Abstract
Alpine areas will likely experience an increase in non-native plant species invasions. Increased human activity and environmental changes are expected to lower the environmental constraints for their establishment and spread. To understand and prepare for high elevation plant invasions it is necessary to evaluate the changes in environmental factors that make alpine regions susceptible to potential invaders. The alpine of the Rocky Mountains has very few occurrences of non-native species to date, but anthropogenic environmental changes may facilitate invasion. We tested whether Bromus tectorum (cheatgrass or downy brome) invasion in the Rocky Mountain alpine could be facilitated by increases in mean and minimum growing season temperatures. We also tested whether nitrogen (N) deposition and alpine soil may modify B. tectorum’s responses to climate warming. Our findings suggest that alpine soils inhibited growth of B. tectorum regardless of temperature or simulated N deposition. These results indicate that local alpine invasion by B. tectorum is unlikely in the near future. However, higher minimum growing temperatures and increased N addition did enhance B. tectorum growth for plants grown in upper montane soils. Such changes may promote population growth of B. tectorum within montane elevations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alpine ecosystems are some of the least invaded environments due both to low seed dispersal and environmental constraints such as low temperatures (Lonsdale 1999; Alexander et al. 2016). Increasing human activity, such as road building and recreation, in alpine regions is resulting in the movement of invasive species’ propagules to high elevation ecosystems (Marini et al. 2009; Rundel and Keeley 2016). Concurrent increases in nitrogen (N) deposition and climate warming have the potential to facilitate invasions in high mountain ecosystems (Dukes and Mooney 1999; Pauchard and Alaback 2004; Concilio et al. 2013; Petitpierre et al. 2015; Lembrechts et al. 2016). Invasive species are now established and spreading along alpine roadsides in the Andes (Cavieres et al. 2005), the Northern Calcareous Alps (Dullinger et al. 2003), and the Australian Alps (Johnston and Pickering 2001), demonstrating that the alpine is not immune to invasive species establishment (reviewed by Alexander et al. 2016).
To successfully establish in a new habitat such as the alpine, non-native species must overcome multiple barriers (i.e. ecological filters). Seed availability is the initial filter (i.e. dispersal limitation), which is followed by multiple site-level filters including abiotic conditions and biotic interactions that determine whether individuals establish a population (Theoharides and Dukes 2007). Multiple site-level filters have been hypothesized to confer invasion resistance to alpine ecosystems. A long-standing hypothesis posits that alpine ecosystems are too cold for invasive species to establish (Pauchard et al. 2009). However, anthropogenic climate warming is increasing growing season temperatures which may benefit non-native species that are adapted to the climate at lower elevations (Dukes and Mooney 1999; Hellmann et al. 2008; Walther et al. 2009; Najberek et al. 2017). Both increases in growing season air temperatures as well as an increase in minimum (subfreezing) growing season air temperatures could allow non-native species with established populations in montane and subalpine ecosystems to colonize the alpine. Haider et al. (2011) found that upper elevation limits of non-native species’ populations in the Alps was not related to how the same nine non-native species responded to a gradient of temperature treatments, suggesting that climate extremes or other site-level conditions determine upper elevation limits. A leading hypothesis for non-native species’ elevation limits is that cold temperatures impose physiological constraints (Dukes and Mooney 1999; Pauchard et al. 2009; Alexander et al. 2016). Several studies have demonstrated that increasing air temperatures determine or predict non-native species’ range expansions in low elevation ecosystems (Jarnevich and Stohlgren 2009; Bradley et al. 2010; Verlinden and Nijs 2010; Hou et al. 2014). Yet, few studies have addressed temperature constraints of non-native species in montane ecosystems (Haider et al. 2011; Barni et al. 2012). Additional experiments are needed to isolate the unique effects of temperature and other site-level filters on non-native species’ abilities to germinate, survive, grow, and reproduce because causation cannot be assessed using a correlational or modeling approach.
Elevated N availability has also been implicated in enhanced growth and reproduction of non-native species (Dukes and Mooney 1999). Invasive, non-native species often grow best in nutrient rich soils and compete well for limiting nutrients relative to native species (Davis et al. 2000; Levine et al. 2003; Funk and Vitousek 2007; Rao and Allen 2010). While native alpine plant species grow slowly and exhibit conservative changes in N use with increasing N availability (Bowman and Bilbrough 2001), the opposite is generally true for non-native species in their invaded ranges (Milberg et al. 1999; Vasquez et al. 2008). Given that N is a limiting nutrient for plant growth, anthropogenic N deposition could benefit non-native species that are able to overcome both dispersal and environmental constraints (Davis et al. 2000; Gross et al. 2005; Flores-Moreno et al. 2016; Liu and van Kleunen 2017).
While air temperature and N deposition may operate as independent invasion filters, shifts in multiple site-level factors in the alpine (i.e. increases in propagule pressure, N availability, and air temperatures) may be necessary to increase the likelihood of invasions. This idea has been examined in the context of community assembly with some evidence supporting it (Myers and Harms 2009; Pinto et al. 2014), but only recently have multiple, potentially interacting factors been tested in invasion biology (Maron et al. 2014; Eskelinen et al. 2017; Lembrechts et al. 2018). In the southern Rocky Mountains, a continued trend toward earlier spring snowmelt (Schwartz et al. 2006; Clow 2010), higher minimum temperatures (McGuire et al. 2012), and increased N availability from N deposition (Sievering 2001) in alpine ecosystems may operate together to make suitable conditions for some non-native plant species (i.e. species that are not dispersal limited and are able to grow in alpine soils).
Bromus tectorum L. (cheatgrass or downy brome) is an invasive, self-pollinating, winter annual grass that is likely to benefit from anthropogenic changes occurring in alpine ecosystems of the Rocky Mountains (Kao et al. 2008; Bromberg et al. 2011). Bromus tectorum is a widespread invasive species in the western United States which does not occur above treeline in the Rocky Mountains (Mack 1981; Chapin et al. 2000). Bromus tectorum is a facultative winter annual and thus pre-adapted to spend the seedling stage under snow and grow quickly in early spring. This species exhibits high phenotypic variability across growing conditions, which includes an ability to produce seed when plants are quite small (Chambers et al. 2007; Griffith et al. 2014). We expect that these traits make B. tectorum well suited for short, cold growing seasons (Leger et al. 2009; Concilio et al. 2013), and thus a candidate species for alpine invasion. While B. tectorum has been predicted to be temperature limited at its upper elevation range on the Colorado Plateau (Chambers et al. 2007), experimental evidence for this assertion is lacking. Additionally, Colorado’s mountain ecosystems are expected to experience an increase in mean air temperatures between 3 and 5 °C between 2035 and 2064 (Lukas et al. 2014). Evidence from N addition studies also demonstrates that B. tectorum is a strong competitor for N in its invaded range compared to native and agricultural plant species (Ball et al. 1996; Vasquez et al. 2008; He et al. 2011).
We experimentally test how environmental conditions may affect B. tectorum invasion in the alpine using growth chamber experiments in which we manipulate temperature, nitrogen (N) availability, and soil type. We hypothesized that cold growing season temperatures or spring subfreezing temperatures, or both, currently inhibit B. tectorum establishment in the alpine. Thus, warmer temperatures should enhance growth and reproduction of B. tectorum. Two experiments were conducted to address how increases in mean growing season temperature and increases in minimum (subfreezing) growing season temperatures affect B. tectorum growth and spikelet production. We also hypothesized that increased N availability from N deposition could promote invasive species establishment when temperature is not the primary constraint on growth, which we tested in both B. tectorum experiments. We additionally expected that alpine soils would be suitable for B. tectorum growth, especially under warmer temperatures and enhanced N availability. Montane soils were used to compare the relative suitability of alpine soils for B. tectorum growth.
Methods
Study species
Bromus tectorum was introduced to the western United States in the late nineteenth century. Populations were established throughout the Intermountain West by 1930 (Mack 1981). In its native range, which includes most of Europe, the northern edge of Africa, and western Asia, it is found at upper montane elevations below 3000 m (Upadhyaya et al. 1986; Novak and Mack 2001). There is no evidence of B. tectorum occurring above treeline in the Rocky Mountains at this time. Treeline occurs at 3400–3800 m in the southern Rocky Mountains (Peet 1978). Verified locations of B. tectorum populations at high elevations are rare, however, the Rocky Mountain Herbarium currently has 19 accessions of B. tectorum collected from 2743 to 3048 m throughout the southern Rocky Mountains (2019). Germination studies have shown that B. tectorum can germinate and grow in 5 °C nighttime and 10 °C daytime temperatures (Aguirre and Johnson 1991; Meyer et al. 1997), and root growth persists below this range and ceases around 3 °C (Harris 1967). Bromus tectorum is also adapted to a relatively wide range of physical soil properties (i.e. soil texture) (Norton et al. 2004; Reisner et al. 2013) and has been shown to respond positively to N when temperature and water availability are not limiting (Uresk et al. 1979; Concilio and Loik 2013).
Growing season experiment
To explore the effect of N availability on B. tectorum growth in alpine growing season temperatures, we conducted a pot experiment in two temperature and light-controlled growth chambers. We recorded establishment (survival to the end of the experiment) and B. tectorum growth in two trials, a current mean growing season temperature (hereafter, current temperature trial) and expected future alpine growing season temperatures (hereafter, warmer temperature trial) for Niwot Ridge, a long-term alpine study site. The 4 °C temperature increase we used for our warmer temperature trial is consistent with estimates for Colorado’s mountain regions, which are predicted to experience an increase in temperatures of 3–5 °C between 2035 and 2064 (Lukas et al. 2014). We tested whether simulated increases in N deposition would enhance the growth of B. tectorum with or without increases in temperature. In this experiment we collected B. tectorum seeds from a single montane population (elevation 1780 m, 40.1262° N, − 105.3078° W). Seeds were sown in a fine sandy loam alpine soil collected from a road cut at a dry meadow site on Niwot Ridge, CO (elevation 3466 m, lat 40.052486° N, long 105.582467° W). This soil type is classified as Moran family-Cryothent Series according to the Web Soil Survey (NRCS 2019). Additional soil characteristics are provided in Table 1 (Eilers et al. 2012). The top 20 cm of soil was collected from the road cut. This included soil from both the A and B horizons and therefore our growing media was a mixture of mineral and organic soil. The soil was mixed and sieved to 2 mm to homogenize and remove rocks and coarse organic material. Then the soil was placed in 164 ml 3.8 × 21 cm conical pots.
We germinated B. tectorum seeds on filter paper in petri dishes in both temperature trials to determine whether temperature influenced germination success. Seedlings were then transplanted into the prepared conical pots. The temperatures for the current growing season temperature trial was set to 12 °C daytime and 8 °C nighttime to simulate average July growing season temperature in the alpine (elevation 3,739 m) on Niwot Ridge (Greenland and Losleben 2001). The warmer summer growing season trial was set to 16 °C daytime and 12 °C nighttime temperatures. Both temperature trials were applied by growing plants in growth chambers, where plants received 14 h of daylight at 400 mmol photons m−2 s−1. These conditions were maintained throughout the germination and growth phases of the experiment.
Half of the pots began receiving the N addition treatment 40 days after transplanting, resulting in a total of 40 pots with 10 replicates for each temperature and N level combination. The N addition treatment of 20 kg N ha−1 year−1 was applied as NH4+NO3− dissolved in tap water at a concentration of 1 mmol N L−1 applied at 30 ml increments. Niwot Ridge receives approximately 8–9 kg N ha−1 year−1 (wet + dry) (NADP 2018). Given that alpine soils in the Front Range have been receiving N deposition at nearly this rate for many years, we added a higher rate of N above ambient N deposition in order to determine whether increases in the quantity or further accumulation of N would increase B. tectorum growth or reproduction compared to ambient N availability. The control treatment (ambient N) received the same volume of tap water without N addition. Pots were randomized in the growth chambers and we allowed the plants to grow for 78 days total to simulate the short alpine growing season. We then measured establishment, shoot length (longest leaf), dry shoot mass, and dry root mass.
Freeze recovery experiment
The goal of the freezing experiment was to determine whether frost events influence establishment and reproduction of B. tectorum, and whether soil type and N addition influence B. tectorum’s responses to simulated frost events. For this experiment, seeds from a different (higher elevation) montane population of B. tectorum (elevation 2632 m, lat 40.0024° N, long 105.5013° W) were collected in August of 2015. The alpine soils used in this experiment were from the same source as the soils used for the growing season experiment. Two additional gravely, sandy loam montane soils were collected from within a population of B. tectorum (elevation 2611 m, location 40.0481° N, 105.4671° W) and just outside of that population (elevation 2,611 m, 40.0481° N, 105.4665° W). These soils are classified as Ratake-Cathedral families-Rock outcrop complex according to Web Soil Survey (NRCS 2019). Additional soil characteristics are provided in Table 1 (Eilers et al. 2012). A primary goal of the study was to determine whether alpine conditions could be suitable for B. tectorum’s growth including both alpine soil properties and abiotic conditions. With this goal in mind, results from the growing season experiment indicated that a non-alpine soil treatment could be useful for interpreting growth results in alpine soil. The addition of the soil treatment for which soil from within the B. tectorum population allowed for comparing alpine soil to a soil known to be suitable for growth of B. tectorum. Addition of the non-conditioned, uninvaded soil, on the other hand, allowed for comparison of two uninvaded soils. These two soil types will be referred to as ‘conditioned’ and ‘non-conditioned’ hereafter. Each soil type was sieved and placed in conical pots (see growing season experiment section above). Seeds were germinated on filter paper and seedlings were planted in the prepared pots of all three soil types which were then randomly arranged to achieve a completely randomized experimental design.
Both temperature treatments were applied using a growth chamber where plants received 12 h of daylight at 400 mmol photons m−2 s−1. Seedlings were grown in 10 °C daytime and 5 °C nighttime temperatures for 50 days to mimic early growing season alpine temperatures. Watering and N additions were performed in the same manner as the growing season experiment (N addition was equivalent to 20 kg N ha−1 year−1). We measured plant height as length of inflorescence or length of longest leaf, whichever was longer.
Plants were then subjected to one of four subfreezing treatments. Plants were randomly assigned to a subfreezing temperature, − 8 °C, − 6 °C, − 4 °C or control (5 °C nighttime temperature). This range of temperatures were centered around the annual minimum June temperature at a high alpine weather station (3,739 m elevation) on Niwot Ridge, averaged across 1994–2014 minimums (mean = − 6 °C, median = − 6.5 °C) (Losleben 1994). The range of temperatures was intended to reveal whether a specific temperature threshold exists for survival or growth of B. tectorum or whether growth response is linear with increasing temperature. Freezing events occurred in an incubator for 3 h between 3:00 am and 6:00 am to simulate a realistic time period when minimum diurnal temperatures would naturally occur. Pots were randomly arranged within the incubator during freezing events.
After freezing events were conducted, pot locations in pot racks were re-randomized across all treatments and plants were allowed to recover from the freezing event in growth chambers with the same watering frequency for an additional 37 ± 3 days to determine whether soil type or N addition affected plants’ recovery from freezing. For this growth period, plants experienced 14 h per day with lights on to simulate lengthening daylight in the growing season. At the end of the experiment, establishment was recorded as survival from the freezing event to the end of the experiment. Plant heights were measured and plants were harvested to measure dry shoot mass and dry root mass. Additionally, reproduction potential was estimated as the number of fully emerged spikelets per plant. This estimate has been used previously (Griffith and Loik 2010; Concilio et al. 2013), and was used for this experiment because, while many plants matured to this flowering stage, none produced fruit over this time interval. For each treatment, between 13 and 16 plants survived for a total of 349 total plants that were included in analysis.
Data analyses
Total plant mass (root + shoot) was used as a response variable in ANOVAs for both experiments. A two-way ANOVA was conducted to determine the effects of N treatment in the two temperature trials on plant mass in the growing season experiment. The two temperature trials were not replicated and therefore we only report p-values for the N treatment effect. For the freeze recovery experiment, a three-way ANOVA was used to assess the response of plant mass to N, temperature, and soil. This ANOVA was performed using type III sum of squares (car package in R, Fox and Sanford 2011). Type III sum of squares is justified because of unbalanced sample sizes after a few plants died in various treatment groups as well as the presence of significant interactions in the model. Omega squared was calculated for the effect sizes (sjstats package in R, Lüdecke 2018; Yigit and Mendes 2018). The effects of freezing temperature and N addition were also assessed within the alpine soil treatment using a two-way ANOVA because a main objective of the experiment was to test for conditions that would allow alpine conditions to be suitable for B. tectorum establishment. We examined residuals for normality and sample sizes within treatments were sufficiently large for ANOVAs. All statistical analyses were performed using R statistical software (R Core Team 2016).
An additional test was conducted on spikelet number data in the freeze recovery experiment to assess treatment effects on potential reproduction. A zero-inflated negative binomial general linear model with a logit link function was used for these zero inflated count data (pscl package in R, Jackman 2017).
Results
Growing season experiment
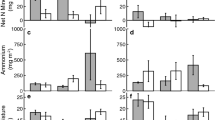
Under the current growing season temperature treatment, 46% of seeds germinated, while 88% of seeds germinated in the warmer temperature treatment. Establishment, which we defined as survival to the end of experiment, was 100%. At the end of the experiment, plant dry mass was greater for plants in the warmer temperature trial compared to the current temperature trial (Fig. 1), however we were unable to statistically separate the temperature affect from a potential growth chamber affect as a consequence of conducting the experiment one time in two different growth chambers. Plants in both temperature trials were small at the end of the experiment (mean height, 4.7 ± 0.15 cm; mean total mass, 0.015 ± 0.001 g) relative to individuals grown in the field from the populations where the seeds were collected (> 20 cm). The N addition treatment did not influence plant mass in either temperature trial (Table 2, Fig. 1). None of the plants in the experiment produced flowers.
Means and standard errors of B. tectorum total dry plant mass from the growing season experiment. The N addition treatment pots received the equivalent of 20 kg N ha−1 year−1. The current growing season treatment was set to 12 °C daytime, 8 °C nighttime, and the warmer growing season treatment was set to 16 °C daytime, 12 °C nighttime
Freeze recovery experiment
Two plants died within the first 10 days after the freezing events, and subtle changes in leaf coloration immediately following the freezing event returned to pre-freezing appearance within this time period. Post-freezing treatment establishment (survival) was 99% for the whole experiment. Subfreezing temperatures significantly affected total dry mass per plant (Table 2, Fig. 2). However, the effect of freezing temperature on plant dry mass differed depending on the soil type in which plants were grown (Table 2, Fig. 2). The model with all predictor variables (temperature, N, and soil) resulted in a significant effect of soil type on plant mass (Table 2, Fig. 2), with significant interactions between soil type and N level (Table 2, Fig. 2), as well as a three-way interaction among temperature, N, and soil type (Table 2, Fig. 2). Total plant mass was 38% lower in alpine soil compared to upper montane soil (Fig. 2).
Means and standard errors of B. tectorum total dry plant mass at the end of the freeze recovery experiment. The N addition treatment pots received the equivalent of 20 kg N ha−1 year−1. The control freezing temperature was 5 °C which was the nighttime temperature experienced by all plants during the growing and recover phases of the experiment
The different treatments did not influence potential reproduction in the same way as total plant mass for all predictor variables. Subfreezing temperatures did not influence the number of spikelets produced per plant (Table 2, Fig. 3). Spikelet production was, however, significantly greater in the N addition treatment relative to ambient N (Table 2, Fig. 3), and there was a significant interaction between soil type and N in the full model (Table 2, Fig. 3) despite soil types not having unique effects on spikelet production (F2,325 = 1.3, p = 0.273).
Means and standard errors of B. tectorum spikelet production per plant (including plants with zero spikelets). We pooled freezing temperature treatments because we did not detect an effect of freezing temperature on spikelet production. The N addition treatment pots received the equivalent of 20 kg N ha−1 year−1
Because the effect of temperature differed depending on the soil type (Fig. 2), and an objective for this experiment was to determine the suitability of alpine soils for B. tectorum, we conducted ANOVAs with the subset of plants grown in alpine soil. Within the alpine soil treatment, N addition decreased total plant mass (F1,106 = 13.1, p < 0.001) and the warmer temperatures resulted in marginally non-significant lower biomass than colder subfreezing temperatures (F3,106 = 2.5, p = 0.061). There was no significant interaction between N treatment and subfreezing temperature (F3,106 = 0.5, p = 0.678). For plants grown in the non-conditioned soil, there was a significant decrease in plant mass after exposure to lower subfreezing temperatures (F3,109 = 45.5, p < 0.001). Nitrogen addition also increased total mass in the non-conditioned soil type (F1,109 = 23.1, p < 0.001), and the magnitude of the effect of the different subfreezing temperatures on mass was greater in the N addition treatment (F3,109 = 13.6, p < 0.001) with the control and least cold freezing treatment (− 4 °C) showing increased biomass with N addition (Fig. 2). For plants grown in the conditioned soil from the upper montane B. tectorum population, N addition again significantly enhanced total mass (F1,110 = 166.2, p < 0.001), but exposure to subfreezing temperatures did not impact mass accumulation during the recovery period (F3,110 = 0.6, p = 0.63).
Discussion
The goal of this study was to evaluate multiple alpine invasion filters which we hypothesized may fail to prevent establishment of non-native species’ populations in the future as alpine regions experience more environmental changes. We found that alpine growing season temperatures, exposure to subfreezing temperatures, and N addition conditionally increased plant mass and reproduction of B. tectorum grown in montane soils, while alpine soil inhibited growth regardless of the other treatments.
Alpine soils inhibit mass and reproduction
Evidence from both experiments indicates that the alpine soil used in this experiment would be the most effective site-level invasion filter for inhibiting B. tectorum establishment. In the freezing experiment, we found that total biomass of plants grown in montane soils was more than 2.5 times greater than plants grown in alpine soil. Nitrogen addition and freezing treatments had no effect on this difference. In the growing season experiment, where all plants were grown in alpine soil, plant height and plant mass were small (plants were < 7 cm tall) and the plants did not produce flowers. Bromus tectorum is generally thought to be well adapted to a wide range of soil conditions (Bradford and Lauenroth 2006; Blank 2008), and yet our results suggest that the transition between upper montane and alpine soil may be an effective invasion barrier. Soil characteristics of the alpine soil source used in this study are on the periphery of soil characteristics for what is typically reported for B. tectorum populations in the invaded range (Bradford and Lauenroth 2006; Miller et al. 2006; Concilio et al. 2013). However, we note that the vast majority of studies reporting soil characteristics in B. tectorum populations were conducted in low elevation, arid ecosystems of the Great Basin. In comparison, the alpine soil used in our study is lower in pH and higher in organic matter than cool desert soils, and this may have contributed to poorer growth (mass) of B. tectorum in this study (Miller et al. 2006; Perkins et al. 2011).
The two montane soils were used in the freeze recovery experiment to provide both invaded soil and uninvaded soil as points of reference for the ability of B. tectorum to grow in alpine soil. Because B. tectorum performed worse in alpine soil compared to both montane soils, the inclusion of both non-conditioned and conditioned montane soil types in this study cannot help us determine whether a microbial mechanism may be involved in the inhibitory effect of alpine soils. Comparing B. tectorum mass in unconditioned montane and conditioned montane soils does suggest that negative microbial feedback loops may occur within the montane ecosystem (Evans et al. 2001; Concilio et al. 2015). Further investigations are necessary to determine whether alpine soil inhibits growth of invasives across different alpine soils in the Rocky Mountains and determine whether soil microbes or other attributes of alpine soil inhibit B. tectorum growth (North 2019). To our knowledge, soil characteristics that are not suitable for B. tectorum growth have not been assessed and therefore a number of potential characteristics including soil texture, bulk density, pH, and C:N may contribute to the inhibitory effect of the alpine soil (Table 1).
Interacting effects of nitrogen, soil, and temperature
Contrary to our hypothesis, N did not enhance the growth of B. tectorum in alpine soils. Thus, it is unlikely that N deposition would improve establishment or spread, or both, of B. tectorum into the alpine. In the growing season experiment, plants did not respond to N addition, even in the warmer than average growing season trial. The amount of N added (20 kg N ha−1 year−1) is near the upper end of the range of forecasted rates near urban and agricultural centers in the western United States for the middle of the twentyfirst century (Dentener et al. 2006). Thus, we interpret this result as evidence that N deposition will not facilitate B. tectorum establishment in this alpine ecosystem, even if rates of N deposition increase locally. This finding differs from lower elevations biomes in B. tectorum’s invaded range wherein B. tectorum often responds positively to high N availability, alters N cycling and changes competitive outcomes in invaded communities (Sperry et al. 2006; He et al. 2011; Concilio and Loik 2013). For example, Uresk et al. (1979) showed that soil temperature constrained growth (measured as dry mass) of B. tectorum below 11 °C, and that above this temperature, N availability influenced mass (Uresk et al. 1979).
Although N addition had no effect on B. tectorum grown in alpine soils, N addition did have a significant positive effect on total biomass accumulation and spikelet production for plants grown in the two montane soil treatments. For plants grown in the non-conditioned soil treatment, N increased plant mass in the control treatment (no freeze) and the least cold subfreezing treatment (− 4 °C), whereas temperature appeared to impede a response to N in the colder subfreezing treatments (− 6 °C and − 8 °C). These results show some similarities to an experiment with B. tectorum in the eastern Sierra Nevada, California, USA where N addition enhanced biomass accumulation but not spikelet production when water availability was not limiting (Concilio and Loik 2013). The positive response of B. tectorum to higher minimum temperatures and N addition in our study may suggest that increased N deposition and increased minimum temperatures during growing seasons could promote persistence and spread of current populations within montane ecosystems. This response would depend partly on current genetic variation among montane populations and differences in abilities of populations to adapt to montane conditions (Kao et al. 2008; Leger et al. 2009; Germino et al. 2016). Larger, persistent populations in the montane are cause for concern because they may provide more opportunities for adaptation to alpine conditions over time (Haider et al. 2010).
We originally hypothesized that cold alpine temperatures currently prevent establishment of non-native species in the alpine, as has been proposed previously (Pauchard et al. 2009). We found that the variation in growing season and extreme minimum temperatures had different effects on B. tectorum’s growth and reproduction respectively. This implies that there are multiple ways in which temperature could act as an invasion filter (Haider et al. 2011). First, no B. tectorum plants reached reproductive maturity under current or warmer growing season temperatures, which may be due to the inhibiting effect of the alpine soil and less to do with growing season temperature. Concilio et al. (2013) found that late melting snowpack delayed phenology but did not ultimately limit spikelet production compared to plots with earlier snow melt. In the context of climate warming, a shift in plant phenology with early spring snowmelt would also increase plants’ exposure to subfreezing temperatures, which is the second way temperature may act as an invasion filter (Synder and de Melo-Abreu 2005). However, none of the subfreezing temperature treatments resulted in B. tectorum mortality and 25% of plants produced flowers, suggesting that spring freezing events may not prevent B. tectorum from establishing in the alpine. This outcome differs from a transplant experiment where high rates of B. tectorum mortality occurred at a montane site but not at a lower elevation site. Abiotic constraints were not tested to determine the cause of mortality in the experiment (Leger et al. 2009).
Conclusion
Our study of plants grown from seed originating from a single population of B. tectorum suggests that locally the alpine in the Colorado Front Range is not at immediate risk of invasion by B. tectorum. Alpine soil was also associated with significantly lower plant biomass compared to montane soils in a related experiment that assessed B. tectorum growth in additional montane and alpine soils in the Front Range (North 2019). Warmer growing season temperatures that are expected with climate warming as well as anthropogenic N deposition may have little impact on non-native species’ establishment if site-level factors like soil strongly inhibit growth and reproduction. Further research is needed to determine the mechanism or mechanisms of soil inhibition and whether this inhibition may occur in other alpine soils from different geographic locations beyond the Front Range. The spread of B. tectorum populations at lower elevations within montane ecosystems is also a concern, and our results suggest that fewer freezing events, warmer freezing temperatures, or both, could enhance growth and reproduction of B. tectorum within montane populations. Haider et al. (2010, 2011) demonstrated that upslope range expansion of non-native plant species relies on genetic changes for species to become adapted to high elevation conditions, indicating that adaptation within current montane B. tectorum populations may be necessary for alpine invasions to occur. Acquiring beneficial genetic changes could be a slow process given that outcrossing is quite rare for B. tectorum (Leger et al. 2009; Haider et al. 2011). However, the discovery of relatively high genetic variability and outcrossing frequency (compared to other selfing plant species) within some montane populations of B. tectorum in the Rocky Mountains (Kao et al. 2008; Leger et al. 2009) indicates that adaptation to alpine conditions is a feasible trajectory for some populations.
Low temperatures and resource availability have been hypothesized as potentially important invasion filters in high elevation systems (Pauchard et al. 2009), but to our knowledge, this is the first time they have been experimentally tested together. Contrary to our expectations, warmer growing season temperatures, warmer minimum temperatures and increased N availability did not lower constraints on growth and reproduction when plants are grown in alpine soil. Our results demonstrate that alpine soil could be an effective invasion barrier for B. tectorum if the inhibitory attributes of the alpine soil we used are widespread.
References
Aguirre L, Johnson DA (1991) Influence of temperature and cheatgrass competition on seedling development of two bunchgrasses. J Range Manag 44:347–354. https://doi.org/10.2307/4002397
Alexander JM, Lembrechts JJ, Cavieres LA et al (2016) Plant invasions into mountains and alpine ecosystems: current status and future challenges. Alp Bot 126:89–103. https://doi.org/10.1007/s00035-016-0172-8
Ball DA, Wysocki DJ, Chastai TG (1996) Nitrogen application timing effects on downy brome (Bromus tectorum) and winter wheat (Triticum aestivum) growth and yield. Weed Technol 10:305–310
Barni E, Bacaro G, Falzoi S et al (2012) Establishing climatic constraints shaping the distribution of alien plant species along the elevation gradient in the Alps. Plant Ecol 213:757–767. https://doi.org/10.1007/s11258-012-0039-z
Blank RR (2008) Biogeochemistry of plant invasion: a case study with downy brome (Bromus tectorum). Invasive Plant Sci Manag 1:226–238. https://doi.org/10.1614/IPSM-07-026.1
Bowman WD, Bilbrough CJ (2001) Influence of pulsed nitrogen supply on growth and nitrogen uptake in alpine graminoids. Plant Soil 233:283–290. https://doi.org/10.1023/A:1010571920890
Bradford JB, Lauenroth WK (2006) Controls over invasion of Bromus tectorum: the importance of climate, soil, disturbance, and seed availability. J Veg Sci 17:693–704. https://doi.org/10.1658/1100-9233(2006)17%5b693:coiobt%5d2.0.co;2
Bradley BA, Wilcove DS, Oppenheimer M (2010) Climate change increases risk of plant invasion in the eastern United States. Biol Invasions 12:1855–1872. https://doi.org/10.1007/s10530-009-9597-y
Bromberg JE, Kumar S, Brown CS, Stohlgren TJ (2011) Distributional changes and range predictions of downy brome (Bromus tectorum) in Rocky Mountain National Park. Invasive Plant Sci Manag 4:173–182. https://doi.org/10.1614/IPSM-D-10-00022.1IPSM-D-10-00022.1
Cavieres LA, Quiroz CL, Molina-montenegro MA et al (2005) Nurse effect of the native cushion plant Azorella monantha on the invasive non-native Taraxacum officinale in the high-Andes of central Chile. Perspect Plant Ecol Evol Syst 7:217–226. https://doi.org/10.1016/j.ppees.2005.09.002
Chambers JC, Roundy BA, Blank RR et al (2007) What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum? Ecol Monogr 77:117–145. https://doi.org/10.1890/05-1991
Chapin FS, Zavaleta ES, Eviner VT et al (2000) Consequences of changing biodiversity. Nature 405:234–242. https://doi.org/10.1038/35012241
Clow DW (2010) Changes in the timing of snowmelt and streamflow in Colorado: a response to recent warming. J Clim 23:2293–2306. https://doi.org/10.1175/2009JCLI2951.1
Concilio AL, Loik ME (2013) Elevated nitrogen effects on Bromus tectorum dominance and native plant diversity in an arid montane ecosystem. Appl Veg Sci 16:598–609. https://doi.org/10.1111/avsc.12029
Concilio AL, Loik ME, Belnap J (2013) Global change effects on Bromus tectorum L. (Poaceae) at its high-elevation range margin. Glob Change Biol 19:161–172. https://doi.org/10.1111/gcb.12032
Concilio A, Vargas T, Cheng W (2015) Rhizosphere-mediated effects of the invasive grass Bromus tectorum L. and native Elymus elymoides on nitrogen cycling in Great Basin Desert soils. Plant Soil 393:245–257. https://doi.org/10.1007/s11104-015-2482-9
Davis MA, Grime JP, Thompson K et al (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534. https://doi.org/10.1046/j.1365-2745.2000.00473.x
Dentener F, Drevet J, Lamarque JF et al (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Glob Biogeochem Cycles 20:1–21. https://doi.org/10.1029/2005GB002672
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139
Dullinger S, Dirnböck T, Grabherr G (2003) Patterns of shrub invasion into high mountain grasslands of the northern calcareous Alps, Austria. Arct Antarct Alp Res 35:434–441
Eilers KG, Debenport S, Anderson S, Fierer N (2012) Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol Biochem 50:58–65. https://doi.org/10.1016/j.soilbio.2012.03.011
Eskelinen A, Kaarlejarvi E, Olofsson J (2017) Herbivory and nutrient limitation protect warming tundra from lowland species’ invasion and diversity loss. Glob Change Biol 23:245–255. https://doi.org/10.1111/gcb.13397
Evans RD, Rimer R, Sperry L, Belnap J (2001) Exotic plant invasion alters nitrogen dynamics in an arid grassland. Ecol Appl 11:1301–1310. https://doi.org/10.1890/1051-0761(2001)011%5b1301:epiand%5d2.0.co;2
Flores-Moreno H, Reich PB, Lind EM et al (2016) Climate modifies response of non-native and native species richness to nutrient enrichment. Philos Trans R Soc B Biol Sci 371:20150273. https://doi.org/10.1098/rstb.2015.0273
Fox J, Sanford W (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Germino MJ, Chambers JC, Brown CS (2016) Exotic brome-grasses in arid and semiarid ecosystems of the western US. Springer, New York
Greenland D, Losleben M (2001) Climate. In: Structure and function of an alpine ecosystem. Oxford University Press, New York and Oxford, pp 15–31
Griffith AB, Loik ME (2010) Effects of climate and snow depth on Bromus tectorum population dynamics at high elevation. Oecologia 164:821–832. https://doi.org/10.1007/s00442-010-1749-3
Griffith AB, Andonian K, Weiss CP, Loik ME (2014) Variation in phenotypic plasticity for native and invasive populations of Bromus tectorum. Biol Invasions. https://doi.org/10.1007/s10530-014-0692-3
Gross KL, Mittelbach GG, Reynolds HL (2005) Grassland invasibility and diversity: responses to nutrients, seed input, and disturbance. Ecology 86:476–486. https://doi.org/10.1890/04-0122
Haider S, Alexander J, Dietz H et al (2010) The role of bioclimatic origin, residence time and habitat context in shaping non-native plant distributions along an altitudinal gradient. Biol Invasions 12:4003–4018. https://doi.org/10.1007/s10530-010-9815-7
Haider S, Alexander JM, Kueffer C (2011) Elevational distribution limits of non-native species: combining observational and experimental evidence. Plant Ecol Divers 4:363–371. https://doi.org/10.1080/17550874.2011.637973
Harris GA (1967) Some competitive relationships between Agropyron spicatum and Bromus tectorum. Ecol Monogr 37:89–111
He WM, Yu GL, Sun ZK (2011) Nitrogen deposition enhances Bromus tectorum invasion: biogeographic differences in growth and competitive ability between China and North America. Ecography (Cop) 34:1059–1066. https://doi.org/10.1111/j.1600-0587.2011.06835.x
Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS (2008) Five potential consequences of climate change for invasive species. Conserv Biol 22:534–543. https://doi.org/10.1111/j.1523-1739.2008.00951.x
Hou Q, Chen B, Peng S, Chen L (2014) Effects of extreme temperature on seedling establishment of nonnative invasive plants. Biol Invasions 16:2049–2061. https://doi.org/10.1007/s10530-014-0647-8
Jackman S (2017) pscl: classes and methods for R developed in the political science computational laboratory. United States Studies Centre, University of Sydney. Sydney, New South Wales, Australia. R package version 1.5.2. https://github.com/atahk/pscl/
Jarnevich CS, Stohlgren TJ (2009) Near term climate projections for invasive species distributions. Biol Invasions 11:1373–1379. https://doi.org/10.1007/s10530-008-9345-8
Johnston FM, Pickering CM (2001) Alien plants in the Australian Alps. Mt Res Dev 21:284–291. https://doi.org/10.1659/0276-4741(2001)021%5b0284:apitaa%5d2.0.co;2
Kao RH, Brown CS, Hufbauer RA (2008) High phenotypic and molecular variation in downy brome (Bromus tectorum). Invasive Plant Sci Manag 1:216–225. https://doi.org/10.1614/ipsm-07-045.1
Leger EA, Espeland EK, Merrill KR, Meyer SE (2009) Genetic variation and local adaptation at a cheatgrass (Bromus tectorum) invasion edge in western Nevada. Mol Ecol 18:4366–4379. https://doi.org/10.1111/j.1365-294X.2009.04357.x
Lembrechts JJ, Pauchard A, Lenoir J et al (2016) Disturbance is the key to plant invasions in cold environments. Proc Natl Acad Sci 113:1–6. https://doi.org/10.1073/pnas.1608980113
Lembrechts JJ, Lenoir J, Nuñez MA, Pauchard A, Geron C, Bussé G, Milbau A, Nijs I (2018) Microclimate variability in alpine ecosystems as stepping stones for non-native plant establishment above their current elevational limit. Ecography 41:900–909. https://doi.org/10.1111/ecog.03263
Levine JM, Vilà M, Antonio CMD, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond Ser B Biol Sci 270:775–781
Liu Y, van Kleunen M (2017) Responses of common and rare aliens and natives to nutrient availability and fluctuations. J Ecol 105:1111–1122. https://doi.org/10.1111/1365-2745.12733
Lonsdale W (1999) Global patterns of plant invasion and the concept of invasibility. Ecology 80:1522–1536. https://doi.org/10.1890/0012-9658
Losleben M (1994) D-1 (3743 m) climate station at Niwot Ridge LTER: CR21X data
Lüdecke D (2018) sjstats: statistical functions for regression models (version 0.17.2). Zenodo. https://doi.org/10.5281/zenodo.1489175
Lukas J, Barsugli J, Doesken N, Rangwala I, Wolter K (2014) Climate change in Colorado: a synthesis to support water resources management and adaptation. In: A report for the Colorado Water Conservation Board, University of Colorado Boulder, Westen Water Assessment, Cooperative Institute for Research in Environmental Sciences (CIRES), pp 1–114
Mack RN (1981) Invasion of Bromus tectorum L. into western North America: an ecological chronicle. Agro-Ecosystems 7:145–165. https://doi.org/10.1016/0304-3746(81)90027-5
Marini L, Gaston KJ, Prosser F, Hulme PE (2009) Contrasting response of native and alien plant species richness to environmental energy and human impact along alpine elevation gradients. Global Ecol Biogeogr 18:652–661
Maron J, Auge H, Pearson D et al (2014) Staged invasions across disparate grasslands: effects of seed provenance, consumers and disturbance on productivity and species richness. Ecol Lett 17:499–507. https://doi.org/10.1111/ele.12250
McGuire CR, Nufio CR, Bowers MD, Guralnick RP (2012) Elevation-dependent temperature trends in the Rocky Mountain Front Range: changes over a 56- and 20-year record. PLoS ONE 7:e44370. https://doi.org/10.1371/journal.pone.0044370
Meyer SE, Allen PS, Beckstead J, Seed J (1997) Seed germination regulation in Bromus tectorum (Poaceae) and its ecological significance. Oikos 78:475–485
Milberg P, Lamont BB, Pérez-fernández MA et al (1999) Survival and growth of native and exotic composites in response to a nutrient gradient. Plant Ecol 145:125–132. https://doi.org/10.1023/A:1009817804815
Miller ME, Belnap J, Beatty SW, Webb BL (2006) Performance of Bromus tectorum L. in relation to soil properties, water additions, and chemical amendments in calcareous soils of southeastern Utah, USA. Plant Soil 288:19–29. https://doi.org/10.1007/s11104-006-9014-6
Myers JA, Harms KE (2009) Seed arrival, ecological filters, and plant species richness: a meta-analysis. Ecol Lett 12:1250–1260. https://doi.org/10.1111/j.1461-0248.2009.01373.x
NADP (2018) National atmospheric deposition program. In: Total Depos. Maps, v2018.01. http://nadp.slh.wisc.edu/committees/tdep/tdepmaps/
Najberek K, Nentwig W, Olejniczak P et al (2017) Factors limiting and promoting invasion of alien Impatiens balfourii in alpine foothills. Flora Morphol Distrib Funct Ecol Plants 234:224–232. https://doi.org/10.1016/j.flora.2017.08.002
North H (2019) Cheating the (eco)system: potential for invasive grass growth above treeline. Undergraduate honors thesis, University of Colorado. https://scholar.colorado.edu/honr_theses/1850
Norton JB, Monaco TA, Norton JM et al (2004) Soil morphology and organic matter dynamics under cheatgrass and sagebrush-steppe plant communities. J Arid Environ 57:445–466. https://doi.org/10.1016/S0140-1963(03)00104-6
Novak SJ, Mack RN (2001) Tracing plant introduction and spread: genetic evidence from Bromus tectorum (cheatgrass). Bioscience 51:114–122. https://doi.org/10.1641/0006-3568(2001)051%5b0114:tpiasg%5d2.0.co;2
NRCS (2019) Web soil survey. In: Natural resources conservation service. United States Department of Agriculture. http://websoilsurvey.nrcs.usda.gov
Pauchard A, Alaback BP (2004) Influences of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of southcentral Chile. Conserv Biol 18:238–248. https://doi.org/10.1111/j.1523-1739.2004.00300.x
Pauchard A, Kueffer C, Dietz H et al (2009) Ain’t no mountain high enough: plant invasions reaching new elevations. Front Ecol Environ 7:479–486. https://doi.org/10.1890/080072
Peet R (1978) Latitudinal variation in southern Rocky Mountain forests. J Biogeogr 5:275–289. https://doi.org/10.2307/3038041
Perkins LB, Johnson DW, Nowak RS (2011) Plant-induced changes in soil nutrient dynamics by native and invasive grass species. Plant Soil 345:365–374. https://doi.org/10.1007/s11104-011-0788-9
Petitpierre B, MacDougall K, Seipel T et al (2015) Will climate change increase the risk of plant invasions into mountains? Ecol Appl 26:150709023716008. https://doi.org/10.1890/14-1871.1
Pinto SM, Pearson DE, Maron JL (2014) Seed dispersal is more limiting to native grassland diversity than competition or seed predation. J Ecol. https://doi.org/10.1111/1365-2745.12282
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rao LE, Allen EB (2010) Combined effects of precipitation and nitrogen deposition on native and invasive winter annual production in California deserts. Oecologia 162:1035–1046
Reisner MD, Grace JB, Pyke DA, Doescher PS (2013) Conditions favouring Bromus tectorum dominance of endangered sagebrush steppe ecosystems. J Appl Ecol 50:1039–1049. https://doi.org/10.1111/1365-2664.12097
Rocky Mountain Herbarium (2019) In: Specimen database. http://rmh.uwyo.edu/data/search.php
Rundel PW, Keeley JE (2016) Dispersal limitation does not control high elevational distribution of alien plant species in the southern Sierra Nevada, California. Nat Areas J 36:277–287. https://doi.org/10.3375/043.036.0308
Schwartz MD, Ahas R, Aasa A (2006) Onset of spring starting earlier across the northern hemisphere. Glob Change Biol 12:343–351. https://doi.org/10.1111/j.1365-2486.2005.01097.x
Sievering H (2001) Atmospheric chemistry and deposition. In: Bowman WD, Seastedt TR (eds) Structure and function of an alpine ecosystem. Oxford University Press, New York, pp 32–44
Sperry LJ, Belnap J, Evans RD (2006) Bromus tectorum invasion alters nitrogen dynamics in an undisturbed arid grassland ecosystem. Ecology 87:603–615. https://doi.org/10.1890/05-0836
Synder RL, de Melo-Abreu JP (2005) Frost protection: fundamentals, practice and economics. Food and Agriculture Organization of the United Nations, Rome
Theoharides K, Dukes J (2007) Plant invasion across space and time: factors affecting nonindigenous species success during four stage of invasion. New Phytol 176:256–273. https://doi.org/10.1111/j.1469-8137.2007.02207.x/pdf
Upadhyaya MK, Turkington R, McIlvride D (1986) The biology of Canadian weeds. 75. Bromus tectorum L. Can J Plant Sci 66:689–709
Uresk D, Cline J, Rickard W (1979) Growth rates of a cheatgrass community and some associated factors. J Range Manag 32:168–170
Vasquez E, Sheley R, Svejcar T (2008) Nitrogen enhances the competitive ability of cheatgrass (Bromus tectorum) relative to native grasses. Invasive Plant Sci Manag 1:287–295. https://doi.org/10.1614/IPSM-08-062.1
Verlinden M, Nijs I (2010) Alien plant species favoured over congeneric natives under experimental climate warming in temperate Belgian climate. Biol Invasions 12:2777–2787. https://doi.org/10.1007/s10530-009-9683-1
Walther GR, Roques A, Hulme PE et al (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693. https://doi.org/10.1016/j.tree.2009.06.008
Yigit S, Mendes M (2018) Which effect size measure is appropriate for one-way and two-way ANOVA models? A Monte Carlo simulation study. Revstat Stat J 16:295–313. https://doi.org/10.1111/j.1468-2958.2002.tb00828.x
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Potter, T.S., Bowman, W.D. Testing invasion filters for the alpine: the roles of temperature, nitrogen deposition and soil. Biol Invasions 22, 1889–1901 (2020). https://doi.org/10.1007/s10530-020-02225-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02225-5