Abstract
One theory concerning the invasiveness of exotic plants suggests that they exude phytotoxic compounds that are novel in areas being invaded. For most invasive plants, however, little is known about the effects of their bioactive chemicals and how novel they are in invaded areas. From a methodological point of view, it also remains largely untested whether phytotoxicity found in vitro translates into allelopathic effects in more complex ecological settings. In this study, we tested for allelopathic effects of root exudates of the invasive plant Heracleum mantegazzianum (giant hogweed), its native congener Heracleum sphondylium (common hogweed) and two less-related native species. We also performed chemical analyses of the invader’s root exudates to identify bioactive compounds. We found that root exudates of H. mantegazzianum contain allelopathic compounds which are not likely to be furanocoumarins, but other as yet unidentified molecules. Allelopathy of the invader detected in vitro conditions and in our garden experiment did not, however, differ from the allelopathy of the native species tested. A meta-analysis of two independent garden experiments indicated significantly negative, though similar, phytotoxic effects of H. mantegazzianum, its native congener and Dactylis glomerata in the absence of activated carbon. Our study thus indicates that allelopathy by producing unique compounds, as predicted by the novel weapons hypothesis, is not a principal driver of the invasion success of H. mantegazzianum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant invasions are among the main concerns of contemporary ecology. Numerous hypotheses have been proposed to explain the success of plant invaders (Catford et al. 2009). Most of these hypotheses assume that invasive species carry certain advantages over native species that make them successful in new areas. The mechanisms invoked by these hypotheses include lower enemy pressure (enemy release hypothesis—Keane and Crawley 2002; Colautti et al. 2004; Joshi and Vrieling 2005), evolutionary changes (evolution of increased competitive ability—Blossey and Notzgold 1995; Joshi and Vrieling 2005), alteration of the soil microbial community to the detriment of native species (accumulation of local pathogens hypothesis—Eppinga et al. 2006) or chemical inhibition through secondary metabolites (novel weapons hypothesis). According to the last theory, allelopathy may facilitate invasions of exotic plants especially if the newcomers’ bioactive compounds affect unadapted native species (Callaway and Aschehoug 2000; Hierro and Callaway 2003; Callaway and Ridenour 2004). Increasing attention has been paid to this theory in the last decade (reviewed in Hierro and Callaway 2003; Inderjit et al. 2008; Duke 2010). Although several studies have shown allelopathy as a possible mechanism facilitating plant invasions (Hierro and Callaway 2003; Prati and Bossdorf 2004; Cappuccino and Arnason 2006; Abhilasha et al. 2008), the extent to which novel weapons are responsible for the invasiveness of exotic species is still unknown.
The novel weapons hypothesis has usually been tested by comparing an invader’s phytotoxic effects on other plants in its native range with the effects it has on other plant species in its invaded range (Callaway and Aschehoug 2000). Along with biogeographical comparisons, it is relevant to compare the composition and effects of secondary compounds of invaders with those of species in the invaded range (Cappuccino and Arnason 2006; Dostál 2011; Del Fabbro et al. 2014). This approach (community comparison sensu Colautti et al. 2004) is often used to estimate differences in herbivore load between invasive and native species in invaded communities. Similarly, it has been used to estimate the advantage of invaders over native relatives due to stronger allelopathic effects (discussed by Inderjit et al. 2008). Some of these studies indicate that some invaders may produce the same secondary compounds (Cappuccino and Arnason 2006) as their close relatives present in the invaded range and that these compounds have the same effects (Dostál 2011; Del Fabbro et al. 2014). This may apply to the invader Heracleum mantegazzianum (giant hogweed), whose congeneric species H. sphondylium (common hogweed) is native to Central Europe. Heracleum mantegazzianum is an invasive plant that is well-known for its negative impact on the structure of native communities (Thiele and Otte 2007; Hejda et al. 2009; Dostál et al. 2013) and because it alters the properties of entire ecosystems (Thiele et al. 2010; Jandová et al. 2014). It remains unclear, however, how allelopathy contributes to the invasiveness of this species (but see Junttila 1975, 1976; Myras and Junttila 1981; Wille et al. 2013). It can be argued that if an invader does not release compounds that have stronger phytotoxic effects than those released by its congeners or other less-related native species, then allelopathy through the production of unique, novel compounds, as predicted by the novel weapons hypothesis, is not very likely to be a principal driver of the invader’s invasion success.
Recently, there has been a lot of debate concerning methodological issues in the field of allelopathy, and examples of incongruent results have been brought up (e.g. Bais et al. 2003, but Blair et al. 2006; Bertin et al. 2007, but Kaur et al. 2009). It is therefore necessary to test the repeatability of allelopathic effects by performing several independent experimental runs. Besides, many substances and plant extracts have been proved to be allelopathic only in vitro, and their effects under natural conditions often remain untested. Concentrations of bioactive compounds released as root exudates or aboveground biomass leachates are much lower under natural conditions than in vitro because they are subject to sorption on soil particles as well as chemical and microbial decomposition (Kaur et al. 2009; Lankau 2010). To obtain sufficient proof of allelopathy, in vitro bioassays should therefore be accompanied by more ecologically relevant approaches such as garden experiments.
Bioassays of plant exudates, leachates or extracts can provide information on the bioactivity of secondary metabolites in mixtures. Estimation of total phenolic content as a parameter of allelopathic potential can then follow (Inderjit and Nilsen 2003). Today, fractionation and chromatographic methods coupled with mass spectrometry or nuclear magnetic resonance are the methods of choice when attempting to describe the composition of particular compounds (Blair et al. 2009; Duke 2010). Separation and identification of bioactive molecules, however, remains a very difficult task. We can nevertheless distinguish among ecological treatments using techniques of metabolomics and by directly comparing fingerprint chromatograms.
In this study, we tested root exudates of the invasive plant H. mantegazzianum and of its native congener H. sphondylium for allelopathic effects, both in vitro and in a garden experiment. In addition, we tested for allelopathy in two other, less-related native species. We also performed chemical analyses of the exudates of H. mantegazzianum to identify bioactive compounds. By doing so, we addressed the following questions: (1) Do root exudates of H. mantegazzianum suppress the germination or root development of native plants in vitro? (2) Does H. mantegazzianum have stronger allelopathic effects than its native congener and two other less-related native species? (3) Are the allelopathic effects also detectable under ecologically more relevant conditions, such as in a garden experiment? (4) What is the chemical composition of putative allelochemicals released by H. mantegazzianum?
Methods
Study species
In our study, we included four species that served as a source of root exudates (Heracleum mantegazzianum, H. sphondylium, Dactylis glomerata and Plantago lanceolata), hereafter called root exudate species.
Heracleum mantegazzianum Sommier et Levier (Apiaceae; giant hogweed) is a monocarpic perennial with leaves up to 2.5 m long and flowering stems up to 5 m tall (Tiley et al. 1996). It is native to the Western Greater Caucasus (Russia, Georgia) and has been introduced to Europe as a garden ornamental in the nineteenth century (Jahodová et al. 2007). Since then it has spread to a number of European countries (Tiley et al. 1996), Canada (Page et al. 2006) and the USA (Kartesz and Meacham 1999). The species readily colonizes disturbed habitats, which offer favourable conditions for the dispersal and establishment of its seeds, but it also invades semi-natural vegetation. It is able to form extensive stands with negative effects on biodiversity (Pyšek and Pyšek 1995; Dostál et al. 2013). The chemistry of the Apiaceae as a whole is very complex, and H. mantegazzianum contains a plethora of secondary metabolites (Tiley et al. 1996). For instance, linear furanocoumarins (e.g. bergapten, psoralen, xanthotoxin), which are present in most parts of the plant, have been studied extensively as a defence mechanism against herbivory (Berenbaum 1981; Hattendorf et al. 2007) and the cause of photodermatitis in people and animals (Nielsen 1971; Molho et al. 1971; Kavli et al. 1983). Angular furanocoumarins possess remarkable fungistatic and antimicrobial properties and are responsible for below-ground protection (Fischer et al. 1978; Ivie 1978). Also present are flavonoids (Harborne 1971) and essential oils (Jain 1969).
Heracleum sphondylium L. (Apiaceae; common hogweed) is a mainly polycarpic perennial with stems up to 2–3 m tall. The species is widespread throughout Europe, except in the extreme north, in much of the Mediterranean region and on certain Atlantic and Mediterranean islands (Sheppard 1991). It produces flavonoids, essential oils and furanocoumarins, which are typical of the genus Heracleum (Harborne 1971). There are much lower amounts of linear furanocoumarins than angular furanocoumarins (Molho et al. 1971).
Dactylis glomerata (Poaceae; cock’s-foot) and Plantago lanceolata (Plantaginaceae; ribwort plantain) served as two less-related species releasing root exudates. These native plants have been observed to occur in communities invaded by H. mantegazzianum (Dostál et al. 2013).
We examined the effects of root exudates released by the invader H. mantegazzianum, its native congener H. sphondylium, and two less-related native species D. glomerata and P. lanceolata. We also used the latter two species as assay species together with Centaurea jacea, a grassland species, and Arabidopsis thaliana, a widely used standard assay species.
Seeds of C. jacea, D. glomerata and P. lanceolata came from a commercial supplier (Planta Naturalis, Markvartice, Czech Republic), so they had no prior experience with the invader. Seeds of A. thaliana belonged to the Columbia-0 ecotype. Seeds and plant material of both Heracleum species were obtained as described below.
Root exudate collection
Root exudates of species examined for allelopathy were collected in three independent runs during the vegetative seasons of 2011 and 2012 (for the overall experimental design, see Table 1). Each time, the root exudates were obtained from an aeroponic growing system (Amazon 32, Nutriculture, Lancashire, UK) placed in a greenhouse with a 20°/10 °C temperature regime at the Institute of Botany, Průhonice, Czech Republic (322 masl; 49°99′N, 14°57′E). The collected exudates were always stored in the dark at 4 °C.
In the first run carried out in May 2011, only H. mantegazzianum was examined. Seeds were collected in autumn 2010 from four different populations of the western part of the Czech Republic (near Lázně Kynžvart, 50°01′N, 12°38′E), mixed randomly and stored in paper bags at room temperature. Later, they were cold-stratified in wet sterilized sand at 4 °C in the dark for 2.5 months and then let to germinate on wet sterilized sand in the greenhouse. After 2 weeks of growth, the seedlings were transplanted into 50-mm mesh pots filled with 1-cm3 rockwool cubes (Grodan, Roermond, the Netherlands) and placed in a greenhouse on trays with a 0.125-strength Hoagland solution (Hoagland and Arnon 1950). After another 3 weeks, 32 randomly chosen seedlings were transferred to an aeroponic growing system and grown in a 0.5-strength Hoagland solution for 1 month. Finally, the Hoagland solution enriched with root exudates (10 L per system) was collected.
In the second run carried out in August 2011, both H. mantegazzianum and H. sphondylium were used as root exudate species. Despite the three-month-long cold stratification, the seeds of H. sphondylium did not start to germinate, so seedlings of both species dug out in three different populations (again from near Lázně Kynžvart) were used instead. The seedlings were thoroughly washed in water, dipped in 10 % bleach for 30 min to assure sterilization and then placed in 50-mm mesh pots filled with 1-cm3 rockwool cubes. Thirty-two randomly chosen seedlings of each species were transferred to separate aeroponic systems—two per species, four in total. The plants were grown and treated as in the first run.
In the third run carried out in May 2012, both H. mantegazzianum and H. sphondylium were examined together with two native species, D. glomerata and P. lanceolata. Seeds of H. mantegazzianum were collected in autumn 2011 from three different populations near Lázně Kynžvart, Czech Republic, and handled as in the first run. Seeds of H. sphondylium were collected in autumn 2009 near Dobříš (49°47′N, 14°11′E), Czech Republic, and in autumn 2011 near Koniz bei Bern (49°55′N, 7°24′E), Switzerland, and then mixed and stored in paper bags at room temperature. Randomly selected seeds were sown into pots with a mixture of sterilized garden soil and sterilized sand in the ratio 2:1. These were left in the garden over winter to ensure cold stratification. After this 4-month-long-cold stratification, the pots were moved to the greenhouse and the seeds germinated. Seeds of D. glomerata and P. lanceolata were allowed to germinate on wet sterilized sand without any additional treatment in the greenhouse. After 2 weeks of growth, the seedlings were washed with water, transplanted into 50-mm mesh pots filled with 1-cm3 rockwool cubes and placed in the greenhouse on trays with a 0.125-strength Hoagland solution. After another 3 weeks, 32 randomly chosen seedlings of each species were transferred to separate aeroponic systems—two per species, eight in total. The plants were grown and treated as described above.
In vitro bioassays
Allelopathic effects of root exudate species were examined in three independent in vitro bioassays on seeds of assay species C. jacea, D. glomerata, P. lanceolata and A. thaliana (for the overall experimental design, see Table 1). A 0.5-strength Hoagland solution was used as a control. Petri dishes (6 cm diameter) were fitted with sheets of qualitative filter paper (Papírna Perštejn, Czech Republic). For each assay species, ten seeds per dish were placed onto the paper, wetted with the examined or control solution and placed in a chamber with a 25°/10 °C temperature and 12-h/12-h light and dark regime. The number of germinants was thereafter recorded twice a week; each time the respective solution was added to keep the filter paper wet. The bioassay was terminated when no increase in the number of germinating plants was observed for at least 1 week. This took 2 weeks for A. thaliana and a month for the other native species. Finally, fresh root lengths of three largest seedlings per dish were determined (for A. thaliana under an OLYMPUS SZX12 microscope). Bioassays always started immediately after exudates were collected, and each treatment had six replications in the first run, and five replications in the second and third run.
Garden experiments
Allelopathic effects of root exudate species were assayed in two independent garden experiments using the seeds of assay species C. jacea, D. glomerata and P. lanceolata (for the overall experimental design, see Table 1). Both experiments were carried out in the experimental garden of the Institute of Botany, Průhonice, Czech Republic. The experiments involved treatments with root exudates, a 0.5-strength Hoagland solution being used as a control, soil microbiota treatments (added vs. sterilized) and activated carbon treatments (with vs. without). The treatments were fully crossed. First, 1-L pots were filled with a mixture of sterilized soil and sterilized sand in the ratio 2:3. The activated carbon treatment consisted of an addition of 20 mL of finely ground activated carbon powder (particle size <0.075 mm; Resorbent Ostrava, Czech Republic) per litre of the soil–sand mixture prior to filling the pots. The activated carbon was used because it is documented to have a high affinity for organic compounds, such as potentially toxic or allelopathic chemicals (Callaway and Aschehoug 2000, Inderjit and Nilsen 2003). Allelopathic effects are also documented to be modified by the presence of a living soil microbiota (e.g. Lankau 2010). Therefore, 1 month prior the start of the experiment, half of the pots were inoculated with a soil suspension (100 mL per pot). Soil from a semi-natural grassland habitat adjacent to the experimental garden in Průhonice, Czech Republic, was used to prepare the suspension (100 g soil per litre of water). Secondly, 10 seeds of the respective assay species were sown per pot. The pots were then randomly placed in beds covered with geotextile in the garden and shaded with 30 % shade cloth. The plants were watered regularly with tap water, and 50 mL of root exudates or control solution per a pot were added weekly for 6 weeks in total. The number of germinants was recorded twice a week. The plants were harvested after 2 months. Finally, the total biomass in each pot was dried (70 °C for 48 h) and weighed. There were four replicates for each treatment combination.
The first run was carried out between 31 August and 17 October 2011 and consisted of 144 pots in total (two different root exudates plus the control × three assay species × two soil microbiota treatments × two activated carbon treatments × four replications). The second run was carried out between 23 May and 16 July 2012 and consisted of 240 pots in total (addition of four different exudates plus control × three assay species × two soil microbiota treatments × two activated carbon treatments × four replications).
Chemical analyses of root exudates
We analysed root exudates of H. mantegazzianum plants used for the May 2011 and August 2011 bioassay. First, we checked for the presence of furanocoumarins, as they are typical of the Apiaceae family. We selected the standards bergapten and xanthotoxin because several studies have proved their presence in tissues of H. mantegazzianum (Tiley et al. 1996; Berenbaum 1981; Herde 2005; Hattendorf et al. 2007). Root exudates were filtered through a 390 Munktell quantitative filter paper, then extracted using SupelSelect HLB SPE 200 mg 6 mL tubes (Sigma Aldrich, USA), which were selected according to the method used in Abhilasha et al. (2008), and later on tested for extraction efficiency of compounds of interest (bergapten and xanthotoxin) using a spectrophotometer (UNICAM UV/VIS UV4). The column was conditioned twice with 6 mL of methanol, equilibrated with 6 mL of water, then the sample was loaded, washed with 6 mL of water, and the absorbed substances were eluted with 6 mL of methanol. The eluent was evaporated under nitrogen flow to 2 mL, after which 1 mL was taken for further LC analyses into a new vial. The other half was evaporated to dryness before being fully redissolved in 1 mL of ethyl acetate in an ultrasonic bath for 1 h for further GC analyses. Prior to the analyses, all samples were filtered through 0.2-μm nylon filters (Costar, USA) in a centrifuge (10 min, 5,000 rpm). Initially, 500 mL of root exudates from the first collection (May 2011) were extracted; however, in order to improve analytical responses, the volume was increased to 1,600 mL in the second run (August 2011). The Hoagland solution was processed in the same manner and used as a control. Commercial standards of bergapten (Aldrich) and xanthotoxin (Fluka) were directly dissolved in methanol or ethyl acetate.
One μL of the sample was injected into a GC–MS device (Varian 3400, ITS-40, Finnigan) equipped with a split/splitless injector. An HP-5 column was used for separation (30 m, 0.25 mm I.D., 0.25-μm film thickness). The temperature programme started at 60 °C and was held for 1 min in splitless mode. After 1 min, the splitter was opened, and the oven was heated to 100 °C at the rate of 25 °C/min, then increased to 135 °C at 1 °C/min and finally to 240 °C at 10 °C/min. The injector temperature was 240 °C, and helium flowing 1 mL/min was used as the carrier gas. The solvent delay time was set to 5 min. The transfer line temperature was set to 280 °C. Mass spectra were recorded at 1 scan/s under electron impact at 70 eV, mass range 50–450 amu. The data were processed using Varian MS Work Station version 691. Peaks of bergapten and xanthotoxin were monitored using their specific even ion m/z 216.
The sample was then analysed using an Acquity UPLC system with a LCT premier XE time-of-flight mass spectrometer (Waters) with the LC Column Acquity UPLC BEH Shield RP18 (50 mm × 2.1 mm I.D., particle size 1.7 μm, Waters) using a two-component mobile phase. The mobile phases A and B consisted of 0.1 % HCOOH in water and 0.1 % HCOOH in acetonitrile, respectively. The analyses were performed under a linear gradient programme (min/ %B) 0/5, 1.5/5, 15/70 followed by a 1.0-min column clean-up (100 % B) and a 1 min equilibration time (5 % B). The total analysis time was 18 min. The column temperature was set to 40 °C, flow rate to 0.4 mL/min, and the injection volume was 5 μL. The mass spectrometer operated in the “W” mode with capillary voltage set at +2800 or −2500 V, cone voltage +40 or −40 V, desolvation gas temperature, 350 °C; ion source block temperature, 120 °C; cone gas flow, 50 L/h; desolvation gas flow, 800 L/h; ion guide 1 and 2 RFs, 200 and 400 V, respectively; hexapole RF, 150 V. The signal was acquired with a scan time of 0.1 s; interscan delay was 0.01 s (0.1 s for lock spray and voltage switch). Mass accuracy was kept below 5 ppm using lock spray technology with leucine enkephalin as the reference compound (2 ng/μL, 5 μL/min). The UV/visible detection was carried out from 194 to 700 nm. The data were processed using MassLynx V4.1 software (Waters).
To evaluate the data, we monitored the peaks of bergapten and xanthotoxin using their specific even ion m/z 216 in the GC–MS chromatograms. We also searched for psoralen, bergapten, imperatorin, petroselinic acid, their glucosyl- and also glucosyloxy derivatives in both ionization modes using the masses calculated by MassLynx software based on respective molecular formulae in the UHPLC–TOF-MS chromatograms. In addition, we compared UHPLC–TOF-MS chromatograms of root exudates and the Hoagland solution that served as the control in both ionization modes and searched for compounds present only in the exudate samples. The molecular formulae of these compounds within the error range were calculated by MassLynx software based on accurate mass and isotopic pattern recognition. Each suggested molecular formula was matched with putative structures using the Reaxys (Reaxys database 2014) and KNapSAcK (KNapSAcK 2014) databases.
Statistical analyses
First, the analyses were performed separately for each run. In vitro bioassay responses of C. jacea, D. glomerata and P. lanceolata were analysed together and that of A. thaliana was analysed separately. In the analysis of the number of germinated seeds, binomial models were fitted and subsequently substituted by quasibinomial models to avoid problems with overdispersion (Crawley 2007). Components of the model were exudates in the case of A. thaliana and exudates, assay species and their interaction in the case of the other native assay species. When necessary, significant effects were further examined by treatment contrasts. Root length (entered as a mean of the three largest seedlings per Petri dish) was analysed by a one-way ANOVA in the case of A. thaliana. In the case of the other native assay species, it was analysed by a two-way ANOVA with the model components exudates, assay species and their interaction. Post hoc comparisons (Tukey contrasts) were applied to examine significant effects. In addition, the responses of C. jacea, D. glomerata and P. lanceolata were analysed together with the only component of the model being exudates to evaluate the general impact of root exudates.
Germination in the garden experiment was analysed using quasibinomial models, biomass using linear models. Initially, a full model including all components (exudates, assay species, soil microbiota, activated carbon) and their interactions was constructed. Non-significant components were then removed to obtain the minimum adequate model. When necessary, significant effects were further examined by treatment contrasts.
Secondly, we carried out a within-study meta-analysis to obtain a summary effect of root exudate species across different experimental runs. We expressed effect size as the standardized mean difference (Hedge’s g):
where df is the degrees of freedom used to estimate S within (i.e. the within-groups standard deviation, pooled across groups), which for two independent groups is n 1 + n 2 − 2 (Borenstein et al. 2009). \(\bar{X}_{\text{exudates}}\) and \(\bar{X}_{\text{control}}\) are means of response variables of plants in Petri dishes or garden pots with added exudates and Hoagland solution, respectively. For in vitro bioassays, the response variable was a product of the number of germinated seeds and of the mean of root lengths of three largest seedlings per a Petri dish. A summary effect was calculated separately for each root exudate species (e.g. a summary effect for H. mantegazzianum included runs performed in May 2011, August 2011 and May 2012) and was obtained separately for the assay species A. thaliana and for the other native assay species. For garden experiments, harvested biomass was used as the response variable. Effect sizes were calculated separately for pots with and without addition of activated carbon, and for pots with sterile and non-sterile soil.
Prior to the analyses, all dependent variables were inspected and, if necessary, data were log- or square-root-transformed to meet the assumptions of homoscedasticity and normality of residuals. We used R 2.14.0 (R Development Core Team 2011) to carry out the ANOVA, linear models, generalized linear models and within-study meta-analysis (metafor package; Viechtbauer 2010).
Results
In vitro bioassays
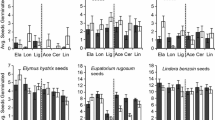
We found a strong allelopathic effect of H. mantegazzianum root exudates in the May 2011 in vitro bioassay (Table 2). The effect of H. mantegazzianum exudates on the germination of C. jacea, D. glomerata and P. lanceolata was strongly negative (F = 16.03; P < 0.001) and species-specific (F = 8.25; P < 0.01; Table 2). The effect of the invader’s exudates on the root length of C. jacea, D. glomerata and P. lanceolata was also significantly negative (F = 211.977; P < 0.001); however, this effect was not species-specific (F = 2.345; NS; Table 2). Only two out of sixty seeds of A. thaliana germinated upon the addition of H. mantegazzianum root exudates (F = 143.73; P < 0.001), and no roots developed at all (Fig. 2).
During the second run, in the August 2011 in vitro bioassay, we observed no effect of H. mantegazzianum (Table 2; Figs. 1, 2). On the other hand, H. sphondylium exudates significantly stimulated the root length of A. thaliana (F = 6.543; P < 0.05). In this bioassay, the responses of native plants were not species-specific (Table 2).
During the third run, in the May 2012 in vitro bioassay, none of the root exudates affected the number of germinants or the root length of A. thaliana (Table 2; Fig. 2). The number of germinants and the root length of native species was also unaffected by the root exudates of H. mantegazzianum (Fig. 1). The number of germinants of native species was, however, stimulated by H. sphondylium, D. glomerata and P. lanceolata (F = 6.01; P < 0.001; Fig. 1). All these effects were species-specific (Table 2).
Native assay species (C. jacea, D. glomerata and P. lanceolata) responses to root exudates of invasive (HM) and native (HS, DG, PL) plant species in three independent in vitro bioassays (May 2011, August 2011, May 2012). Mean ± SE of the number of germinated seeds and of root length are displayed. Columns marked by the same letter are not significantly different (P < 0.05). C stands for control (Hoagland solution), HM for H. mantegazzianum, HS for H. sphondylium, DG for D. glomerata and PL for P. lanceolata (root exudate species)
Responses of A. thaliana to root exudates of invasive (HM) and native (HS, DG, PL) plant species in the three independent in vitro bioassays (May 2011, August 2011, May 2012). Mean ± SE of the number of germinated seeds and of root length are displayed. Columns marked by the same letter are not significantly different (P < 0.05). C stands for control (Hoagland solution), HM for H. mantegazzianum, HS for H. sphondylium, DG for D. glomerata and PL for P. lanceolata (root exudate species)
The summary effect sizes did not indicate significant allelopathic effects of any exudate species included in the experiment. This result was found regardless whether A. thaliana or all three native species were considered as assay species (Fig. 3).
Means and 95 % confidence intervals of parameter estimates (standardized mean difference) of in vitro bioassays. Negative values indicate lower values of the response variables (a product of the number of germinated seeds and mean root lengths of three largest seedlings per Petri dish) when treated with respective root exudates (HM, HS, DG, PL) than when treated with Hoagland’s solution used as a control. Effect sizes are calculated separately for A. thaliana and for the native species. None of the mean effect sizes significantly (P < 0.05) differed from zero (indicated by the dotted line). HM stands for H. mantegazzianum, HS for H. sphondylium, DG for D. glomerata and PL for P. lanceolata (root exudate species)
Garden experiments
The number of germinated seeds and the biomass of assay species were not affected by root exudate species in the garden experiment carried out in 2011 (Table 3). Only Dactylis glomerata biomass was significantly suppressed by the root exudates of both the invasive H. mantegazzianum and the native H. sphondylium in sterile soil (F = 2.95; P = 0.023); however, this effect diminished in non-sterile soil (Fig. 4a). Otherwise, we observed only differences in growth among our assay species, and P. lanceolata biomass was stimulated by the addition of activated carbon regardless of the root exudate treatment.
Effects of root exudates, the soil microbiota and activated carbon on biomass of assay species in garden experiments. Mean ± SE are displayed. C stands for control (Hoagland solution), HM for H. mantegazzianum, HS for H. sphondylium, DG for D. glomerata and PL for P. lanceolata (root exudate species). a D. glomerata biomass was inhibited by root exudates of Heracleum species in 2011 in sterile soil, but not in non-sterile soil. b C. jacea biomass was inhibited by root exudates of D. glomerata in 2012 and activated carbon obviated the effect
In the garden experiment of 2012, the number of germinated seeds was not affected by any of the root exudate species (Table 3). The addition of activated carbon, however, suppressed the germination of native species regardless of the root exudate treatment (F = 4.43; P = 0.036). Neither the exudates of invasive H. mantegazzianum nor those of native H. sphondylium had any effect on the biomass of the assay species. Exudates of D. glomerata nevertheless did suppress the biomass of C. jacea in soil without activated carbon (F = 2.70; P = 0.004); however, the addition of activated carbon inverted the effect (Fig. 4b). Moreover, P. lanceolata exudates significantly negatively affected the biomass of the species itself (F = 5.72; P < 0.001). Furthermore, activated carbon addition stimulated the biomass of D. glomerata and P. lanceolata regardless of the exudate treatment (F = 11.71; P < 0.001).
Overall, there was a significantly negative effect of root exudates of H. mantegazzianum and of two out of three native species in the absence of activated carbon. An additional test, however, did not prove the invader to have stronger phytotoxic effects than the natives (z-value = −0.046; P = 0.963). After addition of activated carbon, the negative effects of all exudate species disappeared (Fig. 5a). Furthermore, the summary effect sizes did not indicate significant allelopathic effects of any of the exudate species whether or not the assay species were grown in non-sterile soil (Fig. 5b).
Means and 95 % confidence intervals of parameter estimates (standardized mean difference) of garden experiments. Negative values indicate lower values of the response variables (biomass of assay species) when treated with respective root exudates (HM, HS, DG, PL) than when treated with Hoagland solution used as a control. Effect sizes are calculated separately for pots with and without addition of activated carbon (a) and for pots with sterile and non-sterile soil (b). Effect sizes significantly (P < 0.05) differed from zero (indicated by the dotted line) for all root exudate species but Plantago lanceolata in the absence of activated carbon (in a). HM stands for H. mantegazzianum, HS for H. sphondylium, DG for D. glomerata and PL for P. lanceolata (root exudate species)
Chemical analyses of root exudates
The peaks of bergapten and xanthotoxin that were monitored using their specific even ion m/z 216 in the GC–MS chromatograms were not detected in the root exudates, although the detection limit of the whole method based on analysis of standards was about 20 ng/L. Accordingly, the monoisotopic weights of psoralen, bergapten, imperatorin, petroselinic acid, their glucosyl and also glucosyloxy derivatives in both ionization modes in the UHPLC–TOF-MS chromatograms were not present either. The compounds that were present only in the exudate samples from the collection used for the May 2011 bioassay and not in the control (Fig. 6) are listed in Table 4. The root exudates from the collection used for the August 2011 bioassay showed very little difference from the control, and we failed to detect any ions in the exudates which would not be present in the control. The UHPLC–TOF-MS peak annotation process that included molecular adduct identification, molecular formula calculation and database matching has not allowed a full identification of compounds of interest; however, the molecular formulae within the error range were calculated based on accurate mass and isotopic pattern recognition in both ionization modes (calculations based only on one ionization mode are marked; Table 4). The database search has not revealed any compound previously reported from any Heracleum species. Nevertheless, compounds C17H26O4 and C27H36O5 were reported in species from the Apiaceae family as derivatives of sesquiterpenes daucanes. Compound C17H26O4 was reported either as siol acetate in Sium latifolium (Casinovi et al. 1983) and S. latijugum (Pandita et al. 1984) or as 11-(acetyl)torilolone in Daucus carota (Yi et al. 2009), whereas compound C27H36O5 may represent a trisubstituted daucane with angeloyloxy and benzoyloxy moiety that has been reported from Ferula communis (Miski and Mabry 1985). More research is needed to assign bioactivity to these compounds and to clarify their structure.
UHPLC–TOF-MS chromatograms (base-peak intensity against retention time) obtained in (a) ESI + positive and (b) ESI − negative ionization mode of a methanol extract from H. mantegazzianum root exudates used for the May 2011 bioassay. The numbers by the peaks indicate retention times. The numbers in boxes indicate compounds that were present only in exudates and not in the control; their molecular weights and tentative molecular formulae are given in Table 4
Discussion
In this study, we have demonstrated that root exudates of H. mantegazzianum are able to exert phytotoxic effects. These effects were detected not only in in vitro but also under garden conditions as confirmed by a meta-analytical approach. However, phytotoxicity was not consistently found across all experimental runs, and this large variation resulted in a non-significant summary effect of in vitro experiments. In some cases of garden experiment, the effects were manifested in the absence of living soil microbiota only. Therefore, it remains unclear to what extent are competitive interactions of H. mantegazzianum with native species determined by allelopathy.
The identity of the compounds involved in allelopathy of the invader remains unknown, but we can rule out the furanocoumarins. Furanocoumarins had previously been indicated as possible agents responsible for allelopathy (Baskin et al. 1967; Macias et al. 1993; Garcia et al. 2002). Junttila (1976), who studied inhibitory effects of a seed extract of H. laciniatum, reported three bioactive fractions including furanocoumarins to suppress germination of assay species although other unknown compounds were also involved. In the highly phytotoxic exudates used for the May 2011 bioassay, we found compounds that were not previously reported from a Heracleum species; some of them, however, possibly represent sesquiterpene derivatives related to daucanes, which have been reported from different species of the Apiaceae family (Casinovi et al. 1983; Pandita et al. 1984; Miski and Mabry 1985; Yi et al. 2009). As we do not know the identity of the phytotoxic compounds released by the invader, we cannot say whether they are novel and unique in invaded communities, as the novel weapons hypothesis predicts (Callaway and Aschehoug 2000; Hierro and Callaway 2003; Callaway and Ridenour 2004). A further investigation is thus needed to prove the phytotoxicity of the candidate compounds and reveal their chemical identity.
Regardless whether bioactive compounds of the invader differ from allelopathic compounds of native species, they all had a significantly negative effect on the performance of assay species used in the garden experiment. Importantly, the allelopathy of the invader did not differ from the allelopathy of its native congener or from that of less-related native species. Although Cappuccino and Arnason (2006) found that some invasive plants are phytochemically unique in their new habitats based on a survey of the literature, Lind and Parker (2010) found no difference in generalist insect herbivore preference between 19 invasive and 21 co-occurring native plant species. However, there is no such study of a similar scale in the field of plant–plant interactions. Allelopathy is mostly studied in invasive plants, so we lack information about allelopathy in native species in their native ranges. Our results nevertheless corroborate those of Del Fabbro et al. (2014) who investigated the effect of three invasive species on germination of native species in a field study and found that invasive species do not suppress seed germination of native species more than species from native plant communities. Overall, we assume that mechanisms other than allelopathy through the production of unique compounds, as predicted by the novel weapons hypothesis (Callaway and Aschehoug 2000; Hierro and Callaway 2003; Callaway and Ridenour 2004), are more likely to explain the invasion success of H. mantegazzianum.
The rapid spread (Pergl et al. 2011) and strong impact of H. mantegazzianum on invaded communities (Jandová et al. 2014) can stem not only from different properties of the invader, but also from attributes of invaded areas. The species has an enormous reproductive capacity. It can produce 20,000 seeds per individual on average (Perglová et al. 2006). Seeds then play an important role in long-distance dispersal, being an important component of population dynamics of invasive species (Pergl et al. 2011). Moreover, Jandová et al. (2014) showed that H. mantegazzianum considerably alters the amount of light available to native species by reducing photosynthetically active radiation and red/far-red ratio in stands it invades. The decreased light levels correlated with observed decreases in native species richness. Moreover, Müllerová et al. (2005) identified the lack of appropriate landscape management associated with disturbances and favourable climatic conditions as the probable reasons for the rapid spread of the species. All these factors, together with allelopathy found in this study, can contribute to the vast invasiveness of H. mantegazzianum.
Several experimental caveats should be borne in mind when drawing conclusions based on results of our study. Firstly, we only used the polar fraction of exudates. Since the solvent present in the root environment is the soil solution that is very close to water and also of high polarity, we decided to study exclusively the polar fraction of root exudates. It may be true that if we had included the non-polar fraction by employing some organic solvent, we would have found out more metabolites; however, these would not necessarily be ecologically relevant for our study. Secondly, we bioassayed the root exudates at only one concentration (that resulting from the collection of material from our growing systems). We therefore do not know whether and how different concentrations would affect our findings. However, our objective was not to estimate the amount of root exudates produced or to estimate the proportion of bioactive compounds present. Finally, our results could have been influenced by using a soil microbiota from a single source in the garden experiment. Nonetheless, it is unlikely that using a soil microbiota from multiple sources would significantly affect our conclusions. Dostál et al. (2013) searched for allelopathic effects of hogweed seedlings cultivated in soil from 20 different sites, including both invaded and uninvaded ones. They failed to prove phytotoxity of hogweed plants, irrespective of the soil origin.
On the other hand, our study provides a comprehensive and repeated test of the novel weapons hypothesis on the example of one of the worst invasive species in Europe. We performed a series of experiments both in vitro and in the garden. In our garden experiments, we considered several factors which had previously been found to modify phytotoxic effects, such as activated carbon and a living soil microbiota. Previous studies have mostly considered allelopathy merely as a species trait, and when intraspecific differences were tested, they focused only on biogeographical comparisons (but see Lankau et al. 2009). Our results indicate that the allelopathic effect of H. mantegazzianum exudates in the species’ introduced range is not a constant trait, but a highly variable one. This finding has prompted a more detailed evaluation of different populations and genotypes, which has revealed substantial variation among genotypes (Jandová et al., preliminary results). Future research is needed to shed light on intraspecific variation in allelopathy of invasive species in their introduced ranges.
To conclude, though root exudates of the invader H. mantegazzianum are able to inhibit the performance of native plant competitors, we found these effects to be comparable with the same effects of native species. We therefore suggest that allelopathy through the production of unique and novel compounds, as predicted by the novel weapons hypothesis, is not very likely to be a principal driver of the invasion success of giant hogweed.
References
Abhilasha Quintana DN, Vivanco J, Joshi J (2008) Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? J Ecol 96:993–1001
Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM (2003) Allelopathy and exotic plants: from genes to invasion. Science 301:1377–1380
Baskin JM, Ludlow CJ, Harris TM, Wolf FT (1967) Psoralen, an inhibitor in the seeds of Psoralea subacaulis (Leguminoseae). Phytochemistry 6:1209–1213
Berenbaum M (1981) Patterns of furanocoumarin distribution and insect herbivory in the umbelliferae—plant chemistry and community structure. Ecology 62:1254–1266. doi:10.2307/1937290
Bertin C, Weston LA, Huang T, Jander G, Owens T, Meinwald J, Schroeder FC (2007) Grass roots chemistry: meta-Tyrosine, an herbicidal nonprotein amino acid. PNAS 104:16964–16969. doi:10.1073/pnas.0707198104
Blair AC, Nissen SJ, Brunk GR, Hufbauer RA (2006) A lack of evidence for an ecological role of the putative allelochemical (±)-catechin in spotted knapweed invasion success. J Chem Ecol 32:2327–2331
Blair AC, Weston LA, Nissen SJ, Brunk GR, Hufbauer RA (2009) The importance of analytical techniques in allelopathy studies with the reported allelochemical catechin as an example. Biol Invasions 11:325–332
Blossey B, Notzgold R (1995) Evolution of increased competitive ability in invasive non-indigenous plants: a hypothesis. J Ecol 83:887–889
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to meta-analysis. Wiley, Chichester
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Cappuccino N, Arnason JT (2006) Novel chemistry of invasive exotic plants. Biol Lett 2:189–193
Casinovi CG, Cerrini S, Motl O, Fardella G, Walter F et al (1983) On terpenes 274. The structure of a new sesquiterpene siol acetate from Sium latifolium L. Collect Czech Chem C 48:2411–2422
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Crawley MJ (2007) The R Book. Wiley, Chichester
Del Fabbro C, Güsewell S, Prati D (2014) Allelopathic effects of three plant invaders on germination of native species: a field study. Biol Invasions 16:1035–1042
Dostál P (2011) Plant competitive interactions and invasiveness: searching for the effects of phylogenetic relatedness and origin on competition intensity. Am Nat 177:655–667
Dostál P, Müllerová J, Pyšek P, Pergl J, Klinerová T (2013) The impact of an invasive plant changes over time. Ecol Lett 16:1277–1284
Duke SO (2010) Allelopathy: current status of research and future of the discipline: a commentary. Allelopathy J 25:17–29
Eppinga MB, Rietkerk M, Dekker SC, Ruiter PCD, van der Putten WH (2006) Accumulation of local pathogens: a new hypothesis to explain exotic plant invasions. Oikos 114:168–176
Fischer FC, Van Doorne H, Dannenberg G (1978) Glycosides and glycosidase in Heracleum mantegazzianum—their possible role in resistance against fungi. In: Cauwet-Marc AM, Carbonnier J (eds) Actes du 2e Symposium International sur les Ombelliferes. CNRS, Perpignan, pp 783–792
Garcia C, Moyna P, Fernandez G, Heinzen H (2002) Allelopathic activity of Ammi majus L. fruit waxes. Chemoecology 12:107–111
Harborne JB (1971) Flavonoid and phenylpropanoid patterns in the Umbelliferae. Bot J Linnean Soc 64(Suppl. 1):293–314
Hattendorf J, Hansen SO, Nentwig W (2007) Defense system of Heracleum mantegazzianum. In: Pyšek P, Cock MJW, Nentwig W, Ravn HP (eds) Ecology and management of giant hogweed (Heracleum mantegazzianum). CAB International, Wallingford, pp 209–225
Hejda M, Pyšek P, Jarošík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403
Herde A (2005) Untersuchung der Cumarinmuster in Fruchten ausgewahlter Apiaceae. Dissertation, University of Hamburg
Hierro JL, Callaway RM (2003) Allelopathy and exotic plant invasion. Plant Soil 256:29–39
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn 347:1–32
Inderjit, Nilsen ET (2003) Bioassays and field studies for allelopathy in terrestrial plants: progress and problems. Crit Rev Plant Sci 22:221–238
Inderjit, Seastedt TR, Callaway RM, Pollock JL, Kaur J (2008) Allelopathy and plant invasions: traditional, congeneric, and bio-geographical approaches. Biol Invasions 10:875–890
Ivie GW (1978) Toxicological significance of plant furocoumarins. In: Keeler RF, van Kamper KR, James LF (eds) Effects of poisonous plants on livestock. Academic Press, New York, pp 475–485
Jahodová S, Trybush S, Pyšek P, Wade M, Karp A (2007) Invasive species of Heracleum in Europe: an insight into genetic relationships and invasion history. Divers Distrib 13:99–114
Jain SR (1969) Investigations on essential oil of Heracleum mantegazzianum L. Planta Med 17:230–235
Jandová K, Klinerová T, Müllerová J, Pyšek P, Pergl J, Cajthaml T, Dostál P (2014) Long-term impact of Heracleum mantegazzianum invasion on soil chemical and biological characteristics. Soil Biol Biochem 68:270–278
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714
Junttila O (1975) Allelopathy in Heracleum laciniatum: inhibition of lettuce seed germination and root growth. Physiol Plant 33:22–27
Junttila O (1976) Allelopathic inhibitors in seeds of Heracleum laciniatum. Physiol Plant 36:374–378
Kartesz JT, Meacham CA (1999) Synthesis of the North American Flora, Version 1.0. North Carolina Botanical Garden, Chapel Hill, NC
Kaur H, Kaur R, Kaur S, Baldwin IT, Inderjit (2009) Taking ecological function seriously: soil microbial communities can obviate allelopathic effects of released metabolites. PloS One 4:e4700
Kavli G, Krokan H, Midelfart K, Volden G, Raa J (1983) Extraction, separation, quantification and evaluation of the phototoxic potency of furocoumarins in different parts of Heracleum laciniatum. Photobioch Photobiop 5:159–168
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
KNApSAcK (2014) A Comprehensive Species-Metabolite Relationship Database. Version 1.000.01 (2008/06/23). Accessed 28 Apr 2014
Lankau R (2010) Soil microbial communities alter allelopathic competition between Alliaria petiolata and a native species. Biol Invasions 12:2059–2068
Lankau RA, Nuzzo V, Spyreas G, Davis AS (2009) Evolutionary limits ameliorate the negative impact of an invasive plant. PNAS 106:15362–15367
Lind EM, Parker JD (2010) Novel weapons testing: Are invasive plants more chemically defended than native plants? Plos One 5:e10429
Macias FA, Galindo JCG, Massanet GM, Rodriguez-Luis F, Zubia E (1993) Allelochemicals from Pilocarpus goudotianus leaves. J Chem Ecol 19:1371–1379
Miski M, Mabry T (1985) Daucane esters from Ferula communis subsp. communis. Phytochemistry 24:1735–1742
Molho D, Jossang P, Jarreau MC, Carbonnier J (1971) Derives furannocoumariniques du genre Heracleum. Bot J Linnean Soc 64(Suppl. 1):337–360
Müllerová J, Pyšek P, Jarošík V, Pergl J (2005) Aerial photographs as a tool for assessing the regional dynamics of the invasive plant species Heracleum mantegazzianum. J Appl Ecol 42:1042–1053
Myras H, Junttila O (1981) Interaction between Heracleum laciniatum and some other plants. Holarctic Ecol 4:43–48
Nielsen BE (1971) Coumarin patterns in the Umbelliferae. Bot J Linnean Soc 64(Suppl. 1):325–336
Page NA, Wall RE, Darbyshire SJ, Mulligan GA (2006) The biology of invasive alien plants in Canada. 4. Heracleum mantegazzianum Sommier & Levier. Can J Plant Sci 86:569–589
Pandita K, Agarwal SG, Thappa RK, Dhar KL (1984) 1-alpha-acetoxy-6-alpha-hydroxy-9-oxo-carot-2-ene, a new derivative from Sium latijugum seeds. Indian J Chem B 23:956–957
Pergl J, Mullerová J, Perglová I, Herben T, Pyšek P (2011) The role of long-distance seed dispersal in the local population dynamics of an invasive plant species. Divers Distrib 17:725–738
Perglová I, Pergl J, Pyšek P (2006) Flowering phenology and reproductive effort of the invasive alien plant Heracleum mantegazzianum. Preslia 78:265–285
Prati D, Bossdorf O (2004) Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am J Bot 91:285–288
Pyšek P, Pyšek A (1995) Invasion by Heracleum mantegazzianum in different habitats in the Czech Republic. J Veg Sci 6:711–718
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Reaxys database (2014) version 2.15212.2, Elsevier. Accessed 28 Apr 2014
Sheppard AW (1991) Biological flora of the British Isles: Heracleum sphondylium L. J Ecol 79:235–258
Thiele J, Otte A (2007) Impact of Heracleum mantegazzianum on invaded vegetation and human activities. In: Pyšek P, Cock MJW, Nentwig W, Ravn HP (eds) Ecology and management of giant hogweed (Heracleum mantegazzianum). CAB International, Wallingford
Thiele J, Kollmann J, Markussen B, Otte A (2010) Impact assessment revisited: improving the theoretical basis for management of invasive alien species. Biol Invasions 12:2025–2035
Tiley GED, Dodd FS, Wade PM (1996) Heracleum mantegazzianum Sommier et Levier. J Ecol 84:297–319
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48
Wille W, Thiele J, Walker EA, Kollmann J (2013) Limited evidence for allelopathic effects of giant hogweed on germination of native herbs. Seed Sci Res. doi:10.1017/S096025851300007X
Yi T, Zhang L, Fu HW, Yang SL, Tian JK (2009) Two new guaiane sesquiterpenes from the fruits of Daucus carota. Helv Chim Acta 92:2769–2773
Acknowledgments
This study was funded by grant P504/10/0132 from the Czech Science Foundation and grant GAUK512712 from the Charles University Grant Agency. Zdeněk Kameník from the Laboratory for Biology of Secondary Metabolism, Institute of Microbiology, Academy of Sciences of the Czech Republic carried out the LC–MS analyses. Frederick Rooks kindly improved our English. We thank Tereza Klinerová, Zdena Křesinová, Dana Parysová and Daniel Samek for their technical assistance, Lenka Moravcová for her advice during the germination experiments and three anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jandová, K., Dostál, P. & Cajthaml, T. Searching for Heracleum mantegazzianum allelopathy in vitro and in a garden experiment. Biol Invasions 17, 987–1003 (2015). https://doi.org/10.1007/s10530-014-0771-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-014-0771-5