Abstract
Biological invasions have become a major global issue in ecosystem conservation. As formalized in the “novel weapon hypothesis”, the allelopathic abilities of species are actively involved in invasion success. Here, we assume that allelopathy can also increase the biotic resistance of native species against invasion. We tested this hypothesis by studying the impact of the native species Sambucus ebulus on the colonization of propagules of the invasive species Fallopia x bohemica and the subsequent development of plants from these. Achenes and rhizome fragments from two natural populations were grown in a greenhouse experiment for 50 days. We used an experimental design that involved “donor” and “target” pots in order to separate resource competition from allelopathy. An allelopathic treatment effect was observed for plant growth but not for propagule establishment. Treatment affected, in particular, the growth of Fallopia plants originating from achenes, but there was less influence on plants originating from rhizomes. By day 50, shoot height had decreased by 27 % for plants originating from rhizomes and by 38 % for plants originating from achenes. The number of leaves for plants originating from achenes had only decreased by 20 %. Leaf and above- and below-ground dry masses decreased with treatment by 40, 41 and 25 % for plants originating from rhizomes and 70, 61 and 55 % for plants originating from achenes, respectively. S. ebulus extracts were analysed using high-performance chromatography, and the choice of test molecules was narrowed down. Our results suggest native species use allelopathy as a biotic containment mechanism against the naturalization of invasive species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many invasive plants are not dominant in their native habitat but may become invasive and develop mono-specific patches when introduced into a new environment (Ridenour and Callaway 2001). Direct competition between invasive and native plants is an ongoing process through which invasive species replace native ones and threaten biodiversity (Gurevitch and Padilla 2004). Among direct interactions, competition for resources is often regarded as the main limiting factor. However, other forms of direct competition, such as allelopathy, can also have a significant impact (Michalet et al. 2006).
Allelopathy is defined as a process of plant-plant or plant-microorganism chemical interaction with either positive or negative effects (Rice 1984). This phenomenon is actively involved in invasive success (Hierro and Callaway 2003). Various studies have led to the “novel weapon hypothesis” (NWH, Callaway and Aschehoug 2000; Callaway and Ridenour 2004; Cappuccino and Arnason 2006) stating that plants that have co-evolved with an allelopathic species may be less susceptible to allelopathic compounds, while newly exposed species (such as those in an invaded range) may exhibit less resistance (Callaway and Aschehoug 2000; Callaway et al. 2008). This theory assumes that the success of invasive species exists because native plants do not have a defence system against the toxic substances produced by the invasive species (Hierro and Callaway 2003). However, a corollary to this theory is that invasive species that have not co-evolved with native species could be sensitive to the toxic compounds produced by these natives. Allelopathic compounds in native plants could affect the growth of invasive species and would thus constitute a new natural method of control (Popovici et al. 2011) and a new type of biotic resistance against invasion (Weidenhamer and Romeo 2005; Cummings et al. 2012). However, the allelopathic effects of native plants on invasive species have rarely been studied.
Fallopia x bohemica (Polygonaceae) is one of the most widespread invasive species in Europe (Bailey and Wisskirchen 2006). This is a rhizomatous herbaceous plant, showing a high growth rate resulting in colonial stands with a huge above-ground and below-ground biomass. In France, this taxon results mostly from multiple hybridizations and from introgression between Fallopia sachalinensis and Fallopia japonica, originating from East Asia (Bailey et al. 2009). It has the ability to disperse using two types of propagules: achenes and rhizomes, making it very difficult to control. It has recently been shown that Sambucus ebulus (Adoxaceae) could limit the colonization and subsequent development of two types of propagules of F. x bohemica (achenes and rhizome fragments) in direct pot competition (Rouifed 2011). This effect could be explained by a direct allelopathic effect, a limited access to light or by competition for resources. Indeed, S. ebulus is an herbaceous pioneer plant, with the ability to colonize the same natural habitat than F. x bohemica (e.g. road verges or riverbanks). It also shows a similar growth form than Fallopia spp.: asexual propagation with a rhizome and a high above-ground growth rate of an erected stem.
Invasion is considered as a staged process, the numbers involved varying depending on the author. Based on the process described by Catford et al. (2009), we can assume that sexual propagules such as achenes or seeds and vegetative propagules such as rhizomes are involved in various invasion stages. Nevertheless, the relative roles played by vegetative and sexual reproduction in F. x bohemica dispersal are still controversial (Tiébré et al. 2008). However, we can assume that both seeds and rhizomes are involved in colonization (survival of introduced plants) and spread (dispersal of propagules and spread of populations outside of area where first introduced) during invasion (Rouifed et al. 2011).
We put forward the hypothesis that the native species S. ebulus has an allelopathic effect on the invasive species F. x bohemica, thus limiting the growth of the invasive species. We tested this hypothesis with achenes and rhizomes, on the assumption that they corresponded to different dispersal strategies. Using an experimental design adapted from Viard-Crétat et al. (2009) that involved watering “target” pots with leachates from “donor” pots, we measured the effect of S. ebulus leachates on Fallopia traits.
Methods
Propagule sampling
On December 13 (2010), rhizomes of S. ebulus were collected in the field on different plants of the same population, in Chambéon (France, Loire Département). This population was separated from the nearest populations of F. x bohemica by at least 30 m. In January–February, rhizomes of F. x bohemica were collected from two populations in France: Feyssine Park in Lyon (Rhône Département) and Veauchette (Loire Département) named populations 1 and 2, respectively. For each of the two populations sampled, all the rhizomes were collected from one individual (i.e. clones). Rhizomes 1 and 2 had different genotypes (ISSR determination, unpublished data). All plants were cautiously washed in order to remove the soil and grown at 20 °C in a greenhouse under identical conditions. Achenes were collected in January 2009 at random from several F. x bohemica shoots of the same stand along the river Dorlay in Lorette (France, Loire Département). Rhizomes and achenes were stored under dry conditions at +4 °C until they were used for the experiment. Preliminary tests showed that these sampling and storage methods did not affect the germination rates of achenes (Rouifed et al. 2011).
Experimental design
We adapted the experimental design of Viard-Crétat et al. (2009) involving donor and target pots used to separate resource competition from allelopathy (Fig. 1). We used 1 L pots filled with 100 % Klasmann potting compost. We used a potting compost to test whether a plant could have a direct chemical effect on another independently of effects related to the microbial community, probably poorly represented in this type of substrate. Donor pots were put on 15-cm diameter plastic funnels that were placed on shelves 30 cm above the target pots. One flask (120 mL) was placed under each funnel to collect the leachates. Thirty pots were randomly assigned to controls (15 pots without plants) or treatment (15 pots containing S. ebulus rhizomes). Rhizomes were selected with one node and a biomass of about 5 ± 0.1 g. Rhizomes of S. ebulus had been planted 2 months before we started the experiment in order to obtain an aerial shoot from each rhizome. Control donor pots were likewise prepared and watered during these 2 months before the beginning of the experiment. At the beginning of the experiment, the 30 donor pots were saturated with water and then watered every 2 or 3 days, respectively, to obtain 100 mL of leachate. Thirty target pots contained propagules of F. x bohemica: 10 with achenes (9 achenes per pot), 10 with rhizome fragments from population 1 and 10 with rhizome fragments from population 2 (1 fragment per pot). Rhizome fragments were selected with one node and a biomass of 1.5 ± 0.1 g. These pots were regularly watered with leachates from donor pots harvested by gravity (for each set of 10 target pots, 5 were watered with leachates from control donor pots and 5 with donor pots containing S. ebulus plants). Target pots were randomly moved every week within a treatment (control or S. ebulus) to avoid a possible localization effect or an effect of S. ebulus development. The experiment lasted 50 days.
Experimental design of the pot experiment. Two types of donor pots (bare compost and S. ebulus in compost) under which were placed three types of target pots containing compost planted with F. x bohemica achenes, or rhizome fragments from populations 1 or 2, for a total of six treatments with five replicates each (30 target pots)
Measurements on F. x bohemica
Two variables were used to represent colonization success: achene germination rate and rhizome regeneration time. The height, number of leaves and dry masses of F. x bohemica were measured to test the effect of S. ebulus leachates on its growth.
The achene germination rate and the time for both achenes and rhizomes to emerge were expressed in days from the beginning of the experiment. The final leaf number and height up to the apical meristem were measured at day 50. Final leaf (using all leaves) and above- and below-ground dry masses were measured after drying for 3 days at 70 °C. One plant from population 1 under treatment was removed from the results because of unexpected growth.
Chemical analysis of S. ebulus
In parallel, rhizome samples from S. ebulus were collected in the experiment and freeze-dried. Seventy-five milligrams of freeze-dried sample was crushed using a ball mill TissueLyser II (Qiagen) and extracted with 1 mL of water/methanol (50:50; v/v) by ultrasonication for 15 min. After centrifugation (10 min at 16,000g), the supernatant was collected and another extraction was carried out on the pellet with 0.75 mL of pure methanol. Both supernatants obtained were pooled, filtered and concentrated to 1 mg mL−1. Rhizome extracts were stored at −20 °C before HPLC analyses. The rhizome extracts were analysed by HPLC using an Agilent 1100 series coupled to a G1315b DAD (Agilent Technologies). The system was run using Chemstation software (Agilent Technologies). Separation was carried out using a Zorbax Eclipse XDB-C8 100 A° (5 μm; 250 × 4.6 mm; Agilent Technologies) at room temperature. The mobile phase was a linear gradient of 1 % formic acid in water (solvent A) and 1 % formic acid in methanol (solvent B). The linear gradient at the flow rate of 1 mL min−1 was 0 to 16 min for 20 % solvent B, 16 to 26 min for 20 to 45 % solvent B, 26 to 40 min for 45 to 80 % solvent B, 40 to 50 min for 80 to 100 % solvent B and 50 to 55 min for 100 % solvent B. The system was equilibrated between samples. Injection volume was 35 μL. Spectra were recorded between 200 and 600 nm, and the response at 280 nm was used for identification analysis.
Statistical analyses
The effect of treatment on the germination rate of achenes was analysed using the t test. The effect of treatment on the number of days to emergence, below- and above-ground dry masses, leaf dry weight, height and total number of leaves were analysed separately for plants originating from achenes and from rhizomes. For plants originating from achenes, the effect of treatment on the number of days to emergence, total final leaf, and below- and above-ground dry masses per pot (i.e. total for all individuals in each pot) were analysed using ANOVA. The effect of treatment on height and number of leaves at 50 days were analysed using mixed model analyses of variance, with the treatment as fixed effect and pot as random effect to take into account the grouping of individuals in the same pot. For plants originating from rhizomes, the effects of treatment, population of origin and their interaction on the number of days to emergence, total final leaf, below- and above-ground dry mass, height and total number of leaves at 50 days were tested with two-way ANOVAs.
For both types of plant (originating from achenes and rhizomes), the number of days to emergence and height were log-transformed to improve the homogeneity of variance. All statistical analyses were made with R 2.12.0 software (R Development Core Team 2010).
Results
Leachate treatment had no effect on the emergence of Fallopia propagules. Shoots from rhizomes 1 and rhizomes 2 emerged around day 7 and day 11 after planting, respectively (Fig. 2a). Achenes germinated around 9 days after planting (Fig. 2a). Leachate treatment also had no significant effect on the germination rate of achenes (t 5.66 = 1.87, p = 0.11, Fig. 2b).
Number of days to emergence of rhizomes (a) and germination rate of achenes (b) of F. x bohemica. Data are shown for the three types of propagule watered by leachates from S. ebulus pots (treatment) or water from compost only pots (control). Bars represent standard deviations (n = 4 and 5 for rhizomes 1, n = 5 and 5 for rhizomes 2, and n = 39 and 33 for achenes, for treatment and control, respectively). Effect of treatment is never statistically significant
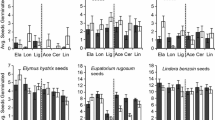
In the case of plants originating from achenes, S. ebulus treatment significantly decreased Fallopia below-ground dry mass, above-ground dry mass, leaf dry mass, height and total number of leaves (Table 1). Below-ground dry mass decreased significantly by 55 % (Table 1, Fig. 4). The treatment significantly reduced above-ground dry mass by 61 %, and leaf dry mass decreased by 70 % for plants originating from achenes (Table 1). Height decreased by 38 % for achenes under treatment (Fig. 3a). By day 50, treatment had significantly decreased, by 20 %, the total number of leaves of plants originating from achenes (Table 1, Fig. 3b).
Final height (a) and total number of leaves (b) of plants originating from three types of propagule watered with leachates from S. ebulus pots (treatment) or compost only pots (control), on day 50. Bars represent standard deviations (n = 4 and 5 for rhizomes 1, n = 5 and 5 for rhizomes 2, and n = 39 and 33 for achenes, for treatment and control, respectively). Significant treatment effect after variance analysis (Tables 1 and 2) is shown with the symbol (asterisk)
In the case of plants originating from rhizomes, interaction between treatment and the rhizome populations was not significant for any of the traits measured (Table 2). However, the number of days to emergence, plant height and total number of leaves differed significantly between plants originating from the two rhizome populations (Table 2): Plants originating from rhizome population 2 were significantly shorter, with fewer leaves and took longer to emerge (Figs. 2, 3 and 4). The S. ebulus treatment significantly decreased Fallopia below-ground dry mass, above-ground dry mass, leaf dry mass and height (Table 2). S. ebulus treatment significantly decreased the total dry mass of below-ground, above-ground and leaves of plants originating from both rhizome populations (Table 2). Below-ground dry mass decreased by 25 % (Fig. 4). Treatment significantly reduced above-ground dry mass by 41 % (Table 2). Leaf dry mass decreased by 40 % for plants originating from rhizomes. By day 50, treatment had significantly reduced the height of Fallopia plants from both rhizome populations (Table 2). Height decreased by 31 % for rhizomes 1 and by 26 % for rhizomes 2 (Fig. 3a). There was no significant decrease in the number of leaves under treatment (Table 2).
Final dry masses of the plants grown from three types of propagule watered using leachates from S. ebulus pots (treatment) or compost pots (control), on day 50. Total below-ground plant dry mass (a), total above-ground plant dry mass (b) and plant leaf dry mass only (c) are represented. Bars represent standard deviations (n = 4 and 5 for rhizomes 1, n = 5 and 5 for rhizomes 2 and n = 5 and 5 for achenes, for treatment and control, respectively, for all types of dry mass). Significant treatment effect after variance analysis (Tables 1 and 2) is shown with symbol (asterisk)
High-performance liquid chromatography at 280 nm of S. ebulus rhizome extraction showed seven main peaks. Retention time and UV spectra (280 nm) were used to identify the compounds. Two compounds were identified as catechin and epicatechin, and this was confirmed with a subsequent co-chromatography with commercial standards (Sigma-Aldrich). Four compounds showed characteristic UV spectra of di-hydroxycinnamic acid family (absorption maxima at 320 nm, shoulder at 310 nm). The last of the detected phenolic compounds could not be identified on the basis of its UV spectrum (absorption maxima at 280 nm).
Discussion
Allelopathic experiments are often controversial due to the experimental design choice. Here, we used the experimental design of Viard-Crétat et al. (2009), involving target pots watered by leachates from donor pots, an appropriate setup for studying allelopathy, assuming that the leachates contain the allelopathic compounds released by donor plants. Activated carbon has been used in many previous experiments (Callaway and Aschehoug 2000). Nevertheless, recent studies have shown that the addition of activated carbon can stimulate microbial activity and increase soil phosphate availability (Weisshuhn and Prati 2009). Another approach used extracts of root systems (Dorning and Cipollini 2006). Nevertheless, extracted compounds are not necessarily exuded by root systems under natural conditions. Using this Viard-Crétat et al. design (2009) allowed us to validate our hypothesis on the involvement of allelopathy in native-invasive plant interactions due to root system exudates from the native species. In a previous study, Fallopia growth was affected by competition from S. ebulus (Rouifed 2011). This direct competition in the same pot was probably due to allelopathic interference, maybe combined with some other form of competition, such as competition for resources.
F. x bohemica can spread both vegetatively and sexually. The potential dispersal of Fallopia achenes along rivers has recently been highlighted (Rouifed et al. 2011). Nevertheless, it is still thought that it usually spreads vegetatively. In a previous study, achenes seemed much more sensitive to biotic resistance than rhizomes (Rouifed 2011). Our results also support this hypothesis. Allelopathic treatment to achenes resulted in a greater decrease in the height, number of leaves or below-ground dry weight of end plants than to rhizomes. This sensitivity could participate to the predominance of clonal dispersal.
Since F. x bohemica is a clonal plant, we can assume that weaker growth implies a decrease in fitness. Allelopathic treatment has no effect on the emergence of a shoot from rhizome fragments or achenes. Nevertheless, during the next stage of development, there is an allelopathic impact. Fallopia plant growth, height and dry weight are all affected. In natural communities, we would assume that such an impact would considerably decrease its fitness. It is well known that communities may resist invasion through a diversity of biotic processes, including predation, competition, herbivory, disease or species diversity (Levine et al. 2004). Biotic resistance would therefore act in different stages of the invasion process, limiting establishment (Theoharides and Dukes 2007), but also colonization.
Non co-evolution has been shown as a determinant for possible allelopathic effects and invasive success (Callaway and Aschehoug 2000). One much studied species is Centaurea maculosa. Other species found in the native range of C. maculosa may have become adapted to its particular biochemical traits, raising the possibility that interactions between plant species may be affected by a common evolutionary history (Thorpe et al. 2009). In the invasive range, native plant species may evolve to tolerate the effects of an exotic invader and in particular to an invader’s novel allelochemistry (Callaway et al. 2005). Theoretical arguments in favour of the novel weapon hypothesis can also be looked at in reverse. Because they have not co-evolved, exotic species are less adapted to potential phytotoxic compounds released by native species. Allelopathy could be involved in invasion resistance, in accordance with a novel weapon hypothesis in reverse, or “Homeland Security” hypothesis (Cummings et al. 2012). In a recent study, we identified an allelopathic compound from the fruits and the leaves of Myrica gale, a species native to the European and American continents, which is phytotoxic to F. x bohemica (Popovici et al. 2011). This study was conducted using a bioassay system and therefore is a little removed from natural conditions. The results of the present study support the allelopathic effect as a resistance to the invasion process. We show that F. x bohemica is sensitive to chemicals from another native plant, in conditions that are closer from conditions in the field. Allelopathic resistance against invasion has also been investigated in Florida scrub (Weidenhamer and Romeo 2005). In this latter case, it appears that environmental factors could intensify the allelopathic effects and play a role in the resistance of this community against invasion. Environmental stress factors, such as high temperatures and nutrient limitation, are known to increase the toxicity or concentration of allelochemicals (Einhellig and Eckrich 1984; Einhellig 1987). Thus, allelopathy may also be a mechanism more common than previously thought, particularly in communities of resource-poor environments. To generalize these findings to a general ecological process, experiments involving various native species and several invasive species are needed.
In our experiment, we observed similar effects of the treatment on functional traits in both rhizome populations. Nevertheless, the F. bohemica taxon exhibits a high genetic variation (Mandák et al. 2005), due to its occurrence at three ploidy levels and because the hybrid has been produced a number of times at different places (Pyšek et al. 2003). More information about the geographical interaction between Fallopia populations and potentially allelopathic Sambucus populations could be useful in formulating different evolution models. Such interactive events could make Fallopia populations evolve to tolerate the effect of the native plant’s novel allelochemistry. Similarly, the genetic variability of S. ebulus also needs to be studied. Potential links between variability in the allelochemical compositions of populations and invasion resistance also has to be explored.
Chromatography of extracts of rhizomes of S. ebulus revealed compounds known to be potentially allelopathic (Macıas et al. 2007). Flavanols (catechin, epicatechin) are a family of bioactive compounds present in a wide range of botanical sources (Hackman et al. 2008). Catechin is a strong allelochemical involved in the invasive success of C. maculosa (Thorpe et al. 2009). Cinnamic acids are considered as phytotoxic allelochemicals too (Li et al. 1993). The secondary metabolite composition of S. ebulus validates our hypothesis that this species has an allelopathic role. However, it has been really poorly studied so far, and more analyses are necessary to improve the method of the chromatography and to detail the composition and the effects of its secondary metabolites. It is indeed possible that the allelopathic effect of S. ebulus is due to a combination of the identified compounds or other minor unidentified compounds. A way to identify which particular compounds in S. ebulus are involved in the competition against Fallopia would be to supplement F. x bohemica target pots with some of the key secondary metabolites identified in the cocktail of S. ebulus.
These metabolites can also target microorganisms, and observed allelopathic effects may result from indirect interactions involving microorganisms (Inderjit et al. 2011). Here, the experimental design did not allow concluding between a direct plant-plant interaction and an effect of Sambucus on microorganisms associated with Fallopia. Besides, even if we took precautions and used potting compost, we acknowledge that microorganisms present in the different treatments in donor pots may modify the biochemical cocktail from S. ebulus. Further experiments are needed to deepen the mechanisms involving microorganisms, whether they are targets in allelopathic process or interfering organisms from the soil.
To conclude, our study validates a new type of design in allelopathic experiments, avoiding the possible alternative effects in previous experimental designs. We have shown that the invasive weed F. x bohemica is sensitive to allelochemicals produced by the native species, S. ebulus. These results suggest a new type of biotic resistance at work, poorly studied so far, following a novel weapon hypothesis in reverse.
References
Bailey J, Wisskirchen R (2006) The distribution and origins of Fallopia x bohemica (Polygonaceae) in Europe. Nord J Bot 24:173–199
Bailey JP, Bimova K, Mandak B (2009) Asexual spread versus sexual reproduction and evolution in Japanese Knotweed s.l. sets the stage for the “Battle of the Clones”. Biol Invasions 11:1189–1203
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Callaway RM, Ridenour WM, Laboski T, Weir T, Vivanco JM (2005) Natural selection for resistance to the allelopathic effects of invasive plants. J Ecol 93:576–583
Callaway RM, Cipollini D, Barto K, Thelen GC, Hallett SG, Prati D, Stinson K, Klironomos J (2008) Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 89:1043–1055
Cappuccino N, Arnason JT (2006) Novel chemistry of invasive exotic plants. Biol Lett 2:189–193
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40
Cummings JA, Parker IM, Gilbert GS (2012) Allelopathy: a tool for weed management in forest restoration. Plant Ecol 213:1975–1989
Dorning M, Cipollini D (2006) Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol 184:287–296
Einhellig FA (1987) Interaction among allelochemicals and other stress factors of the plant environment. ACS Symp Ser 330:343–357
Einhellig FA, Eckrich PC (1984) Interactions of temperature and ferulic acid stress on grain-sorghum and soybeans. J Chem Ecol 10:161–170
Gurevitch J, Padilla DK (2004) Response to Ricciardi. Assessing species invasions as a cause of extinction. Trends Ecol Evol 19:620–620
Hackman RM, Polagruto JA, Zhu QY, Sun B, Fujii H, Keen CL (2008) Flavanols: digestion, absorption and bioactivity. Phytochem Rev 7:195–208
Hierro JL, Callaway RM (2003) Allelopathy and exotic plant invasion. Plant Soil 256:29–39
Inderjit, Wardle DA, Karban R, Callaway RM (2011) The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol 26:655–662
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Li H-H, Inoue M, Nishimura H, Mizutani J, Tsuzuki E (1993) Interactions of trans-cinnamic acid, its related phenolic allelochemicals, and abscisic acid in seedling growth and seed germination of lettuce. J Chem Ecol 19:1775–1787
Macıas FA, Molinillo JM, Varela RM, Galindo JC (2007) Allelopathy—a natural alternative for weed control. Pest Manag Sci 63:327–348
Mandák B, Bímová K, Pyšek P, Štepánek J, Placková I (2005) Isoenzyme diversity in Reynoutria (Polygonaceae) taxa: escape from sterility by hybridization. Plant Syst Evol 253:219–230
Michalet R, Brooker RW, Cavieres LA, Kikvidze Z, Lortie CJ, Pugnaire FI, Valiente-Banuet A, Callaway RM (2006) Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol Lett 9:767–773
Popovici J, Bertrand C, Jacquemoud D, Bellvert F, Fernandez MP, Comte G, Piola F (2011) An allelochemical from Myrica gale with strong phytotoxic activity against highly invasive Fallopia x bohemica taxa. Molecules 16:2323–2333
Pyšek P, Brock JH, Bímová K, Mandák B, Jarošik V, Koukoliková I, Pergl J, Štepánek J (2003) Vegetative regeneration in invasive Reynoutria (Polygonaceae) taxa: the determinant of invasibility at the genotype level. Am J Bot 90:1487–1495
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rice EL (1984) Allelopathy, 2nd edn. Academic, New York
Ridenour WM, Callaway RM (2001) The relative importance of allelopathy in interference: the effects of an invasive weed on a native bunchgrass. Oecologia 126:444–450
Rouifed S (2011) Bases scientifiques pour un contrôle des Renouées asiatiques. PhD thesis, Université Lyon1
Rouifed S, Puijalon S, Viricel M-R, Piola F (2011) Achenes buoyancy and germinability of the terrestrial invasive Fallopia x bohemica in aquatic environment: a new vector of dispersion? Ecoscience 18:79–84
Theoharides KA, Dukes JS (2007) Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytol 176:256–273
Thorpe AS, Thelen GC, Diaconu A, Callaway RM (2009) Root exudate is allelopathic in invaded community but not in native community: field evidence for the novel weapons hypothesis. J Ecol 97:641–645
Tiébré M-S, Saad L, Mahy G (2008) Landscape dynamics and habitat selection by the alien invasive Fallopia (Polygonaceae) in Belgium. Biodivers Conserv 17:2357–2370
Viard-Crétat F, Gallet C, Lefebvre M, Lavorel S (2009) A leachate a day keeps the seedlings away: mowing and the inhibitory effects of Festuca paniculata in subalpine grasslands. Ann Bot 103:1271–1278
Weidenhamer JD, Romeo JT (2005) Allelopathy as a mechanism for resisting invasion: the case of Polygonella myriophylla. International Symposium on Ecology of Biological Invasions (ed I. Inderjit). Unive Delhi, CEMDE, Delhi, India
Weisshuhn K, Prati D (2009) Activated carbon may have undesired side effects for testing allelopathy in invasive plants. Basic Appl Ecol 10:500–507
Acknowledgments
The authors would like to thank Denis Desbouchages for help during greenhouse experiments (FR 41, Université Lyon 1). This study was allowed thanks to the financial support of the project “Scientific basis for a control of Asian knotweeds” by FEDER (Plan Loire Grandeur Nature), Agence de l’Eau Loire Bretagne, Conseil Général du département de la Loire and Région Rhône-Alpes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Beverley Glover
Rights and permissions
About this article
Cite this article
Christina, M., Rouifed, S., Puijalon, S. et al. Allelopathic effect of a native species on a major plant invader in Europe. Sci Nat 102, 12 (2015). https://doi.org/10.1007/s00114-015-1263-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-015-1263-x