Abstract
An important factor influencing whether or not a non-native plant species becomes invasive is the climate in the area of introduction. To become naturalised in the new range, a species must either be climatically pre-adapted (climate matching), have a high phenotypic plasticity, or be able to adapt genetically, which in the latter case may take many generations. Furthermore, patterns of successful establishment across species might vary with habitat context. To address the interaction of these factors on non-native species richness, we recorded the presence of non-native annual plant species along an altitudinal gradient on Tenerife (Canary Islands, Spain). We compared the distributions of species differing in bioclimatic origin (Mediterranean and temperate) and time since introduction (old and recent introductions), and compared richness patterns of these groups in anthropogenic and natural habitats. Non-native species richness increased strongly from lowlands to mid-altitudes, but dropped sharply at the transition from anthropogenic to natural habitats, and thereafter declined with altitude in the natural habitat. This pattern indicates that the altitude effects reflected changes in both climate and habitat context. Mediterranean and temperate species were distributed similarly along the altitudinal gradient, and we found no effect of bioclimatic origin on species distributions. As almost all species present at the highest sites also occurred in the lowlands, we conclude that most species were introduced to lowland sites and were therefore pre-adapted to those climatic conditions (lowland introduction filter). The altitudinal ranges of species tended to increase with time since introduction, and the species reaching the highest altitudes were mostly old introductions. This effect of time was more pronounced among Mediterranean than temperate species. Thus, while climatic pre-adaptation is important for establishment along this altitudinal gradient, species tend to extend their altitudinal range with time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The climatic conditions in the area of introduction have recurrently been shown to influence the outcome of plant invasions (e.g. Kitayama and Mueller-Dombois 1995; Kueffer et al. 2010; Thuiller et al. 2005) and are important for predictions made in weed risk assessment systems (Gordon et al. 2008; Tatem and Hay 2007). Consequently, the invasiveness of a plant species may change considerably with climate change (Dukes and Mooney 1999; Walther et al. 2009).
To establish and spread in a new area, a species must be able to tolerate the prevailing climatic conditions. This is possible if the species originates in a region that is climatically similar; indeed, climate matching has emerged in many studies as a consistent and important predictor of the potentially invaded area of a non-native species (Dawson et al. 2009; Kolar and Lodge 2001). However, although ecological niche modelling based on climate may be useful for predicting whether a species will become invasive (Peterson 2003; Thuiller et al. 2005), the climatic niche of some non-native plants appears to have changed in the introduced range (niche shift, Alexander and Edwards 2010; Beaumont et al. 2009; Broennimann et al. 2007; Maron et al. 2007). For instance, Broennimann et al. (2007) showed that the European herb Centaurea maculosa established in the USA within the climatic niche of its native range, but from there it colonized novel niche space. For this reason, the assumption underlying niche modelling—that climatic niches are stable (niche conservatism)—has recently been challenged.
A useful approach for elucidating the role of climate in limiting invasions is to investigate the distribution of non-native species along an altitudinal gradient (e.g. Alexander et al. 2009a; Johnston and Pickering 2001; Marini et al. 2009; Parks et al. 2005; Pauchard et al. 2009; Sullivan et al. 2009). Such studies have consistently shown a strong decrease of non-native species richness with increasing altitude, at least from mid- to high-altitudes (Becker et al. 2005; Daehler 2005; McDougall et al. 2005; Pauchard and Alaback 2004; Pauchard et al. 2009; Wester and Juvik 1983). Studies in other ecosystems have shown that invasibility tends to decline with the severity of environmental conditions (Alpert et al. 2000), and it has therefore been argued that climate is the most important factor limiting the spread of non-native plants to high altitudes (Pauchard et al. 2009), where climate conditions are unfavourable for most species (Körner 2003).
The spread of non-native plants in mountainous regions has usually been studied along roads (Alexander et al. 2009b; Arteaga et al. 2009; Sullivan et al. 2009; Wilson et al. 1992); this is appropriate not only for practical reasons, but because roads are important dispersal corridors (Christen and Matlack 2009; Johnston and Johnston 2004; Lilley and Vellend 2009) and roadsides are usually disturbed habitats (Christen and Matlack 2006; Forman et al. 2003) which favour the establishment of non-native species (Gelbard and Belnap 2003). In addition, with the exception of climate, the most relevant abiotic (e.g. nutrient availability) and biotic conditions (e.g. competition) for non-native species’ establishment success are relatively constant along road verges over the whole altitudinal gradient (Ullmann and Heindl 1989; Wilson et al. 1992). Finally, efficient anthropogenic dispersal along roads makes it unlikely that the altitudinal limits of species are dispersal limited but rather are in equilibrium with their climatic limits (e.g. Alexander et al. 2009b).
Although roadsides offer relatively constant site conditions, the distribution of non-native species is also influenced by neighbouring habitats, and previous studies have shown that species richness depends strongly on the habitat context (e.g. Chytrý et al. 2009; Vilá et al. 2007).
We recorded non-native annual plant species along two roads on the island of Tenerife (Canary Islands, Spain). Tenerife was chosen because oceanic islands are convenient model systems for invasion biology (Daehler 2005; Kueffer et al. 2010), and this particular island offers a steep climatic gradient, ranging from subtropical conditions at the coast to a subalpine climate above 2,000 m a.s.l. To elucidate whether climatic pre-adaptation matters for non-native plant establishment, we compared the altitudinal distributions of non-native plant species of Mediterranean and temperate origin. Within both groups we also discriminated between old and recent introductions to investigate whether residence time is a factor affecting the altitudinal ranges of non-native plants. Such an effect could reflect either the time that it takes for a species to disperse, or the time needed to adapt to changing conditions along an altitudinal gradient (Becker et al. 2005).
In this paper we address the following hypotheses: (1) there is an altitudinal zonation of non-native species due to bioclimatic origin, with Mediterranean species dominating at low altitude roadside communities and temperate species in high altitude ones; (2) within bioclimatic groups old-established non-native plant species have broader altitudinal ranges than recent introductions. We predict that (3) species richness patterns will show a hump-shaped distribution with altitude due to the overlap of species ranges established under hypotheses (1) and (2). However, we expect that (4) these altitudinal distribution patterns also depend on the habitat context, i.e. the response of species to altitude might be modulated by the zonation of habitat types along the altitudinal gradient.
Methods
Study area
The study sites were located in the northern part of Tenerife (Canary Islands, Spain, 28°N, 16°W), which is the largest island (2,033 km²) of the volcanic Canarian archipelago and represents the highest mountain of Spain (Pico de Teide, 3,718 m a.s.l.). The climate of Tenerife is strongly influenced by north-eastern trade winds, and the northern and southern parts of the island differ greatly in temperature and precipitation. The windward northern part, where our study was located, is characterized by a strong climatic zonation along the altitudinal gradient (Whittaker and Fernández-Palacios 2007). Mean annual temperature declines from 19°C at sea level to 11°C at 2,000 m a.s.l. Low altitudes are characterized by a Mediterranean-type climate with mild, wet winters and warm, dry summers (<300 mm annual precipitation) (Sperling et al. 2004). A temperature inversion at mid-altitudes causes a relatively persistent cloud layer, typically between 1,000 and 1,500 m a.s.l., leading to a more humid climate in this altitudinal band (>700 mm/year) (Fernández-Palacios 1992). Above the inversion the climate is again dry and cool (<500 mm/year). The natural vegetation follows the climatic zonation, with semi-desert scrub below the clouds, Erica-Myrica woody heath and humid pine forest (Pinus canariensis) in the cloud layer, and dry pine forest and subalpine scrub above the cloud layer (Fernández-Palacios and de Nicolás 1995). During the growing season, precipitation is mainly very low and differences between altitudes are diminished (Fig. 1). In our study area, relatively undisturbed, natural vegetation started at c. 1,000 m a.s.l. at the lower boundary of the persistent cloud layer; this was entirely composed of pine forest except for the highest sites at 2,000 m a.s.l. where there was a subalpine scrub on loose volcanic gravel. Where the road passes through these vegetation types, referred to as natural habitat (NAT), the herb layer along the roadside is usually sparse (0–16%) due to the dense accumulation of pine needles from the forest. In the study area, the canopy cover in the pine forest ranges from 0 to c. 75%, but varies between the more humid lower part where trees are dense (30–75%) and the drier upper part which is more open (0–20%; S. Haider, unpublished data).
Variation of mean temperature (solid line with open symbols) and mean monthly precipitation (dashed line with filled symbols) with altitude in the study area during the growing season (April to June). Climate data for every site was compiled through Worldclim (Hijmans et al. 2009)
Anthropogenic influences are very high in the zone between the coast and c. 1,000 m a.s.l., with agriculture (e.g. bananas, tomatoes) and dense settlements spread over the entire landscape (anthropogenic habitat, ANT). Roadside communities in this habitat consist mainly of open vegetation, which is only rarely shaded by trees (herb layer cover: 4–56% of ground area, canopy cover: 0–9%; S. Haider, unpublished data).
Soil conditions, especially pH values, differ considerably between the lowest sites below 400 m a.s.l. (pH: 7.0–8.0) and higher altitudes (pH: 5.0–6.3; S. Haider, unpublished data). Our roads passed mostly through lava of intermediate age (i.e. in the order of 100 ka), which corresponds to young basaltic bedrock, except for the lowest sites (100 and 200 m a.s.l. at road A and 100 m a.s.l. at road B) that were placed on very old lava (4–5 Ma). No site was situated on an historic lava flow (i.e. within several hundred years old) (Hoernle and Carracedo 2009).
Species
We recorded all non-native annual, flowering plant species that are known to have originated in a region with either a Mediterranean or temperate climate (Dahl 1998; Schultz 2005). We focused on annual plants to avoid confounding the results with different life forms, since it has been shown that lowland and high altitude non-native floras tend to harbour different proportions of annuals and perennials (McDougall et al. unpublished data). However, we also included species that are not strictly annual (e.g. Tragopogon porrifolius, which can also be biennial). Non-native species were further divided into two groups according to their time since introduction. We considered as old introductions all plant species that might have been introduced by the Romans, Spanish or Portuguese before the year 1,500, in a period before trade with other continents became common. International trade gained importance from the 16th century, and intensified contacts with the New World and Asia led to the introduction of many new plants, which we regard as recent introductions.
To distinguish between native and non-native species, and to determine the time of introduction and bioclimatic origin of non-native species, we compiled information about species distribution and introduction status in the whole Macaronesian floristic region and classified species based on the literature (“Appendix 1”), personal communication with other scientists, and our own expertise. Taxonomy was standardized with the Germplasm Resources Information Network (GRIN) online database (USDA 2009).
Data collection
Data was collected along two paved roads that were similar with respect to traffic intensity and climatic conditions, and extended from 100 to 2,000 m a.s.l. Road A led from Bajamar via La Laguna and La Esperanza to El Portillo where it met road B coming via La Orotava and Aguamansa from El Sauzal (different roads than in Arévalo et al. 2005, 2010; Arteaga et al. 2009). Traffic intensity was highest in the vicinity of the cities of La Laguna and La Orotava (c. 20,000 cars/day; Cabildo Tenerife 2007) and declined towards the coast and towards higher altitudes (c. 2,000–12,000 cars/day in coastal areas and mid-altitudes). Above c. 1,000 m a.s.l. traffic intensity remained constant with c. 1,000–2,000 cars/day (Cabildo Tenerife 2007).
We recorded the vegetation during two growing seasons—from March to May 2007 and May to June 2008—to reduce the risk of bias due to extreme conditions in a single year. The sampling period in the second season was shifted to be sure of sampling species with both early and late phenologies. Data from both years were pooled, i.e. a species was identified as present if it was recorded at least in one year. Sampling sites were placed at 100 m altitudinal intervals (hereafter “site”). At each site we recorded the presence of the target species in two 250 m × 2 m transects along both sides of the road and located immediately adjacent to the paved area. Observations for both transects per site were pooled and data analysis was performed with species richness per site.
Within each of the roadside transects we established a subplot of 12.5 m × 2 m (longer side parallel to the road) to record non-native species and total vegetation cover-abundance using the Domin-Scale (1 = very scarce, ≤4%, 2 = scarce, ≤4%, 3 = scattered, ≤4%, 4 = 4–10%, 5 = 10–25%, 6 = 25–33%, 7 = 33–50%, 8 = 50–75%, 9 = 75–95%, 10 = 95–100%; Bannister 1966). Prior to the analysis classification values were transformed according to Currall (1987). Overall we sampled two roads with 20 sites each. At all sites we recorded the habitat type (anthropogenic vs. natural habitat).
Data analysis
To investigate broad patterns of species richness along the altitudinal gradient, and whether these patterns differed between different habitat types (anthropogenic, ANT; natural, NAT), general linear mixed effects models were first fitted using the “lme” function in R (R Foundation for Statistical Computing, version 2.10.1 for Windows; “nlme” package). Four models of total species richness were fitted containing different fixed effects: (1) altitude only, (2) the second-order polynomial of altitude, (3) altitude, habitat type (ANT; NAT) and their interaction and (4) the second-order polynomial of altitude, habitat type (ANT, NAT) and their interaction. All four models included site nested within road as random effects and were fitted using the maximum likelihood method to enable their comparison based on Akaike’s Information Criterion (AIC). The model with the lowest AIC score, or the most parsimonious model in the case of a difference in scores of less than 2, was favoured. Additional models with the same random effects were fitted using the REML method to investigate differences in the responses of alternative sub-groups of species (cf. Öckinger et al. 2009). These models contained the fixed effects of altitude, habitat type and either bioclimatic origin (Mediterranean, MED vs. temperate, TEMP) or time since introduction (old introductions, OLD vs. recent introductions, NEW), and all 2- and 3-way interactions. Significant 3-way interactions were further explored by re-fitting these models separately for the ANT and NAT habitats.
We extracted the minimum and maximum altitudes for all species and calculated the altitudinal range for all species that were recorded at least twice. We then used non-parametric Wilcoxon rank-sum tests to compare the altitudinal distributions of species groups with different bioclimatic origins (MED vs. TEMP) and different times since introduction (OLD vs. NEW). We also generated a predicted species richness curve based on the altitudinal species ranges and the assumption that each species occurs in every site within its range.
To test whether the non-native species composition of sites was nested, we calculated the NODF metric of Almeida-Neto et al. (2008) using the R-package Vegan (version 1.17–2). We produced two species-site matrices, with sites either maximally packed or ordered by altitude. An additional matrix was constructed assuming species to be present at all sites within their altitudinal range. Tests of nestedness of sites were based on 1,000 randomizations of the matrix using a null model that constrained species richness within sites whilst randomizing the occurrence of species within sites (method R1; Wright et al. 1998).
Results
Non-native roadside flora
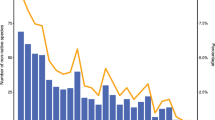
We recorded a total of 58 non-native annual plant species, of which 79% were of Mediterranean (MED) and 21% of temperate origin (TEMP; “Appendix 2”). We found more old (OLD) than recent (NEW) introductions (62% and 38% of the species, respectively). Within the TEMP group there were 58% OLD and 42% NEW introductions, while within the MED group 63% were OLD and 37% NEW. A rank-abundance curve showed a rather smooth decline in species’ abundance (Fig. 2), indicating that the non-native flora is not strongly dominated by a few very abundant species. The most important plant families were Fabaceae (15 species), Asteraceae (11 species), and Brassicaceae (8 species), which comprised together more than half of the sampled species. Altogether the species recorded were from 17 families and 41 genera. Each group contained 11 or 12 families (MED and NEW, and TEMP and OLD, respectively), but the species of the most frequent families were distributed unequally. Almost all Fabaceae, Asteraceae and Brassicaceae species were of MED origin. Whereas Fabaceae and Asteraceae species were more equally distributed between OLD and NEW species, all Brassicaceae species except one belonged to the OLD group.
Rank-abundance distribution of the recorded a Mediterranean (N = 46) and b temperate (N = 12) non-native species along roadsides. The y-axis indicates the proportion of sites in which each species was present. Black bars represent old-introduced (OLD), white bars recently-introduced (NEW) species. Species abbreviations are always composed by the first four letters of genus and species. Complete species names can be found in “Appendix 2”
The cover of individual species was mainly low (≤4% of ground area) and, with few exceptions, constant across sites. Only Hirschfeldia incana (MED-OLD) was recorded with cover class 25–33% in one plot at 1,000 m a.s.l. and Sisymbrium erysimoides (MED-OLD) reached 4–10% in one plot at 100 m a.s.l. There was a positive relationship between non-native species richness and non-native species cover per plot (R² = 0.87, P < 0.001).
Species altitudinal ranges
Eighty-eight percent of the sites contained at least one non-native species. The majority of species were found in plots of the anthropogenic habitat (ANT), and 52% of the species were present only in this habitat (Fig. 3). Only three species—Eschscholzia californica, Tragopogon porrifolius, and Trifolium ligusticum (all MED)—occurred exclusively in the natural habitat (NAT habitat). Fifty-two percent of all MED species and 61% of all OLD species were present in NAT habitat, while these proportions were only 33% and 27% for TEMP and NEW species, respectively (Fig. 3). On average, OLD species reached higher altitudes (Wilcoxon rank-sum test, N = 58, W = 557, P = 0.009, two-tailed) and colonized a wider altitudinal range (Wilcoxon rank-sum test, N = 52, W = 449.5, P = 0.015, two-tailed) than NEW species (Fig. 4). The groups did not differ in their lower altitudinal limit (Wilcoxon rank-sum test, N = 58, W = 406.5, P = 0.869, two-tailed). For the MED and TEMP groups, there were no significant differences in the lower and upper altitudinal limits of species, nor in their altitudinal ranges (Wilcoxon rank-sum test, lower limit: N = 58, W = 288.5, P = 0.813, two-tailed, upper limit: N = 58, W = 289, P = 0.808, two-tailed, range: N = 52, W = 210.5, P = 0.745, two-tailed).
Altitudinal distribution ranges (lines) of non-native a Mediterranean (N = 41) and b temperate (N = 11) species that occurred at least twice along the altitudinal gradient. Old-introduced species (OLD) are indicated with solid lines, recently-introduced species (NEW) with dashed lines. The symbols (filled for OLD, open for NEW) are placed at the mean altitude where the species occurred. Species are sorted according to their altitude of maximum occurrence. The boarder between anthropogenic and natural habitat is at 1,000 m a.s.l.
Comparison of a the maximum altitude and b the colonized altitudinal gradient of old- (OLD) and recently-introduced (NEW) species. The unusual shape of the box for the maximum altitude of NEW species arises from the fact that almost half of the species have a maximum altitude of 1,000 m a.s.l. and that there are only four outliers above (the outlier at 1,400 m a.s.l. occurred twice)
Although sites were significantly nested using the original presence-absence matrix (N sites = 40.2, z = 2.78, P = 0.013), the species composition of high-altitude sites was not significantly nested in low-altitude sites (N sites = 24.4, z = −0.09, P = 0.964). However, the species composition of sites was significantly nested in relation to altitude under the assumption that species were present at every site within their altitudinal range (N sites = 53.4, z = 9.36, P < 0.001).
Variation in non-native species richness along the altitudinal gradient
Species richness showed a strongly humped relationship with altitude, with richness peaking in the middle of the gradient, between 600 and 1,000 m a.s.l. (Fig. 5). However, this relationship was not smoothly polynomial (AIC = 283.49; “Appendix 3”) but rather was best described by a model containing two linear relationships, with a linear increase in richness in the anthropogenic habitat up to c. 1,000 m a.s.l., and a much lower and slightly declining richness above this point (significant interaction between altitude and habitat type, AIC = 238.94, F 1,35 = 31.64, P < 0.001; Fig. 5; “Appendix 3”).
Along the whole altitudinal gradient (in ANT habitat as well as in NAT habitat) MED species were more numerous than TEMP species, with an average of seven (357%) more MED than TEMP species per site (Table 1). MED species were present in 88%, and TEMP species in 75% of sites. Both bioclimatic groups showed the same response to altitude (Fig. 6a, b; Table 2).
Species richness of a Mediterranean (MED), b temperate (TEMP), c old-introduced (OLD) and d recently-introduced (NEW) species along the altitudinal gradient. Dots indicate observed species richness. Crosses show species richness predicted from the altitudinal ranges of the species. Predicted species richness was based on the assumption that species occur at all sites within their altitudinal range
OLD species were present in 88% of sites, and NEW species in 75%. Across both habitat types the mean number of OLD species per site was significantly higher than the number of NEW species (on average five more OLD species per site; Fig. 6c and d). In NAT habitat both groups had a similar decrease in species richness with increasing altitude. However, in ANT habitat the increase of OLD species with increasing altitude was significantly faster than for NEW species (Table 2).
Predicted species richness followed the observed species richness patterns closely with the exception of the lower end of the NAT habitat (Fig. 6). Predicted species richness was based on observed species altitudinal ranges (Fig. 3) and the assumption that a species occurs in every site that is within its observed altitudinal range.
Discussion
Does bioclimatic origin determine species distribution along the altitudinal gradient?
We hypothesised that bioclimatic origin leads to an altitudinal zonation of Mediterranean and temperate non-native annual species. However, Mediterranean and temperate species responded very similarly to altitude, and there was no evidence for any altitudinal separation of the two groups. Indeed, almost all species present at high altitudes also occurred in the lowlands. These patterns may be explained by assuming that most non-native plant species initially establish at low altitudes and thus need to be climatically pre-adapted to lowland conditions (lowland introduction filter, Becker et al. 2005; Pauchard et al. 2009). A lowland introduction filter may also explain why we found overall more Mediterranean than temperate non-native species, since Mediterranean species are pre-adapted to such a lowland climate and may be more likely to establish than temperate species.
Our results are consistent with those from a global survey of non-native floras in mountainous regions compiled by McDougall et al. (unpublished data), which found that high altitude floras tend to be similar to those at low altitudes in the same region, even though the climate may change dramatically along the altitudinal gradient. In contrast, mountain floras in different regions with a similar alpine climate tend to be dissimilar, which the authors interpret as reflecting their differing introduction histories. The distributional patterns in our study differ from those on the oceanic islands of Hawaii, where there is a turnover of species of different bioclimatic origin along the altitudinal gradient (Daehler 2005; Wester and Juvik 1983). A possible explanation is that species introductions in Hawaii took place along a larger altitudinal gradient, including intensely used grasslands at high altitudes (>2,000 m a.s.l.; Daehler 2005); thus many species may have been introduced to higher altitudes rather than dispersing from the lowlands. Further, in accordance with our study, a zonation reflecting bioclimatic origin has been found more commonly for (sub)tropical species than for temperate and Mediterranean species. Finally, the absence of a zonational pattern could reflect the fact that we only studied annual plants. This was a deliberate choice to avoid confounding of the results by different life forms; and an unequal representation of different life forms in different bioclimatic groups may have confounded results in other studies.
Does time since introduction influence altitudinal ranges of non-native species?
In accordance with our second hypothesis, the altitudinal ranges of old introductions tended to be broader than those of recently-introduced species, and with few exceptions the species in natural habitats at high altitudes were old introductions. Becker et al. (2005) also showed a positive correlation between the highest occurrence of non-native species and their time since introduction in the Swiss Alps. Such a relationship could simply reflect the time it takes for propagules to disperse to higher altitudes (i.e. propagule pressure) (Ross et al. 2008), although other studies suggest that roadside distributions of non-native species are unlikely to be dispersal limited (Alexander et al. 2009b). Another explanation is that it reflects the time needed for populations to adapt genetically to the new conditions (Dietz and Edwards 2006; Roy et al. 2000). In the natural habitat the proportion of old introductions was higher amongst Mediterranean than temperate species. This could be because Mediterranean species are less likely to be pre-adapted to cold climatic conditions, so that local adaptation would be necessary for them to grow at high altitudes. In contrast, temperate species that establish at low altitudes have to be climatically plastic, which would explain why recently-introduced species have been able to spread to higher altitudes.
Do species ranges explain species richness patterns with altitude?
In line with our third hypothesis, there was a close match between the species richness observed along the gradient and that predicted from the species’ range sizes (Fig. 6), which led to a hump-shaped richness pattern (Fig. 5).
A decrease in species richness at low and high altitudes can therefore be explained by a loss of species with overlapping ranges. A strong decline in the richness of non-native Mediterranean and temperate plant species at low and high altitudes was previously reported for different islands in the Canary Islands (Arévalo et al. 2005; Arteaga et al. 2009). Studies in other mountainous regions have shown either a monotonic decline of non-native plant species richness with altitude or, as in our case, a hump-shaped pattern (Becker et al. 2005; Jakobs et al. 2010; Marini et al. 2009; McDougall et al. 2005; Pauchard et al. 2009). While at temperate latitudes, the limiting climate factor at high altitudes is likely to be low temperatures (Becker et al. 2005; Marini et al. 2009), on subtropical oceanic islands species may be limited at low altitudes by aridity (Arévalo et al. 2005; Hawkins et al. 2003; Jakobs et al. 2010; Fig. 1). Thus, the hump-shaped pattern in species richness could reflect either the altitudinal pattern of water availability alone or opposing gradients of climatic harshness—aridity at low altitudes and low temperature at high altitudes (or a combination of both).
It cannot be excluded that a decline in species richness at the extremes of the altitudinal gradient is due to increased habitat resistance to invasion because of competition from established vegetation and/or reduced propagule pressure. However, because we surveyed highly disturbed roadsides where total vegetation cover at the extremes of the gradient was only some 10–60%, we do not think that competitive exclusion played an important role. Propagule pressure is unlikely to decline much towards the lowest altitudes, but it cannot be excluded as a relevant factor at high altitudes; however, the roads in the survey are heavily used by tourists, even at the highest altitudes (1,000–2,000 cars/day).
Habitat context influences species distribution patterns
The altitudinal distribution pattern of non-native annual species was modulated by the habitat context. About 30% of the species reached a sharp altitudinal distribution limit at the boarder of the anthropogenic and natural habitats, which resulted in a drop in species richness at the transition of the two habitat types (Figs. 3, 5). When comparing the observed and predicted species richness, based on the assumption that a species occurs everywhere within its altitudinal range, it appears that this drop can be explained only partly by an ultimate altitudinal limit of species ranges; indeed, many Mediterranean species reappeared again at higher altitudes, so that the observed and predicted richness of Mediterranean species differ strongly between c. 1,000 and 1,500 m a.s.l. (Fig. 6).
This separation might be explained by the influence of the cloud layer within the natural habitat, which is most pronounced between c. 1,000 and 1,500 m a.s.l. Moist and shady conditions within the cloud forest at these altitudes might exclude typically light-demanding and drought-adapted Mediterranean ruderal species, which reappear above the cloud layer where the pine forest is more open and light. This habitat effect may also explain contrasting results between this study and previous work in Tenerife. Arévalo et al. (2005), working on the leeward side of Tenerife, where there is no cloud forest, did not find the same mid-altitude drop in numbers of non-native species. Arteaga et al. (2009) found in a narrower altitudinal range between 0 and 650 m a.s.l. a monotonic increase for non-native temperate species richness, consistent with our results for this altitudinal range, but a hump-shaped pattern for non-native Mediterranean species richness. Because of topographic effects, in their study area the transition to cloud forest occurred at c. 600 m a.s.l. (Arévalo et al. 2008, Marzol 2008). This may explain the drop in Mediterranean species richness at a lower altitude.
If, as seems likely, climate change alters the altitudinal distribution of cloud forests on oceanic islands (Loope and Giambelluca 1998), the distribution of non-native plants could indirectly be affected. Indeed, indirect effects of climate change through changes in habitat distribution may prove to be more important than direct climatic effects in shaping non-native species distributions.
Conclusions
Our results suggest that bioclimatic origin does not influence the non-native species richness pattern along an altitudinal gradient. However, climate matching is important for the establishment of non-native species at low altitudes, while plasticity is crucial for species that are not climatically pre-adapted. Niche modelling may thus be useful to predict potential areas of first establishment (cf. Broennimann et al. 2007; Tatem and Hay 2007). Nonetheless, the importance of time since introduction suggests that ongoing adaptation might be important as species extend their ranges upwards along the altitudinal gradient. This could account for the observed time lags between introduction and rapid spread of non-native species (e.g. Richardson and Pyšek 2006). Our results show that the altitudinal distribution of non-native plants is affected both by climatic and habitat conditions. Climate change is therefore likely to affect the occurrence of these species both directly and indirectly, e.g. by altering the distribution of habitats such as cloud forest. So far, this interplay of regional climate and habitat type has not been discussed in studies of non-native species distributions along an altitudinal gradient.
References
Alexander JM and Edwards PJ (2010) Limits to the niche and range margins of alien species. Oikos (in press). doi 10.1111/j.1600-0706.2010.17977.x
Alexander JM, Edwards PJ, Poll M, Parks CG, Dietz H (2009a) Establishment of parallel altitudinal clines in traits of native and introduced forbs. Ecology 90:612–622. doi:10.1890/08-0453.1
Alexander JM, Naylor B, Poll M, Edwards PJ, Dietz H (2009b) Plant invasions along mountain roads: the altitudinal amplitude of alien Asteraceae forbs in their native and introduced ranges. Ecography 32:334–344. doi:10.1111/j.1600-0587.2008.05605.x
Almeida-Neto M, Guimarães P, Guimarães PR Jr, Loyola RD, Ulrich W (2008) A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos 117:1227–1239. doi:10.1111/j.2008.0030-1299.16644.x
Alpert P, Bone E, Holzapfel C (2000) Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol Evol Syst 3:52–66. doi:10.1078/1433-8319-00004
Arévalo JR, Delgado JD, Otto R, Naranjo A, Salas M, Fernández-Palacios JM (2005) Distribution of alien vs native plant species in roadside communities along an altitudinal gradient in Tenerife and Gran Canaria (Canary Islands). Perspect Plant Ecol Evol Syst 7:185–202. doi:10.1016/j.ppees.2005.09.003
Arévalo JR, Peraza MD, Àlvarez C, Bermúdez A, Delgado JD, Gallardo A, Fernández-Palacios JM (2008) Laurel forest recovery during 20 years in an abandoned firebreak in Tenerife, Canary Islands. Acta Oecologica 33:1–9. doi:10.1016/j.actao.2007.06.005
Arévalo JR, Otto R, Escudero C, Fernández-Lugo S, Arteaga M, Delgado JD, Fernández-Palacios JM (2010) Do anthropogenic corridors homogenize plant communities at a local scale? A case studied in Tenerife (Canary Islands). Plant Ecol 209:23–35. doi:10.1007/s11258-009-9716-y
Arteaga MA, Delgado JD, Otto R, Fernández-Palacios JM, Arévalo JR (2009) How do alien plants distribute along roads on oceanic islands? A case study in Tenerife, Canary Islands. Biol Invasions 11:1071–1086. doi:10.1007/s10530-008-9329-8
Bannister P (1966) The use of subjective estimates of cover-abundance as the basis for ordination. J Ecol 54:665–674
Beaumont L, Gallagher RV, Thuiller W, Downey PO, Leishman MR, Hughes L (2009) Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib 15:409–420. doi:10.1111/j.1472-4642.2008.00547.x
Becker T, Dietz H, Billeter R, Buschmann H, Edwards PJ (2005) Altitudinal distribution of alien plant species in the Swiss Alps. Perspect Plant Ecol Evol Syst 7:173–183. doi:10.1016/j.ppees.2005.09.006
Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson AT, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709. doi:10.1111/j.1461-0248.2007.01060.x
Cabildo Tenerife (2007) Mapa de Intensidades Medias Diarias de Tráfico
Christen D, Matlack G (2006) The role of roadsides in plant invasions: a demographic approach. Conserv Biol 20:385–391. doi:10.1111/j.1523-1739.2006.00315.x
Christen DC, Matlack GR (2009) The habitat and conduit functions of roads in the spread of three invasive plant species. Biol Invasions 11:453–465. doi:10.1007/s10530-008-9262-x
Chytrý M, Pyšek P, Wild J, Pino J, Maskell LC, Vilá M (2009) European map of alien plant invasions based on the quantitative assessment across habitats. Divers Distrib 15:98–107. doi:10.1111/j.1472-4642.2008.00515.x
Currall JEP (1987) A transformation of the Domin scale. Vegetatio 72:81–87
Daehler CC (2005) Upper-montane plant invasions in the Hawaiian Islands: patterns and opportunities. Perspect Plant Ecol Evol Syst 7:203–216. doi:10.1016/j.ppees.2005.08.002
Dahl E (1998) The phytogeography of Northern Europe (British Isles, Fennoscandia and adjacent areas). Cambridge University Press, Cambridge
Dawson W, Burslem DFRP, Hulme PE (2009) Factors explaining alien plant invasion success in a tropical ecosystem differ at each stage of invasion. J Ecol 97:657–665. doi:10.1111/j.1365-2745.2009.01519.x
Dietz H, Edwards PJ (2006) Recognition of changing processes during plant invasions may help reconcile conflicting evidence of the drivers. Ecology 87:1359–1367. doi:10.1890/0012-9658(2006)87[1359:RTCPCD]2.0.CO;2
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139
Fernández-Palacios JM (1992) Climatic responses of plant species on Tenerife, The Canary Islands. J Veg Sci 3:595–602
Fernández-Palacios JM, de Nicolás JP (1995) Altitudinal pattern of vegetation variation on Tenerife. J Veg Sci 6:183–190
Forman RTT, Sperling D, Bissonette JA, Clevenger AP, Cutshall CD, Dale VH, Fahrig L, France R, Goldman CR, Heanue K, Jones JA, Swanson FJ, Turrentine T, Winter TC (2003) Road ecology: science and solutions. Island Press, Washington
Gelbard JL, Belnap J (2003) Roads as conduits for exotic plant invasions in a semiarid landscape. Conserv Biol 17:420–432. doi:10.1046/j.1523-1739.2003.01408.x
Gordon DR, Onderdonk DA, Fox AM, Stocker RK (2008) Consistent accuracy of the Australian weed risk assessment system across varied geographies. Divers Distrib 14:234–242. doi:10.1111/j.1472-4642.2007.00460.x
Hawkins BA, Field R, Cornell H, Currie DJ, Guégan JF, Kaufman DM, Kerr JT, Mittelbach GG, Oberdoorff T, O’Brien EM, Porter EE, Turner JRG (2003) Energy, water, and broad-scale geographic patterns of species richness. Ecology 84:3105–3117. doi:10.1890/03-8006
Hijmans RJ, Cameron S, Parra J (2009) Worldclim. http://www.worldclim.org. Accessed 17 Nov 2009
Hoernle K, Carracedo J-C (2009) Canary Islands, Geology. In: Gillespie R, Clague DA (eds) Encyclopedia of islands. University of California Press, Berkeley, pp 133–143
Jakobs G, Kueffer C and Daehler CC (2010) Introduced weed richness across altitudinal gradients in Hawai’i: humps, humans and water-energy dynamics. Biol Inv (in press)
Johnston FM, Johnston SW (2004) Impacts of road disturbance on soil properties and on exotic plant occurrence in subalpine areas of the Australian Alps. Arct Antarct Alp Res 36:201–207. doi:10.1657/1523-0430(2004)036[0201:IORDOS]2.0.CO;2
Johnston FM, Pickering CM (2001) Alien plants in the Australian Alps. Mt Res Dev 21:284–291. doi:10.1659/0276-4741(2001)021[0284:APITAA]2.0.CO;2
Kitayama K, Mueller-Dombois D (1995) Biological invasion on an oceanic island mountain: do alien plant species have wider ecological ranges than native species? J Veg Sci 6:667–674. doi:10.2307/3236436
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Körner C (2003) Alpine plant life. Functional plant ecology of high mountain ecosystems, 2nd edn. Springer, Berlin
Kueffer C, Daehler CC, Torres-Santana CW, Lavergne C, Meyer J-Y, Otto R, Silva L (2010) A global comparison of plant invasions on oceanic islands. Perspect Plant Ecol Evol Syst 12:145–162. doi:10.1016/j.ppees.2009.06.002
Lilley PL, Vellend M (2009) Negative native-exotic diversity relationship in oak savannas explained by human influence and climate. Oikos 118:1373–1382. doi:10.1111/j.1600-0706.2009.17503.x
Loope LL, Giambelluca TW (1998) Vulnerability of island tropical montane cloud forest to climate change, with special reference to east Maui, Hawaii. Clim Change 39:503–517. doi:10.1023/A:1005372118420
Marini L, Gaston KJ, Prosser F, Hulme PE (2009) Contrasting response of native and alien plant species richness to environmental energy and human impact along alpine elevation gradients. Glob Ecol Biogeogr 18:652–661. doi:10.1111/j.1466-8238.2009.00484.x
Maron JL, Elmendorf SC, Vilà M (2007) Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum. Evolution 61:1912–1924. doi:10.1111/j.1558-5646.2007.00153.x
Marzol MV (2008) Temporal characteristics and fog water collection during summer in Tenerife (Canary Islands, Spain). Atmos Res 87:352–361. doi:10.1016/j.atmosres.2007.11.019
McDougall KL, Morgan JW, Walsh NG, Williams RJ (2005) Plant invasions in treeless vegetation of the Australian Alps. Perspect Plant Ecol Evol Syst 7:159–171. doi:10.1016/j.ppees.2005.09.001
Öckinger E, Franzén M, Rundlöf M, Smith HG (2009) Mobility-dependent effects on species richness in fragmented landscapes. Basic Appl Ecol 10:573–578. doi:10.1016/j.baae.2008.12.002
Parks CG, Radosevich SR, Endress BA, Naylor BJ, Anzinger D, Rew LJ, Maxwell BD, Dwire K (2005) Natural and land-use history of the northwest mountain ecoregions (USA) in relation to patterns of plant invasions. Perspect Plant Ecol Evol Syst 7:137–158. doi:10.1016/j.ppees.2005.09.007
Pauchard A, Alaback PB (2004) Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of south-central Chile. Conserv Biol 18:238–248. doi:10.1111/j.1523-1739.2004.00300.x
Pauchard A, Kueffer C, Dietz H, Daehler CC, Alexander JM, Edwards PJ, Arévalo JR, Cavieres LA, Guisan A, Haider S, Jakobs G, McDougall K, Millar CI, Naylor BJ, Parks CG, Rew LJ, Seipel T (2009) Ain’t no mountain high enough: plant invasions reaching new elevations. Front Ecol Environ 7:479–486. doi:10.1890/080072
Peterson AT (2003) Predicting the geography of species’ invasions via ecological niche modeling. Q Rev Biol 78:419–433. doi:10.1086/378926
Richardson DM, Pyšek P (2006) Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog Phys Geogr 30:409–431. doi:10.1191/0309133306pp490pr
Ross LC, Lambdon PW, Hulme PE (2008) Disentangling the roles of climate, propagule pressure and land use on the current and potential elevational distribution of the invasive weed Oxalis pes-caprae L on Crete. Perspect Plant Ecol Evol Syst 10:251–258. doi:10.1016/j.ppees.2008.06.001
Roy S, Simon J-P, Lapointe F-J (2000) Determination of the origin of the cold-adapted populations of barnyard grass (Echinochloa crus-galli) in eastern North America: a total-evidence approach using RAPD DNA and DNA sequences. Can J Bot 78:1505–1513. doi:10.1139/cjb-78-12-1505
Schultz J (2005) The ecozones of the world. The ecological divisions of the geosphere. Springer, Berlin
Sperling FN, Washington R, Whittaker RJ (2004) Future climate change of the subtropical North Atlantic: implications for the cloud forests on Tenerife. Clim Change 65:103–123. doi:10.1023/B:CLIM.0000037488.33377.bf
Sullivan JJ, Williams PA, Timmins SM, Smale MC (2009) Distribution and spread of environmental weeds along New Zealand roadsides. N Z J Ecol 33:190–204
Tatem AJ, Hay SI (2007) Climatic similarity and biological exchange in the worldwide airline transportation network. Proc R Soc Lond B Biol Sci 274:1489–1496. doi:10.1098/rspb.2007.0148
Thuiller W, Richardson DM, Pyšek P, Midgley GF, Hughes GO, Rouget M (2005) Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob Chang Biol 11:2234–2250. doi:10.1111/j.1365-2486.2005.01018.x
Ullmann I, Heindl B (1989) Geographical and ecological differentiation of roadside vegetation in temperate Europe. Bot Acta 102:261–340
USDA, ARS, National Genetic Resources Program. Germplasm Resources Information Network - (GRIN) [Online Database]. National Germplasm Resources Laboratory, Beltsville, Maryland. http://www.ars-grin.gov/cgi-bin/npgs/html/index.pl. Accessed 19 Nov 2009
Vilá M, Pino J, Font X (2007) Regional assessment of plant invasions across different habitat types. J Veg Sci 18:35–42. doi:10.1111/j.1654-1103.2007.tb02513.x
Walther G-R, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H, Czúcz B, Dauber J, Hickler T, Jarošík V, Kenis M, Klotz S, Minchin D, Moora M, Nentwig W, Ott J, Panov VE, Reineking B, Robinet C, Semenchenko V, Solarz W, Thuiller W, Vilà M, Vohland K, Settele J (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693. doi:10.1016/j.tree.2009.06.008
Western L, Juvik JO (1983) Roadside plant communities on Mauna Loa, Hawaii. J Biogeogr 10:307–316. doi:10.2307/2844740
Whittaker RJ, Fernández-Palacios JM (2007) Island biogeography. Ecology, evolution, and conservation, 2nd edn. Oxford University Press, Oxford
Wilson JB, Rapson GL, Sykes MT, Watkins AJ, Williams PA (1992) Distributions and climatic correlations of some exotic species along roadsides in South Island, New Zealand. J Biogeogr 19:183–193
Wright DH, Patterson BD, Mikkelson GM, Cutler A, Atmar W (1998) A comparative analysis of nested subset patterns of species composition. Oecologia 113:1–20. doi:10.1007/s004420050348
Acknowledgments
We thank José María Fernández-Palacios, José Ramon Arévalo and Rüdiger Otto (Universidad de La Laguna, Tenerife, Spain) for enabling the field work, helping with species identification and sharing many facilities of the department. Werner Nezadal (University of Erlangen-Nürnberg, Germany) gave support in the decision about the introduction status of the species. The manuscript was improved by comments from Aníbal Pauchard (Universidad de Concepción, Chile) and two anonymous reviewers. SH was funded by a graduate scholarship from Universität Bayern e.V.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
-
Overview of sources used to determine life form, longevity, introduction status, bioclimatic origin and time since introduction for recorded species.
-
Acebes Ginovés JR, del Arco Aguilar M, García Gallo A, León Arencibia MC, Pérez de Paz PL, Rodríguez Delgado O and Wildpret de la Torre W (2001) Pteridophyta, Spermatophyta. In: Izquierdo Zamora I, Martín Esquivel JL, Zurita Pérez N and Arechavaleta Hernández M (eds) Lista de especies silvestres de Canarias (hongos, plantas y animales terrestres) 2001, Consejería de Política Territorial y Medio Ambiente Gobierno de Canarias, pp 98–140
-
Acebes Ginovés JR, del Arco Aguilar M, García Gallo A, León Arencibia MC, Pérez de Paz PL, Rodríguez Delgado O, Wildpret de la Torre W, Martín Osorio VE, Marrero Gómez MdC and Rodríguez Navarro ML (2004) Pteridophyta, Spermatophyta. In: Izquierdo Zamora I, Martín Esquivel JL, Zurita Pérez N and Arechavaleta Hernández M (eds) Lista de especies silvestres de Canarias (hongos, plantas y animales terrestres) 2004, Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canarias, pp 96–143
-
Arévalo JR, Delgado JD, Otto R, Naranjo A, Salas M and Fernández-Palacios JM (2005) Distribution of alien vs. native plant species in roadside communities along an altitudinal gradient in Tenerife and Gran Canaria (Canary Islands). Perspect Plant Ecol Evol Syst 7: 185–202. doi: 10.1016/j.ppees.2005.09.003
-
Arteaga MA, Delgado JD, Otto R, Fernández-Palacios JM and Arévalo JR (2009) How do alien plants distribute along roads on oceanic islands? A case study in Tenerife, Canary Islands. Biol Invasions 11: 1071–1086. doi: 10.1007/s10530-008-9329-8
-
Brandes D and Fritzsch K (2002) Alien plants of Fuerteventura, Canary Islands. Plantas extranjeras de Fuerteventura, Islas Canarias. p 25
-
Bundesamt für Naturschutz. Floraweb. http://www.floraweb.de. Accessed 12 Aug 2009.
-
González Henríquez MN and Kunkel G (1991) Flora y vegetación del archipiélago Canario 3. Tratado Florístico 2. Edirca, Las Palmas de Gran Canaria
-
Hansen A and Sunding P (1993) Flora of Macaronesia. Checklist of vascular plants, 4th edn. Botan. Garden, Oslo
-
Hegi G (1965–1987) Illustrierte Flora von Mittel-Europa. Carl Hansen, München
-
Heß HE, Landolt E and Hirzel R (1967–1972) Flora der Schweiz und angrenzender Gebiete. Birkhäuser, Basel
-
Hohenester A and Welß W (1993) Exkursionsflora für die Kanarischen Inseln mit Ausblicken auf ganz Makaronesien. Ulmer, Stuttgart
-
Jardim R and Menezes de Sequeira M (2008) List of vascular plants (Pteridophyta and Spermatophyta). In: Borges PAV, Abreu C, Franquinho Aguiar AM, Carvalho P, Jardim R, Melo I, Oliveira P, Sérgio C, Serrano ARM and Vieira EP (eds) A list of the terrestrial fungi, flora and fauna of Madeira and Selvagens archipelagos, Direcção Regional do Ambiente da Madeira and Universidade dos Açores, Funchal and Angra do Heroísmo, pp 179–207
-
Klotz S, Kühn I and Durka W (2004) BIOLFLOR—Eine Datenbank mit biologisch-ökologischen Merkmalen zur Flora von Deutschland. Bundesamt für Naturschutz, Bonn
-
Kunkel G (1973) The role of adventitious plants in the vegetation of the Canary Islands. Monogr Biol Canar 4: 103–106
-
Kunkel G (1976) Notes on the introduced elements in the Canary Islands’ flora. In: Kunkel G (ed) Biogeography and ecology in the Canary Islands, Dr. W. Junk b.v. Publishers, The Hague, pp 249–266
-
Kunkel G (1987) Die Kanarischen Inseln und ihre Pflanzenwelt. Gustav Fischer, Stuttgart
-
Lauber K and Wagner G (1996) Flora Helvetica, 4th edn. Paul Haupt, Bern
-
Meusel H (1948) Vergleichende Arealkunde. Listen- und Kartenteil. 2 Bände. Borntraeger, Berlin-Zehlendorf
-
Meusel H and Jäger EJ (1992) Vergleichende Chorologie der zentraleuropäischen Flora. Gustav Fischer, Stuttgart
-
Meusel H, Jäger EJ, Rauschert SW and Weinert E (1978) Vergleichende Chorologie der zentraleuropäischen Flora. Gustav Fischer, Jena
-
Meusel H, Jäger EJ and Weinert E (1965) Vergleichende Chorologie der zentraleuropäischen Flora. Gustav Fischer, Jena
-
Sánchez-Pinto L, Rodríguez L, Rodríguez S, Martín K, Cabrera A and Marrero C (2005) Spermatophyta. In: Arechavaleta M, Zurita N, Marrero MC and Martín JL (eds) Lista preliminar de especies silvestres de Cabo Verde (hongos, plantas y animales terrestres), Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canarias, pp 40–57
-
Schönfelder P, Leon Arencibia MC and Wildpret W (1993) Catálogo de la flora vascular de la Isla de Tenerife (Islas Canarias). In: Díaz González TE, Fernández González F, Géhu JM, Pedrotti F, Rivas-Martínez S and Penas Merino A (eds) Itinera Geobot, Universidad de León, León, pp 375–404
-
Silva L, Pinto J, Press B, Rumsey F, Carine M, Henderson S and Sjögren E (2005) List of vascular plants. In: Borges PAV, Cunha R, Gabriel R, Frias Martins A, Silva L and Vieira V (eds) A List of Terrestrial Fauna (Mollusca and Arthropoda) and Flora (Bryophyta, Pteridophyta and Spermatophyta) from the Azores, Direcção Regional do Ambiente and Universidade dos Açores, Hora, Angra do Heoísmo and Ponta Delgada, pp 131–155
-
Stierstorfer C and von Gaisberg M (2006) Annotated checklist and distribution of the vascular plants of El Hierro, Canary Islands, Spain. Botanic Garden and Botanical Museum Berlin-Dahlem, Berlin
-
Swiss Federal Institute for Forest, Snow and Landscape Research (1999) Swiss web flora. http://www.webflora.ch. Accessed 24 Sep 2009.
-
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM and Webb DA (1968–1980) Flora Europaea. Cambrige University Press, Cambridge
-
Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM and Webb DA (1964) Flora Europaea. Cambrige University Press, Cambridge
-
USDA, NRCS (2009) The PLANTS Database. National Plant Data Center, Baton Rouge, LA 70874-4490 USA. http://plants.usda.gov. Accessed 19 Nov 2009
Appendix 2
See Table 3
Appendix 3
See Table 4
Rights and permissions
About this article
Cite this article
Haider, S., Alexander, J., Dietz, H. et al. The role of bioclimatic origin, residence time and habitat context in shaping non-native plant distributions along an altitudinal gradient. Biol Invasions 12, 4003–4018 (2010). https://doi.org/10.1007/s10530-010-9815-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9815-7