. biarmata, Antennella secundaria, Hebella spp., Plumularia setacea, Campanulariids, Filellum serratum Ansín Agís et al. 2001), the anemones Amphianthus dohrnii (Pérès and Picard 1964; Gamulin-Brida 1967; Pérès 1967) bivalves, bryozoans, gastropod eggs, foraminifers and stalked barnacles. The vagile fauna was represented by caprellids (Ansín Agís et al. 2001), solenogasters (Rhopalomenia aglaopheniae) (Von Salvini-Plawen 1972; Allen 1899) the gastropod Capulus ungaricus (Linnaeus, 1758) (Pérès and Picard 1964), and nudibranchs (Doto koenneckeri McDonald and Nybakken 1996). These two mollusks are predators of L. myriophyllum (Von Salvini-Plawen 1972; McDonald and Nybakken 1996).
Not withstanding L. myriophyllum is scarcely colonized in comparison to Eudendriids or Sertulariids, it may host about 20 taxa, suggesting that the large colonies of this hydroid create a habitat on soft bottoms and provide refuge and food for other organisms.
Conclusions
Some species can be considered either as ecosystem engineers or habitat modifiers for their ability to create, modify or maintain habitats (Piraino et al. 2002; Cerrano et al. 2006). The hydroid L. myriophyllum, with colonies more than 1 m high (Rossi 1958), represents an important secondary substrate in soft bottoms and, similarly to other large cnidarian species, can be considered a habitat former sensu Fraschetti et al. (2008). In Northern Ireland L. myriophyllum is rare and declining because of loss of habitats due to the impact of massive bottom-fishing and it is considered a Northern Ireland Priority Species hydroids (NIPS) (Goodwin et al.2011). Bottom trawling, in fact, damages the seabed, with consequent destruction of the habitats and living communities and causes evident changes in the ecosystem structure (Sacchi 2008).
Concerning Italian waters, no data are available on the effects of physical disturbance of bottom fishing on L. myriophyllum, but it is much probable that the species is extremely vulnerable to bottom trawling, as it is in Ireland. The employment of non destructive techniques, such as remotely operated vehicle (ROV) and SCUBA diving, allows to monitor the status of benthic species in deep waters without negative impact on the living communities. Several surveys of L. myriophyllum should be conducted throughout the Italian coasts to evaluate the hydroid distribution and abundance in order to develop conservation or, if needed, recovery programs of the species. Measures of protection of L. myriophyllum should be envisaged also in the Mediterranean Sea, following the example of Ireland. The geographical and bathymetrical distribution of L. myriophyllum is likely wider in the Mediterranean Sea than reported here, calling for specific programs dedicated to the knowledge of the distribution, the biology, and ecology of soft bottom habitat formers, to redefine marine protected areas bathymetric limits and limit trawl fisheries where these species are present.
References
Allen EJ (1899) On the fauna and bottom-deposits near the thirty-fathom line from the Eddystone Grounds to Start Point. J Mar Biol Assoc 5:365–452
Allman GJ (1877) Report on the Hydroida collected during the exploration of the Gulf Stream by L.F. De Pourtalès, assistant United States Coast Survey. Mem Mus Comp Zoöl 5:1–66
Ammons A, Daly M (2008) Distribution, habitat use and ecology of deepwater anemones (Actiniaria) in the Gulf of Mexico. Deep Sea Res II 55:2657–2666
Ansín Agís J, Ramil F, Vervoort W (2001) Atlantic Leptolida (Hydrozoa, Cnidaria) of the families Aglaopheniidae, Halopterididae, Kirchenpaueriidae and Plumulariidae collected during the CANCAP and Mauretania-II expeditions of the National Museum of Natural History, Leiden, The Netherlands. Zool Verhandel 333:1–268
Baillon S, Hamel JF, Wareham VE, Mercier A (2012) Deep cold-water corals as nurseries for fish larvae. Front Ecol Environ. doi:10.1890/120022
Bavestrello G, Cattaneo-Vietti R, Cerrano C, Sarà M (1996) Relations between Eudendrium glomeratum (Cnidaria, Hydromedusae) and its associated vagile fauna. Sci Mar 60:157–163
Bavestrello G, Puce S, Cerrano C, Zocchi E, Boero F (2006) The problem of seasonality of benthic hydroids in temperate waters. Chem Ecol 22:197–205
Bo M, Di Camillo CG, Bertolino M, Povero P, Misic C, Castellano M, Harriague AC, Gasparini GP, Borghini M, Schroeder K, Bavestrello G (2011) Characteristics of the mesophotic megabenthic assemblage of the Vercelli Seamount (North Tyrrhenian Sea). PlosOne 6:e16357. doi:10.1371/journal.pone.0016357
Boero F (1984) The ecology of marine hydroids and effects of environmental factors: a review. Pubbl Staz zool Napoli I Mar Ecol 5:93–118
Boero F, Fresi E (1986) Zonation and evolution of a rocky bottom hydroid community. Pubbl Staz zool Napoli I Mar Ecol 7:123–150
Bouillon J, Medel MD, Pagès F, Gili JM, Boero F, Gravili C (2004) Fauna of the Mediterranean Hydrozoa. Sci Mar 68:1–449
Bouillon J, Gravili C, Pagés F, Gili JM, Boero F (2006) An introduction to Hydrozoa. Mém Mus Hist Nat 194:1–591
Cattaneo Vietti R, Albertelli G, Aliani S, Bava S, Bavestrello G, Cecchi LB, Bianchi CN, Bozzo E, Capello M, Castellano M, Cerrano C, Chiantore M, Corradi N, Cocito S, Cutroneo L, Diviacco G, Fabiano M, Faimali M, Ferrari M, Gasparini GP, Locritani M, Mangialajo L, Marin V, Moreno M, Morri C, Orsi Relini L, Pane L, Paoli C, Petrillo M, Povero P, Pronzato R, Relini G, Santangelo G, Tucci S, Tunesi L, Vacchi M, Vassallo P, Vezzulli L, Wurtz M (2010) Ligurian Sea: present status, problems and perspectives. Chem Ecol 26:319–340
Cerrano C, Bavestrello G, Puce S, Chiantore M (2000) Unusual trophic strategies of Hydractinia angusta (Cnidaria, Hydrozoa) from Terra Nova Bay, Antarctica. Pol Biol 23:488–494
Cerrano C, Puce S, Chiantore M, Bavestrello G, Cattaneo-Vietti R (2001) The influence of the epizooic hydroid Hydractinia angusta on the recruitment of the Antarctic scallop Adamussium colbecki. Pol Biol 24:577–581
Cerrano C, Arillo A, Azzini F, Calcinai B, Castellano L, Muti C, Valisano L, Zega G, Bavestrello G (2005) Gorgonian population recovery after a mass mortality event. Aquat Conserv Mar Fresh Ecos 15:147–157
Cerrano C, Calcinai B, Bertolino M, Valisano L, Bavestrello G (2006) Epibionts of the scallop Adamussium colbecki in the Ross Sea, Antarctica. Chem Ecol 22:235–244
Cerrano C, Calcinai B, Di Camillo CG, Valisano L, Bavestrello G (2007) How and why do sponges incorporate foreign material? Strategies in Porifera. Porifera Res Biodivers Innov Sustain 20:239–246
Cerrano C, Danovaro R, Gambi C, Pusceddu A, Riva A, Schiaparelli S (2010) Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers Conserv 19:153–167
Claudet J, Fraschetti F (2010) Human-driven impacts on marine habitats: a regional meta-analysis in the Mediterranean Sea. Biol Conserv 143:2195–2206
Di Camillo CG, Bo M, Lavorato A, Morigi C, Segre Reinach M, Puce S, Bavestrello G (2008) Foraminifers epibiontic on Eudendrium (Cnidaria: Hydrozoa) from the Mediterranean Sea. J Mar Biol Assoc UK 88:485–489
Di Camillo CG, Puce S, Bavestrello G (2011) Lytocarpia and Cladocarpus (Cnidaria: Hydrozoa, Aglaopheniidae) from the Bunaken National Marine Park (North Sulawesi, Indonesia). Mar Biodiv 41:517–536
Di Camillo CG, Bo M, Betti F, Martinelli M, Puce S, Vasapollo C, Bavestrello G (2012) Population dynamics of Eudendrium racemosum (Cnidaria, Hydrozoa) from the North Adriatic Sea. Mar Biol 159:1593–1609
EC 2007. Proposal for a Council Regulation on the protection of vulnerable marine ecosystems in the high seas from the adverse impacts of bottom fishing gears. COM(2007) 605 final. 2007/0224 (CNS)
Ellis J (1755) An essay towards a natural history of the corallines, and other marine productions of the like kind, commonly found on the coasts of Great Britain and Ireland. To which is added the description of a large marine polyp taken near the North Pole, by the whale-fishers, in the summer 1753. London. pp. 1–103
Ellison AM, Bank MS, Clinton BD, Colburn EA, Elliott K, Ford CR, Foster DR, Kloeppel BD, Knoepp JD, Lovett GM (2005) Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3:479–486
Fraschetti S, Terlizzi A, Boero F (2008) How Many habitats are there in the sea (and where)? J Exp Mar Biol Ecol 366:109–115
Freitas R, Rodrigues AM, Quintino V (2003) Benthic biotopes remote sensing using acoustics. J Exp Mar Biol Ecol 285/286:339–353
Freiwald A, Beuck L, Rüggeberg A, Taviani M, Hebbeln D, R/V Meteor Cruise M70–1 participants (2009) The white coral community In the central Mediterranean Sea revealed by ROV surveys. Oceanography 22:58–74
Folino NC (1993) Feeding and Growth of the nudibranch Cuthona nana (Alder and Hancock, 1842). J Moll Stud 59:15–22
Gamulin-Brida H (1967) The benthic fauna of the Adriatic Sea. Oceanogr Mar Biol 5:535–568
Genzano GN (2001) Associated fauna and sediment trapped by colonies of Tubularia crocea (Cnidaria, Hydrozoa) from the rocky intertidal of Mar del Plata. Biocencias 9:105–119
Genzano GN (2002) Associations between pycnogonids and hydroids from the Buenos Aires littoral zone, with observations on the semi-parasitic life cycle of Tansystylum orbiculare (Ammotheiidae). Sci Mar 66:83–92
Gili JM (1986) Estudio sistematico y raunistico de 105 cnidarios de la costa catalana. Ph. D. Thesis. Autonomous University of Barcelona
Gili JM, Hughes RG (1995) The ecology of marine benthic hydroids. Oceanogr Mar Biol Ann Rev 33:351–426
Gili JM, Ros JD, Pagès F (1987) Types of bottoms and benthic cnidaria from the trawling grounds (littoral and bathyal) of Catalonia (NE Spain). Vie Milieu 37:85–98
Gili JM, Alvà V, Coma R, Orejas C, Pagès F, Ribes M, Zabala M, Arntz W, Bouillon J, Boero F, Hughes RG (1998) The impact of small benthic passive suspension feeders in shallow marine ecosystems: the hydroids as an example. Zool Verh 323:99–105
Goodwin C, Edwards H, Breen J, Picton B (2011) Rathlin Island - A survey report from the nationally important marine features project 2009–2011. Northern Ireland Environment Agency Research and Development Series No. 11/03
Gravier-Bonnet N (2004) Hydroid nematophores: morphological, structural, and behavioural variety from old knowledge and new data. Hydrobiol 530(531):199–208
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for education and data analysis. Paleontologia Electronica 4:1–9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm. Accessed 8 Feb 2013
Hincks T (1868) A history of the British hydroid zoophytes. London 1:1–338
Hughes RG (1975) The distribution of epizoites on the hydroid Nemertesia antennina (L.). J Mar Biol Ass UK 55:275–294
Kosevich IA (2005) Branching in colonial hydroids. In: Davis J (ed) Branching morphogenesis. Springer, New York, pp 91–112
Kossevitch IA, Herrmann K, Berking S (2001) Shaping of colony elements in Laomedea flexuosa Hinks (Hydrozoa, Thecaphora) includes a temporal and spatial control of skeleton hardening. Biol Bull Mar Biol Lab Woods Hole 201:417–423
Lesser MP, Slattery M, Leichter JJ (2009) Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol 375:1–8
Linnaeus C (1758) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Editio decima, reformata, Holmiae (Stockholm). 1-823
Manaro N, Ungaro N, Vaccarella R (1989) Nota preliminare sulle comunità di macroinvertebrati dei fondi strascicabili dell’adriatico pugliese. Thal Sal 19:3–19
Marinopoulos J (1981) Contribution à la connaissance des hydraires profonds de la Méditerranée. Rapport Commission International pour l’exploration scientifique de la Mer Méditerranée. 27:175–176
Martin R (2003) Management of nematocysts in the alimentary tract and in cnidosacs of the aeolid nudibranch gastropod Cratena peregrina. Mar Biol 143:533–541
McDonald GR, Nybakken JW (1996) A List of the Worldwide Food Habits of Nudibranchs. http://www.theveliger.org/nudibranch_food.html
Morri C, Bavestrello G, Bianchi CN (1991) Faunal and ecological notes on some benthic cnidarian species from the Tuscan archipelago and eastern Ligurian Sea (western Mediterranean). Bollettino Museo Istituto Biologia Università di Genova 54–55:27–47
Nutting C (1900) American hydroids. Pt. 1. The Plumularidae. Special Bull US Natl Mus 4:1–285
Pérès JM (1967) The Mediterranean benthos. Oceanogr Mar Biol Annu Rev 5:449–533
Pérès J, Picard J (1964) Nouveau manuel de bionomie benthique de la mer Méditerranée. Recueil des Travaux de la Station Marine d’Endoume 31:5–137
Piraino S, Fanelli G, Boero F (2002) Variability of species’ roles in marine communities: change of paradigms for conservation priorities. Mar Biol 140:1067–1074
Ramil F, Vervoort W (1992) Report on the Hydroida collected by the ‘BALGIM’ Expedition in and around the Strait of Gibraltar. Zool Verh Leiden 277:1–263
Ramil F, Vervoort W, Ansín JA (1998) Report on the Haleciidae and Plumularioidea (Cnidaria, Hydrozoa) collected by the French SEAMOUNT 1 Expedition. Zool Verh Leiden 322:1–42
Relini G, Giaccone G (2009) Gli Habitat prioritari del Protocollo SPA/BIO (Convenzione di Barcellona) presenti in Italia. Schede descrittive per l’identificazione. Biol Mar Medit 16:1–367
Ritchie J (1911) Contribution to our knowledge of the hydroid fauna of the West of Scotland. Being an account of the collections made by Sir John Murray, K.C.B., on S.Y. ‘Medusa’. Ann Scott Nat Hist 80:217–225
Rossi L (1958) Contributo allo studio della fauna di profondità vivente presso la riviera Ligure di Levante. Doriana 2:113
Sacchi J (2008) The use of trawling nets In the Mediterranean. Problems and selectivity options. Options Méditerranéennes 62:87–96
Schejter L, Bremec C, Hernández D (2008) Comparison between disturbed and undisturbed areas of the Patagonian scallop (Zygochlamys patagonica) fishing ground “Reclutas” in the Argentine Sea. J Sea Res 60:193–200
Schuchert P (2003) Hydroids (Cnidaria, Hydrozoa) of the Danish expedition to the Kei Islands. Steenstrupia 27:137–256
Sokal RR, Rohlf FJ (1981) Biometry: the principle and practice of statistics in biological research. Freeman, New York
Stabili L, Gravili C, Tredici SM, Piraino S, Talà A, Boero F, Alifano P (2008) Epibiotic Vibrio luminous bacteria isolated from some Hydrozoa and Bryozoa species. Microb Ecol 56:625–636
Standing J (1976) Coelenterate ecology and behavior. In: Mackie GO (ed) Fouling community structure: effects of the hydroid Obelia dichotoma on larval recruitment. Plenum, New York, pp 155–164
Taviani M, Remia A, Corselli C, Freiwald A, Malinverno E, Mastrototaro F, Savini A, Tursi A (2005) First geo-marine survey of living cold-water Lophelia reefs in the Ionian Sea (Mediterranean basin). Facies 50:409–417
Tursi A, Mastrototaro F, Matarrese A, Maiorano P, D’Onghia G (2004) Biodiversity of the white coral reefs in the Ionian Sea (Central Mediterranean). Chem Ecol 20:107–116
Vervoort W (2006) Leptolida (Cnidaria:Hydrozoa) collected during the CANCAP and Mauritania-II expeditions of the National Museum of Natural History, Leiden, The Netherlands [Anthoathecata, various families of Leptothecata and addenda]. Zool Med 80:181–318
Von Salvini-Plawen L (1972) Cnidaria as food-sources for marine invertebrates. Cah Biol Mar 13:385–400
Acknowledgments
The research has been partially funded by PRIN 2008 Italian funds (2008YBEANX_002), by the Committee for Research and Exploration of the National Geographic Society (grant Grant Agreement 8876-11), the EC Grant Agreement No. 287844 (FP7/2007-2013) for the project “Towards COast to COast NETworks of marine protected areas (from the shore to the high and deep-sea), coupled with sea-based wind energy potential” (COCONET), the Flagship Project RITMARE—The Italian Research for the Sea—coordinated by the Italian National Research Council and funded by the Italian Ministry of Education, University and Research within the National Research Program 2011–2013. Portofinodivers provided the technical assistance during sampling activities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Di Camillo, C.G., Boero, F., Gravili, C. et al. Distribution, ecology and morphology of Lytocarpia myriophyllum (Cnidaria: Hydrozoa), a Mediterranean Sea habitat former to protect. Biodivers Conserv 22, 773–787 (2013). https://doi.org/10.1007/s10531-013-0449-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-013-0449-9