Abstract
Many plants release allelopathic chemicals that can inhibit germination, growth, and/or survival in neighboring plants. These impacts appear magnified with the invasion of some non-native plants which may produce allelochemicals against which native fauna have not co-evolved resistance. Our objective was to examine the potential allelopathic impact of an invasive non-native shrub/tree on multiple plant species using field observation and experimental allelopathy studies. We surveyed and collected an invasive, non-native tree/shrub (Rhamnus cathartica) at Tifft Nature Preserve (a 107-ha urban natural area near Lake Erie in Buffalo, NY). We also surveyed understory plant communities in the urban forest to examine correlations between R. cathartica abundance and local plant community abundance and richness. We then used experimental mesocosms to test if patterns observed in the field could be explained by adding increased dosages of R. cathartica to soils containing five plant species, including native and non-native woody and herbaceous species. In the highly invaded urban forest, we found that herbaceous cover, shrubs and woody seedlings negatively covaried with R. cathartica basal area and seedlings density. In the mesocosm experiments, R. cathartica resulted in significant decreases in plant community species richness, abundance, and shifted biomass allocation from roots. Our results provide evidence that R. cathartica is highly allelopathic in its invaded range, that R. cathartica roots have an allelopathic effect and that some plant species appear immune. We suggest that these effects may explain the plant’s ability to form dense monocultures and resist competitors, as well as shift community composition with species-specific impacts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants release secondary chemical compounds (not essential for growth, reproduction, and survival) that deter and inhibit herbivores and other plants; the deleterious effect on other plants is called allelopathy (Rice 1984). Allelopathic plants release phytotoxic chemicals into the soil environment through root and leaf leachate that can inhibit the germination, growth, and/or survival of neighboring plants (Fernandez et al. 2016; Lankau 2011; Orr et al. 2005). Some invasive plants appear to gain considerable advantage in novel ecosystems because they produce phytotoxic allelochemicals against which invaded-range native species may not have co-evolved resistance (Callaway and Ridenour 2004; Fernandez et al. 2016; Pisula and Meiners 2010; Thorpe et al. 2009). As a result, some invasive plants may form dense monocultures where native plants cannot compete well.
European or common buckthorn (Rhamnus cathartica L.) is a shrub/small tree native to most of Europe and Western Asia that appears restricted to open and edge habitats in its native range but is able to invade forest understories and dominate native ecosystems which were introduced in northern North America (Knight et al. 2007; Kurylo and Endress 2012). The shrub forms dense thickets that correspond with noticeable declines in native plant abundance and diversity (Archibold et al. 1997; Boudreau and Wilson 1992; Mascaro and Schnitzer 2007). The cause and effect between R. cathartica and native plant communities remains unclear, however, as the shrub appears as an opportunist, which employs multiple potential competitive mechanisms. Rhamnus cathartica tolerates a poor habitat (e.g., dry, shady), and it germinates, grows and reproduces vigorously in disturbed habitats where sunlight is plentiful (Gavier-Pizarro et al. 2010; Knight et al. 2007; Moffatt and McLachlan 2004). Still, R. cathartica invasion success may not be just opportunistic. The shrub leafs out earlier and thus is able to photosynthesize longer than co-occurring native species, allowing invasion into canopy understory, and it produces copious seed-bearing fruits that are dispersed by birds and mammals (Godwin 1936; Harrington et al. 1989).
Rhamnus cathartica also produces secondary compounds that alter soil chemistry, deter native herbivores, and appear to suppress native flora and fauna (Grunzweig et al. 2015; Heneghan et al. 2006; Klionsky et al. 2011; Schuh and Larsen 2015; Seltzner and Eddy 2003; Trial Jr. and Diamond 1979). In particular, phytotoxic compounds leached from the bark, leaves, stems and fruit—including emodin and other anthraquinones, tannins, and flavonoids (see Izhaki 2002 and references therein)—may suppress the germination, survival and growth of other plants (Archibold et al. 1997; Klionsky et al. 2011; Seltzner and Eddy 2003), though these effects require substantiation (Knight et al. 2007). Rhamnus cathartica leaves and fruit demonstrably reduced germination in some native and ornamental species, but R. cathartica bark and roots did not, and the effects appeared species specific (Klionsky et al. 2011; Knight et al. 2007). Hence, R. cathartica allelopathic effects may depend on its tissue type, and the effectiveness may depend on the identity and life stage of the target species.
Our objective was to examine the allelopathic impact of an invasive non-native shrub/tree on plant species using field observation and experimental allelopathy studies. Given the known allelochemical content, and predicted allelopathic effects, of R. cathartica, we predicted that increased soil amendments with R. cathartica leaf and root tissues would inhibit plant germination and growth in five woody and herbaceous plant species.
Methods
Study site
The Tifft Nature Preserve is a 107-ha urban natural area located on the shore of Lake Erie in Buffalo, NY (U.S.). The landscape was used for industrial and residential refuse, and turned into a natural area in the 1970s. The preserve contains deciduous forest (dominated by Populus deltoides W. Bartram ex Marshall), wetlands, and open fields.
Forest survey data collection
Herbaceous and woody plants were surveyed in the Tifft woodlands and grasslands in August 2013. Three random transects were established in each of these two habitats (n = 6 transects); all were 500 m long except one woodland transect which was 250 m long due to forest shape. Plots were established at 50-m intervals (n = 55 plots). Areas with active management and edges were avoided by moving plots 50 m. Total herbaceous percent cover and species richness, as well as woody seedlings (<1 cm dbh), were measured in 1 m2 subplots (n = 55). For woody species, each plot was sampled using the point-center quarter technique (n = 51, Cottam and Curtis 1956) at the same 50-m intervals along the transects. Point-center quarter is a long-used forest sampling protocol in which a random point is selected, a compass used to divide the area into quadrants and the nearest tree (>11.7 cm dbh) and shrub (<11.7 cm dbh) located, and identified, and dbh (stem diameter at 1.4 m height) measured in each quadrant.
Forest survey data analysis
We examined 1-m2-subplot-level variation among herbaceous cover (%) and herbaceous species richness, and point-quarter-plot-level tree richness, shrub richness, woody seedling density (stems · m−2), R. cathartica basal area (stem basal area * stem density), tree basal area, shrub basal area, and R. cathartica basal area using principal component analysis (PCA) using the “prcomp” method and “scale” option (standardizes all variables to unit length) in the “R” statistical package (R Development Core Team 2016).
Mesocosm experimental design
Rhamnus cathartica leaves and roots were haphazardly collected at Tifft in September 2015 by removing whole plants <2.5 cm DBH, which were stored at 15 °C before being prepared for the mesocosm experiments. In January 2016, the R. cathartica plant material was placed in a drying oven for 7 days at 15 °C. The plant material was macerated and mixed with soil in one of four treatments: 0.1 g leaf, 1 g leaf, 10 g leaf, and 5 g root. Fifty mesocosms (12.5 cm clay pots) were filled with 250 g of sterile potting soil and planted with 10 seeds of each of 5 plant species (Pinus strobus L, P. deltoides, Microstegium vimineum (Trin.) A. Camus, Liatris cylindracea Michx., Symphyotrichum oolentangiense (Riddell) G.L. Nesom). These are a combination of herbaceous (M. vimineum, L. cylindracea, S. oolentangiense) and woody (P. strobus, P. deltoides) plants, including a plant from Asia (M. vimineum) that is invasive in North America and generally occurs in open to partly shady conditions (and hence could compete with R. cathartica in disturbed habitat). Pinus strobus and P. deltoides seeds were purchased from Sheffield’s Seed Company (Locke, NY USA); L. cylindracea and S. oolentangiense seeds were purchased from Prairie Moon Nursery (Winona, MN USA). Microstegium vimineum seeds were collected from wild populations in Otto, NC (USA) in 2013 and stored in a dry cabinet. The P. strobus, S. oolentangiense, and L. cylindracea seeds were cold stratified at 4.5 °C for 60 days; the other seeds were not cold stratified.
Ten mesocosms were designated for each of the four treatments (and a control) [n = 50 total]. The mesocosms were placed under full spectrum growth lights and warmed to 21.5 °C using seed heating pads to encourage germination. The location of each mesocosm was randomized and shifted under different grow lights once per week. After 12 weeks, all plants were harvested, above- and belowground matter separated, and dried for 3 days at 15 °C before weighing. The species that germinated survived until the end of the experiment.
Mesocosm data analysis
We evaluated the R. cathartica treatment effects (0.1 g leaf, 1 g leaf, 10 g leaf, 5 g root) on mesocosm species richness, recruitment (seedling abundance), biomass (biomass · total seedlings−1), and root:shoot ratio using analysis of variance (ANOVA) models in the R statistical program (R Development Core Team 2016). We used Tukey’s HSD for post hoc evaluation of the differences in terms. In the root:shoot model, we included belowground biomass as the dependent variable and aboveground biomass as a predictor along with the treatment levels. We included an aboveground biomass × treatment interaction term to analyze how the ratio may vary by treatment level.
We also examined species-specific recruitment success (germinated · planted−1) by R. cathartica treatment (and species × treatment interaction) using ANOVA.
Results
Field surveys
Herbaceous cover at Tifft was 87.3 ± 4% m−2 (mean ± SE) and herbaceous richness 2.5 ± 0.2% m−2. The most common understory species were non-native plants (R. cathartica, Polygonum cuspidatum Siebold & Zucc., and Phragmites spp.) and some native Urtica dioica L. and Solidago spp. Woody seedling abundance was 0.6 ± 0.2 stems m−2, and R. cathartica seedling abundance was 1.7 ± 0.4 stems m−2. Rhamnus cathartica generally occurred as a large shrub/small tree (9.4 ± 0.5 dbh) at Tifft and dominated the understory (relative density; 257 stems · ha−1). Co-occurring shrubs included Cornus racemosa Lam. and Rhus typhina L., but they were a fraction (combined = 33 stems · ha−1) of the R. cathartica-dominated layer. Populus deltoides trees dominated the canopy layer (175 stems · ha−1) and were much larger (92.1 ± 4.5 cm dbh) than R. cathartica. Rhamnus cathartica was more widespread in the forested (459 stems · ha−1) than grassy (56 stems · ha−1) habitats.
The field survey PCA indicated that most variation (34%) occurred along the PC1 axis where herbaceous cover negatively covaried with tree basal area and woody and R. cathartica seedling abundances (Fig. 1). We used the minimum contribution if all variable loadings contributed equally (38% here) to determine the most important loadings for each principle component. These loadings indicated that the most important PC1 components were percent herbaceous cover (−0.472) which negatively covaried with R cathartica seedling (0.416) and other woody seedling density (0.448). On PC2, however, the most important components were R. cathartica basal area (−0.525) and seedling density (−0.393), which negatively covaried with shrub basal area (0.443) and woody seedling density (0.418).
Principal component analysis of plot-level variation in herbaceous coverage (herb cover) and species richness (herb richness), native woody basal area (shrub and tree area) and species richness (shrub and tree richness), native shrub and tree seedlings (woody seedlings) and Rhamnus cathartica basal area (RHCA area) and seedlings (RHCA seedlings). Arrows pointing in the same direction indicate positive covariation and those pointing in opposite directions indicate negative covariation. The component loadings indicated that the most important PC1 components (horizontal axis) were percent herbaceous cover, which negatively covaried with R cathartica and other woody seedling density. On PC2 (vertical axis), the most important components were R. cathartica basal area and seedling density, which negatively covaried with shrub basal area and woody seedling density
Mesocosm experiment
Overall, mesocosm recruitment was 11.6%. Pinus strobus had the highest recruitment rate with 22.6% of the seeds germinating and surviving. Liatris cylindracea also had a high recruitment rate at 20.0% followed by P. deltoides at 11.8%. Recruitment was low for S. oolentangiense (3.6%) and very low for M. vimineum (0.4%).
Mesocosm species richness differed significantly with R. cathartica treatments with a clear decline in response to increased dosage (Table 1a; Fig. 2a). The declines in species richness between the lowest leaf amendment (0.1 g) and control, and between the highest leaf amendment (10 g) and root amendments, did not differ, but the decline across all amendments with increased R. cathartica formed a clear negative trend. Species abundance also differed significantly with R. cathartica treatments, but the decline in response to dosage was not as consistent as species richness (Table 1b; Fig. 2b). Species abundance was significantly less in all of the R. cathartica treatments than the control; however, the two lowest amendments (0.1 and 1 g) did not differ, nor did the highest dosage (10 g) and root amendment.
Biomass seedling−1 did not significantly differ among treatments (Table 1c; Fig. 2c). A significant aboveground biomass × treatment interaction term indicated that the root:shoot ratio (belowground:aboveground biomass) differed by treatment (Table 1d; Fig. 3). The control and low dosage R. cathartica treatment ratios were quite similar with a relatively constant root:shoot ratio indicating that biomass was allocated relatively equally as the plants grew. However, at higher R. cathartica doses, the plants allocated considerably more biomass to shoots than roots.
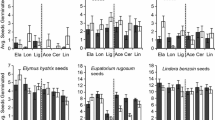
A significant species × treatment interaction term indicated species-specific treatment responses (Table 1e; Fig. 4). Microstegium vimineum had particularly low germination in this study, and it only germinated in the control and lowest R. cathartica dosage mesocosms. Populus deltoides, L. cylindracea, and S. oolentangiense recruitment all decreased with increased R. carthartica dosages (with root being the ‘highest’ dosage); Populus deltoides appeared the most impacted as it only germinated in the control and lowest R. cathartica dosage mesocosms. Pinus strobus was the anomaly as it seemed only slightly negatively impacted by R. cathartica leaf additions, unaffected by increased leaf dosage, and it actually improved germination with root addition.
Species × treatment interaction plot for plant seedling recruitment [Pinus strobus (PIST), Populus deltoides (PODE), Microstegium vimineum (MIVI), Liatris cylindracea (LICY) and Symphyotrichum oolentangiense (SYOO)], and Rhamnus cathartica treatments (control [0], 0.1 g leaf [0.1], 1 g leaf [1], 10 g leaf [10] and 5 g root [Rt])
Discussion
Rhamnus cathartica leaf and root tissue alone appeared sufficient to inhibit potential plant competitors and shift local plant community succession—suggesting an allelochemical mechanism. We found a negative relationship between R. cathartica and herbaceous- and shrub-layer plants in a highly invaded landscape that could indicate that R. cathartica out-competed the other plants or took advantage of habitat disturbance and/or co-occurring non-native facilitators. Mesocosm experiments were far less ambiguous: increased doses of R. cathartica soil amendments resulted in significantly decreased plant community species richness and abundance, and a shift in biomass allocation from roots and shoots. Moreover, the R. cathartica impacts appeared species specific as the study plants differed in their sensitivities to R. cathartica soil amendments.
Our field surveys showing R. cathartica’s dominance beneath the canopy trees are consistent with several other studies in northeastern North America that report the plant in dense monocultures devoid of similarly sized woody plants or an appreciable herbaceous layer (Archibold et al. 1997; Boudreau and Wilson 1992; Mascaro and Schnitzer 2007). Rhamnus cathartica occurs in both sunny and shady habitats, appearing to favor sunny areas (Gavier-Pizarro et al. 2010; Moffatt and McLachlan 2004), but we found it far more extensively beneath canopy trees than in open grassy habitats. Given that the canopy trees at Tifft are mostly P. deltoides, a fast-growing tree associated with habitat disturbance, and R. cathartica also is associated with disturbance, it is likely that both were established when the Tifft landscape reverted from its industrial use in the 1970s. Once established, R. cathartica has many traits that could help it compete against other plants, such as low rates of herbivore attack, a thick, shading canopy, fast growth and reproduction, and an extended phenology allowing relatively greater carbon gain (Knight et al. 2007). We show that allelopathy also may be a key component of its competitive ability.
We targeted R. cathartica allelopathy as one explanation for the plant’s community level effects. We used five wild plant species (four native and one non-native, two woody and three perennials) to examine the potential community-level effects in the mesocosms, and we found that increased R. cathartica leaf soil amendments, with a root amendment showing the most impact, reduced plant richness, and abundance. Interestingly, biomass · seedling−1 did not change with R. cathartica amendments for those plants that germinated, but biomass allocation did. In no- or low-dose R. cathartica treatments, plants allocated about the same biomass to above- and below-ground tissues regardless of size. In higher-dose R. cathartica treatments, biomass allocation was disproportionally shifted aboveground. The biomass shift could be a response to allelopathic chemicals in the soil, but also might have been prompted by the high level of nitrogen typically found in R. cathartica leaves (Harrington et al. 1989). That said, the biomass allocation response was similar between the highest leaf amendment and the root amendment, which would likely introduce different levels of nitrogen from R. cathartica, and increased nitrogen prompts biomass allocation aboveground (Poorter et al. 2012) rather than belowground as we observed. In greenhouse and field studies, Klionsky et al. (2011) recorded reduced growth and germination in native perennials in greenhouse soils amended with R. cathartica leaves and field soils once inhabited by R. cathartica. Seltzner and Eddy (2003) found the greatest inhibitory effect of R. cathartica on crop plants from the drupe exudates with less effect by leaves and no effect from roots. We found that root amendments generally caused the largest inhibitory effects, even with half the biomass (5 g) of the largest leaf amendment (10 g).
Given that most plants die during recruitment (germination and emergence), plant community composition is highly dependent on recruitment (Blaney and Kotanen 2001; Fenner and Kitajima 1999; Moles and Westoby 2004; Warren II and Bradford 2011), and allelopathy appears most inhibitory against competitor seedlings (Izhaki 2002; Orr et al. 2005; Pisula and Meiners 2010). We found species-specific R. cathartica impacts, as have several other researchers in multi-plant allelopathy studies (Cipollini and Bohrer 2016; Izhaki 2002; Klionsky et al. 2011; Small et al. 2010). Our lone non-native species, M. vimineum (an invasive annual) did not germinate well, possibly due to an unknown issue with long-term seed storage. When it did germinate, it only did so in the control and lowest-level R. cathartica treatments. Populus deltoides (a fast-growing canopy tree) germinated much better than M. vimineum, but it also only germinated in the control and lowest-dose treatments. Liatris cylindracea and S. oolentangiense (perennial herbs) recruitment generally decreased with increased leaf amendments. Pinus strobus (a gymnosperm canopy tree) appeared little impacted by R. cathartica leaf treatments and, unlike the other species, none of which germinated in the root treatment, it appeared to thrive with macerated R. cathartica root material. Interestingly, P. strobus seeds are much larger (~15–17 g) than the other plant species used here (~0.5–3 g), which may confer some sort of enhanced protection or resistance to the R. cathartica effects.
These results suggest that multi-species and multi-type plant studies are crucial in -determining community-level allelopathic impacts. Our mesocosm experiment suggested allelopathic effects from macerated R. cathartica tissues, but these results must be considered potential effects as our mode of delivery was a rapid-pulse soil amendment, which may be realistic (Heneghan et al. 2002; Schuh and Larsen 2015), but this mechanism needs to be tested in an ecologically realistic setting. Moreover, the community-level impacts may be direct allelopathy mechanisms or the indirect effects of R. cathartica resistance gave specific species, such as P. strobus, competitive advantages against the other species.
The putative key allelochemical in the genus Rhamnus is emodin, which has been found in all parts of the plant except roots, though it has been found in the rhizomes and roots of other plants (Izhaki 2002). Emodin, and potentially other secondary metabolites in R. cathartica, may be Eurasian allelochemicals against which North American organisms have not co-evolved defenses (Izhaki 2002). As such, invasive non-native plant species may bring novel weapons against which native species have little or no resistance, including novel allelochemicals (Callaway et al. 2008; Callaway and Ridenour 2004; Thorpe et al. 2009). Indeed, Thorpe et al. (2009) demonstrated that Centaurea maculosa (a Eurasian forb) is allelopathic in its invaded but not home range. Rhamnus cathartica’s novel allelochemicals also may provide indirect competitive advantage by deterring local phytophagous herbivores (Izhaki 2002; Ridenour and Callaway 2011). For example, Lepidopterans at the Tifft Nature Preserve feed extensively on native woody species but avoid R. cathartica (Grunzweig et al. 2015). Moreover, white-tailed deer avoid browsing R. cathartica at Tifft and as a result pose a visible burden on native woody seedling and saplings (pers. obs.).
The impacts of non-native invasive species such as R. cathartica likely are scale-dependent (Knight and Reich 2005; Powell et al. 2013). At larger scales, R. cathartica establishment seems driven by long-distance seed dispersal by birds and facilitated by habitat disturbance. Whereas R. cathartica can invade forest interiors, it appears to establish most successfully at forest edges or with canopy disturbance (Harrington et al. 1989; Knight et al. 2007), particularly in urban forests (Schneider and Miller 2014; Zipperer 2002). Indeed, intact, native forests appear to resist R. cathartica invasion (McCay and McCay 2009; Whitfield et al. 2014). However, once established, R. cathartica allelochemicals may protect seedlings from herbivores and augment herbaceous-layer competition against other plants. Eventually, these advantages may promote dense local populations. Hence, R. cathartica ‘s allelochemical abilities may not help it colonize new habitats as much as help the plant hold its ground after invasion.
References
Archibold OW, Brooks D, Delanoy L (1997) An investigation of the invasive shrub European buckthorn, Rhamnus cathartica L., near Saskatoon, Saskatchewan. Can Field Nat 111:617–621
Blaney CS, Kotanen PM (2001) Effects of fungal pathogens on seeds of native and exotic plants: a test using congeneric pairs. J Appl Ecol 38:1104–1113
Boudreau D, Wilson G (1992) Buckthorn research and control at Pipestone National Monument (Minnesota). Restor Manag Notes 10:94–95
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Callaway RM, Cipollini D, Barto K et al (2008) Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 89:1043–1055
Cipollini K, Bohrer MG (2016) Comparison of allelopathic effects of five invasive species on two native species. J Torrey Bot Soc 143:427–436
Cottam G, Curtis JT (1956) The use of distance measure in phytosociological sampling. Ecology 37:451–460
Fenner M, Kitajima K (1999) Seed and seedling ecology. In: Pugnaire FI, Valladares F (eds) Handbook of functional plant ecology. Marcel-Dekker, New York, pp 589–611
Fernandez C, Monnier Y, Santonja M et al (2016) The impact of competition and allelopathy on the trade-off between plant defense and growth in two contrasting tree species. Front Plant Sci 7:594
Gavier-Pizarro GI, Radeloff VC, Stewart SI et al (2010) Rural housing is related to plant invasions in forests of southern Wisconsin, USA. Landsc Ecol 25:1505–1518
Godwin H (1936) Studies in the ecology of Wicken Fen III. The establishment and development of fed scrub (Carr). J Ecol 24:82–116
Grunzweig L, Spiering D, Labatore A et al (2015) Non-native plant invader renders suitable habitat unsuitable. Arthropod Plant Interact 9:577–583
Harrington RA, Brown BJ, Reich PB et al (1989) Ecophysiology of exotic and native shrubs in southern Wisconsin I. Oecologia 80:368–373
Heneghan L, Clay C, Brundage C (2002) Rapid decomposition of Buckthorn litter may change soil nutrient levels. Ecol Restor 20:108–111
Heneghan L, Fatemi F, Umek L et al (2006) The invasive shrub European buckthorn (Rhamnus cathartica L.) alters soil properties in Midwestern US woodlands. Appl Soil Ecol 32:142–148
Izhaki I (2002) Emodin—a secondary metabolite with multiple ecological functions in higher plants. New Phytol 155:205–217
Klionsky SM, Amatangelo KL, Waller DM (2011) Above- and belowground impacts of European buckthorn (Rhamnus cathartica) on bour native forbs. Restor Ecol 19:728–737
Knight KS, Reich PB (2005) Opposite relationships between invasibility and native species richness at patch versus landscape scales. Oikos 109:81–88
Knight KS, Kurylo JS, Endress AG et al (2007) Ecology and ecosystem impacts of common buckthorn (Rhamnus cathartica): a review. Biol Invasions 9:925–937
Kurylo J, Endress AG (2012) Rhamnus cathartica: notes on its Early History in North America. Northeast Nat 19:601–610
Lankau RA (2011) Interpopulation variation in allelopathic traits informs restoration of invaded landscapes. Evol Appl 5:270–282
Mascaro J, Schnitzer SA (2007) Rhamnus cathartica L. (common buckthorn) as an ecosystem dominant in southern Wisconsin forests. Northeast Nat 14:387–402
McCay TS, McCay DH (2009) Processes regulating the invasion of European buckthorn (Rhamnus cathartica) in three habitats of the northeastern United States. Biol Invasions 11:1835–1844
Moffatt SF, McLachlan SM (2004) Understorey indicators of disturbance for riparian forests along an urban-rural gradient in Manitoba. Ecol Ind 4:1–16
Moles AT, Westoby M (2004) What do seedlings die from and what are the implications for evolution of seed size? Oikos 106:193–199
Orr SP, Rudgers JA, Clay K (2005) Invasive plants can inhibit native tree seedlings: testing potential allelopathic mechanisms. Plant Ecol 181:153–165
Pisula NL, Meiners SJ (2010) Relative allelopathic potential of invasive plant species in a young disturbed woodland. J Torrey Bot Soc 137:81–87
Poorter H, Niklas KJ, Reich PB et al (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50
Powell KI, Chase JM, Knight TM (2013) Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science 339:316–318
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rice EL (1984) Allelopathy. Academic Press, Orlando
Ridenour WM, Callaway RM (2011) The relative importance of allelopathy in interference: the effects of an invasive weed on native bunchgrass. Oecologia 126:444–450
Schneider SC, Miller JR (2014) Response of avian communities to invasive vegetation in urban forest fragments. Condor 116:459–471
Schuh M, Larsen KJ (2015) Rhamnus cathartica (Rosales: Rhamnaceae) invasion reduces ground-dwelling insect abundance and diversity in Northeast Iowa forests. Environ Entomol 44:647–657
Seltzner S, Eddy TL (2003) Allelopathy in Rhamnus cathartica, European buckthorn. Mich Bot 42:51–61
Small CJ, White DC, Hargbol B (2010) Allelopathic influences of the invasive Ailanthus altissima on a native and a non-native herb. J Torrey Bot Soc 137:366–372
Thorpe AS, Thelen GC, Diaconu A et al (2009) Root exudate is allelopathic in invaded community but not in native community: field evidence for the novel weapons hypothesis. J Ecol 97:641–645
Trial H Jr, Diamond JB (1979) Emodin in buckthorn: a feeding deterrent to phytophagous insects. Can Entomol 111:207–212
Warren RJ II, Bradford MA (2011) The shape of things to come: woodland herb niche contraction begins during recruitment in mesic forest microhabitat. Proc R Soc B 278:1390–1398
Whitfield TJS, Lodge AG, Roth AM et al (2014) Community phylogenetic diversity and abiotic site characteristics influence abundance of the invasive plant Rhamnus cathartica L. J Plant Ecol 7:202–209
Zipperer WC (2002) Species composition and structure of regenerated and remnant forest patches within an urban landscape. Urban Ecosyst 6:271–290
Acknowledgements
The views expressed in this article do not necessarily represent the views of USACE or the United States. We thank Elise Labatore for field and lab assistance. We also thank two anonymous reviewers and Luke Flory for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Luke Flory.
Rights and permissions
About this article
Cite this article
Warren, R.J., Labatore, A. & Candeias, M. Allelopathic invasive tree (Rhamnus cathartica) alters native plant communities. Plant Ecol 218, 1233–1241 (2017). https://doi.org/10.1007/s11258-017-0766-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-017-0766-2