Abstract
Plant-soil feedback responses for native and invasive plant species are well documented, but little is known about how feedback effects from the soil biota community affect plant interactions with herbivores. Here we examine whether changes of the soil biota community by the successful invader Solidago canadensis influence growth and herbivore susceptibility of two coexisting native plant species (Tanacetum vulgare, Melilotus albus). Root zone soil from two different habitat types (‘urban’ and ‘suburban’) was collected and used as inocula in a plant-soil feedback study. Each plant species was grown either in its own soil biota community or with the community with a history from the competitive invasive or native plant species. To identify potential drivers of responses to the different soil biota communities, we analyzed root colonization by arbuscular mycorrhizal fungi and dark-septate endophytes (DSE), and the community composition of soil inhabiting nematodes at the end of our experiment. Results show that S. canadensis and M. albus were not affected by soil history. In contrast, T. vulgare showed increased plant growth in ‘foreign’ soil derived from S. canadensis root zone compared with its ‘home’ soil suggesting a growth promotion by the soil biota community of S. canadensis. From the examined drivers, the abundance of DSE explained the growth response of T. vulgare to the S. canadensis soil biota community best. However, shoot herbivory by banded snails (Cepaea nemoralis, C. hortensis) was not affected by soil history, but by the habitat type where the soil inocula originated. Our study shows that a native plant species may profit from the presence of an invasive competitor mediated by changes in the soil biota community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The consequences of species invasions are of various kinds, but they are generally recognized as a major environmental problem which can change ecosystem functioning and influence biodiversity on local and global scales (Vitousek et al. 1996; Vilà et al. 2007). Plenty of theories are proposed to better understand the mechanisms of the successful establishment and spread of exotic plant species in native communities (Catford et al. 2009). More recent work suggests that interactions between plants and soil biota may play an important role in the invasion process (Klironomos 2002; Wolfe and Klironomos 2005; Wurst et al. 2011).
The concept of plant-soil feedback has become widely recognized and was also incorporated in the research on plant species invasions (Klironomos 2002; Callaway et al. 2004; Van Grunsven 2007). The basic theoretical background of plant-soil feedback experiments is that plants first influence their soil environment by affecting the composition of the soil biota community and/or soil nutritional properties, which is called soil conditioning (Brinkman et al. 2010). Then, effects of the previous conditioning are measured by assessing the soil effects on subsequent plant growth. Most studies indicate that native plant species show a negative soil feedback (i.e. they perform better in soils that were not conditioned by themselves), whereas exotics exhibit a positive feedback (i.e. they perform better in soils that were conditioned by themselves) (Klironomos 2002) or a less negative feedback than natives (Van Grunsven 2007; Engelkes et al. 2008; Morriën et al. 2011). But there are also studies which found no difference in the effect strength of plant-soil feedback between native and exotic species (Dostál and Palečková 2011), and studies which documented negative soil feedbacks for successful invaders (Nijjer et al. 2007). By considering the numerous examples of impacts of exotic plant species on soil biota communities in their new ranges (Belnap and Phillips 2001; Kourtev et al. 2002; Callaway et al. 2004), the idea of linking the plant-soil feedback concept with the expansion of exotic plants becomes obvious.
The majority of studies on exotic plant invasions and interacting organisms have been conducted in a strictly bi-trophic framework involving plants and their respective antagonists or symbionts (Harvey et al. 2010). Experimental studies with a multitrophic perspective involving both, the above- and the belowground compartment, are lacking in this field, although a growing body of research highlights the importance of interactions between above- and belowground biota for plant performance (van der Putten et al. 2001; Wardle et al. 2004; Soler et al. 2005; van Dam et al. 2005; Wurst and Rillig 2011). As far as we are aware, only one study (Morriën et al. 2011) has looked at consequences of plant-soil feedback for a higher trophic level, i.e. the interaction of native and range-expanding plant species with aboveground insect herbivores. The authors did not find a correlation between the strength of aboveground herbivory and the feedback from the soil. This does not mean that plant-soil feedback effects and aboveground herbivory may not affect each other. A recent study by Bezemer et al. (2013) showed that herbivore presence during the soil conditioning phase has an influence on the soil feedback effect in the subsequent phase.
The present study links research on plant species invasions with the plant-soil feedback approach by integrating both below- and aboveground biotic interactions. We investigated whether plant-soil feedback effects facilitate the invasion of an exotic plant by using one of the most dominant exotic species in disturbed urban areas—Solidago canadensis—and two of its native competitors as target plant species. We used a natural experimental approach by collecting field soil samples of known plant species and using them as inocula for the feedback phase. A strength of this approach is that it uses soils conditioned by plants for long periods of time under natural field conditions (Kulmatiski and Kardol 2008). Some studies have shown that interactions between plants and soil microorganisms depend on soil conditions (Marrs et al. 1991; Marschner et al. 2004; Bezemer et al. 2006). Even within a single soil type, the biomass and activity of the soil microbial community generally depends on soil fertility conditions (Bardgett et al. 1999). In order to assess whether the plant-soil feedback effects are persistent over the range of different habitats in which S. canadensis is able to become abundant, we took the soil samples at sites of two different habitat types (‘urban’ and ‘suburban’). Besides assessing the effects of soil biota history on plant biomass production (i.e. the classical plant-soil feedback approach), we investigated the effects of soil history on plant susceptibility to leaf herbivory by snails, the only herbivores we regularly found feeding on both S. canadensis and the two native target plant species at the study sites. As far as we are aware, only two studies have investigated plant-mediated effects of soil biota on snails (Thompson et al. 1993; Wurst and Rillig 2011), although molluscs have been reported to be the second most important herbivores after rodents in grasslands (Hulme 1996).
To elucidate which component of the soil biota community could have affected plant growth and leaf herbivore attack we focussed on arbuscular mycorrhizal fungi (AMF) and dark-septate endopyhtes (DSE) as two wide-spread root associated groups of fungi, and on soil nematodes as potential drivers of the feedback effects. AMF may affect interactions with aboveground herbivores, but investigations have shown that there is a large amount of specificity and context dependency in the outcome of these interactions, reflecting the influence of fungus and host plant, their genotypes, the insect species used, and environmental factors (Hartley and Gange 2009). Not much is known about the ecological role of DSE and to which extent they interact with plants and other plant colonizing fungi like AMF and fine endophytes (Jumpponen 2001; Postma et al. 2007). They may act as mutualists with positive effects on plant performance (Newsham 2011). But because of the high variability of their effects on plants, which may also range from neutral to negative, it is assumed by some authors that they stretch a continuum from mutualism to antagonism (Jumpponen and Trappe 1998; Jumpponen 2001; Tellenbach et al. 2011). Impacts of root-feeding nematodes on aboveground insect herbivores are varying as well, depending for example on the insect feeding guild (Wurst and van der Putten 2007; Kaplan et al. 2009).
With a full factorial greenhouse study we aimed to answer thus three major questions: (1) whether plant-soil feedback effects facilitate the invasion of an exotic plant in its new range and whether these effects are persistent in two different invaded habitat types, (2) whether feedback effects may cascade up to higher trophic levels (i.e. shoot herbivores) and (3) if any of the observed soil organisms (AMF, DSE and nematodes) may have contributed to the feedback effects.
Methods
We applied a method that differed from the known approaches of plant-soil feedback experiments conducted in other studies (Klironomos 2002; Callaway et al. 2004; Van Grunsven 2007; see Brinkman et al. 2010 for a comparison). One approach (e.g. Klironomos 2002) consists of a conditioning phase in the greenhouse with the experimental plant species growing twice in succession in pots and a subsequent feedback phase with every plant species growing either in ‘home’ treatment (plants growing in soil with their own respective soil history) or ‘foreign’ treatment (soil with history of other plant species). Other researchers obtained the soil directly from the rhizosphere of the target plant species in the field (Callaway et al. 2004; Van Grunsven 2007), sterilized half of the soil volume and used the sterile and the unsterile half as inocula for sterile background soil to compare plant growth with sterile and unsterile inoculation. The approach applied in our study is as follows: soil sampling from the root zone of the experimental plant species took place in the field; and in a subsequent feedback phase in the greenhouse we grew every plant species in sterilized background soil inoculated with field soil with a history of its own (‘home’) or of other plant species (‘foreign’). By obtaining soil samples from the root zones of the plant species in the field and using them for inoculation, we assume that the conditioning phase has already taken place in the field, thus a conventional conditioning phase in the greenhouse is unnecessary in our study.
Plant species
We tested the effects of plant-soil feedback on plant growth and leaf herbivory by snails on three plant species, specifically S. canadensis L. (Asteraceae), Tanacetum vulgare L. (Asteraceae), and Melilotus albus Medik. (Fabaceae). The Canada goldenrod S. canadensis is a successful worldwide invader of North American origin where it is a characteristic species of tall-grass prairies, abandoned farmland, infrequently grazed pastures, and waste land (Werner et al. 1980). Introduced in the eighteenth century in Europe, it began to spread in the nineteenth century in Central Europe where it may become a highly abundant species in a variety of habitats like abandoned fields and disturbed habitats in urban areas (Kowarik 2003). Here it typically co-exists with dense stands of T. vulgare (tansy) and the more dispersed occurring M. albus (white sweet-clover). Both plant species are suggested to be native to Eurasia (Turkington et al. 1978).
Soil sampling and site description
In July 2010, soil samples were collected for each species at six sites across Berlin, Germany. According to the site surroundings, adjacent vegetation and former land-use, the sites were classified as either ‘urban’ or ‘suburban’ sites. The three ‘urban’ sites can be characterized as highly disturbed habitats with a huge impact of human activity. The sites were used mainly as storage areas for building material during the construction of nearby buildings or railroad stations and have been abandoned at least 5 years. The three ‘suburban’ sites were former agricultural fields located in the middle of a forest (Grunewald) on the outskirts of Berlin and thus remained free from a tree layer unlike the surrounding forest vegetation. The three ‘suburban’ sites have been abandoned at least 10 years. It was relevant that sites of one habitat type did not differ considerably among each other in terms of environmental variables which may influence the composition of the soil biota community. Therefore we took plant species composition as a proxy for site environmental characteristics and, eventually, selected three sites per area type that did not differ evidently in plant species composition and used them as replicate sites for the habitat type. Only sites where all three target plant species occurred with a population size of at least ten patches of clonal colonies (in terms of S. canadensis and T. vulgare) or ten individuals (in terms of M. albus) were taken into account.
At each site we sampled soil from the root zone of more than six different plant individuals or six colonies of each focal plant species. Samples were taken with a shovel to a depth of 30 cm while trying to ensure that the sampling locations were only impacted by the target species. To avoid interference caused by the two other target species we only took soil samples from locations with at least 5 m distance to the two other target species. The soil was then transferred to the lab, thoroughly homogenized within sites and species, and then sieved through a 4 mm mesh to remove stones, coarse roots, and other particles. For abiotic soil characterization pH was determined on a subsample of each soil sample with a pH meter. Another subsample was ground (Mill MM 2, Retsch, Germany) and the N and C content were determined with a CN analyzer (Euro EA, HEKAtech GmbH, Germany). An ANOVA detected no differences in pH between root zone samples of the three plant species or the two habitat types (P > 0.05). The C:N ratio did not differ between the root zone samples of the different plant species, but it differed significantly between the two habitat types (P < 0.05), i.e. the soil inocula from the ‘suburban’ sites had a lower C:N ratio (on average 14.50 ± 0.62 SE) than the soil inocula from the ‘urban’ sites (on average 24.85 ± 3.17 SE).
Experimental set-up
To keep abiotic soil conditions homogenous we used steamed soil as background (steamed at 90 °C for at least 3 h) and inoculated it with the collected root zone soil samples. As background soil we used a sandy loam soil (pH 7.1, C:N ratio 15.58) that was collected from an old field located in Berlin-Dahlem. Experimental 2 L plastic pots were filled with 1,840 ± 0.5 g of this mixture (1,405 g background soil + 435 g inoculum). The experimental plants were grown from seeds (Appels Wilde Samen GmbH, Hesse, Germany) germinated on sterilized soil.
In August 2010 seedlings of the three target plant species were transplanted into the pots with the following treatment configuration. Native plant species T. vulgare and M. albus were growing in ‘home’ (sterile background soil with soil inocula from T. vulgare or M. albus, respectively) and ‘foreign’ soil, where the sterile background soil was inoculated with root zone soil of S. canadensis, the invasive plant species. S. canadensis itself grew respectively in ‘home’ soil (sterile background soil inoculated with root zone soil of S. canadensis) or ‘foreign’ soil where the sterile background soil was inoculated with a 1:1 mixture from root zone soils of both native plant species. By using that mixture, we attained that S. canadensis had just one ‘foreign’ soil treatment (instead of two), because we were interested in the difference between ‘home’ soil versus native conditioned ‘foreign’ soil and not in pair-wise species comparisons.
Soil of each of the 6 sites was inoculated to 10 individuals per plant species that were grown individually in pots. Five out of these ten individuals were grown in ‘home’ treatment and five in ‘foreign’ treatment, respectively, resulting in 60 pots per plant species and 180 pots in total. During the whole experiment the pots were kept in a greenhouse with 16 h light a day (~150 μE m−2 s−1 PAR) and 22/18 °C day/night temperature. Their position was completely randomized twice a week.
Leaf herbivores
To examine how plant-soil feedback may cascade up to higher trophic levels we used grove snail Cepaea nemoralis (Linnaeus 1758) and white-lipped snail Cepaea hortensis (O. F. Müller, 1774) as leaf herbivores. Individuals from both land snail species were collected in September 2010 on one of the ‘urban’ sites (Berlin-Südkreuz). Both species are native to Europe and among the most dominant generalist herbaceous snail species in ruderal plant communities in Berlin (personal observation). Both species were selected after herbivore surveys on our three target plant species. It turned out, that C. nemoralis and C. hortensis were the only observed herbivores on shoots of S. canadensis at the field sites. After collection, snail individuals were kept in plastic boxes at room temperature for 4 weeks and fed with lettuce and cucumber. Nine weeks after seedling transplanting the shoots of the experimental plants were covered with perforated plastic bags and two snail individuals (one from each species) were added to the plants according to the following pattern: Within each plant species and each habitat type 12 pots (out of 30) received snail addition. Six of these treated 12 plant individuals grew in ‘home’ soil and the other 6 in ‘foreign’ soil, respectively. Before the transfer, snails were starved for 3 days and weighed afterwards with a precision balance. During snail application plants were sprayed with water every second day to increase humidity inside the plastic bags. This was done to ensure that snails did not fall in an inactive state in the greenhouse due to a lack of air humidity. One day before harvesting the aboveground biomass of the plants we removed the snails and weighed them.
Harvest
Six weeks after snail addition shoots were harvested at ground level and oven-dried at 50° C for 3 days. The roots were carefully washed to remove soil particles and oven-dried at 50° C. After drying, shoots and roots were weighed with a precision balance to determine dry weight. Hereafter we performed statistical analysis on growth data (see section below for details). In the case of any detectable significant differences (P < 0.05) as a response to our ‘home’ and ‘foreign’ soil biota treatments within one of the three plant species, we went on with analyses of the soil nematode community composition and the percentage root colonization with AMF and DSE. This was done in order to investigate if one of these soil biota components could have played a role in achieving the observed feedback effect.
Nematode extraction
At harvest, 50 g soil were sampled from every pot and stored at 4° C for a maximum of 6 days. Nematodes were extracted from soil using Cobb’s decanting and sieving technique (Flegg 1967). The soil samples used for the extraction contained also root material for the inclusion of root associated nematodes into the analysis. After the extraction, nematodes were preserved in 4 % formaldehyde solution and kept at 4° C. They were counted, and 100 individuals per sample were classified to functional groups according to feeding types (plant feeders, fungal feeders, bacterial feeders and predators) after Yeates et al. (1993) by using a microscope (400× magnification).
Root associated fungi
Root subsamples randomly collected from the total root biomass were put in 10 ml 10 % KOH and heated for 30 min to 90° C in a water bath. Afterwards, the KOH was decanted, the roots were rinsed with demineralized water and placed in 1 % HCl for 5 min. After removing the HCL the roots were covered with Trypan Blue staining solution and heated again to 90° C for 15 min. For destaining the roots were placed in lactoglycerol at room temperature (Phillips and Hayman 1970). The roots were checked for percentage root colonization by fungi at 200 times magnification using the magnified intersection method, by checking 100 intersections (McGonigle et al. 1990). Hyphae associated with arbuscules or vesicules, with irregular or none cross-wall septation, and branching typically not at a right angle were noted as AMF hyphae (Rillig et al. 1998). Corresponding AMF structures (arbuscules, vesicles) were counted as well. Darkly-pigmented, septate hyphae together with clusters of inflated, rounded, thick-walled cells (the sclerotia) within the root cortical cells (Jumpponen and Trappe 1998) were associated with DSE. Fungal structures that did not follow these morphological traits were not noted.
Statistical analysis
Due to the imbalance of our experimental design and the circumstance that we incorporated fixed and random predictors that were also nested in each other, we used linear mixed-effects models for data analysis. All analyses were done in R (R Development Core Team 2011) with addition of the ‘nlme’ package. We performed the full model with the fixed factors being habitat type, soil history and leaf herbivory. The sites were entered as a random factor and were nested into the factor habitat type. The model was performed on individual plant species growth data (root, shoot, and total dry weight), AMF data (% -AMF root colonization, arbuscules, vesicules), DSE data (%-DSE root colonization), the nematode data (total number and numbers of fungivorous, bacterivorous, herbivorous, and predatory nematodes) and on the weight gain of the snails. Independent from the model we also performed a single ANOVA to check for differences in total biomass production between the three focal plant species. If there was a matching pattern between the plant biomass data and the colonization data of root or soil associated organisms, we checked for a relationship between them by performing a simple linear regression with plant growth data as the dependent variable.
Results
Growth of the experimental plant species
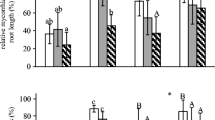
A significant difference in total biomass production in ‘home’ treatment compared to ‘foreign’ treatment was only detected for native T. vulgare. Plants growing in soil inoculated with S. canadensis soil biota performed better than plants growing in soil inoculated with soil biota from the own root zone (Figs. 1, 2b; Table 1). Besides the significant effect of soil history on growth of T. vulgare there was a significant interaction between habitat type and snail addition on shoot and total biomass production (Fig. 2b; Table 1), i.e. the effect of snails depended on the area where the soil was collected. Snails affected the plants growing with soil inocula from ‘urban’ sites positively, but negatively when the plants were growing with soil biota from the ‘suburban’ sites.
Total biomass of S. canadensis, T. vulgare and M. albus plants grown either in a ‘home’ or in a ‘foreign’ soil history treatment (mean ± SE, n = 15 per bar). ‘Suburban’ and ‘urban’ indicate the origin of the soil inocula that were used for the experiment. P values for main and interaction effects are derived from linear mixed-effects models
Shoot biomass production (mean ± SE) of invasive plant species a Solidago canadensis and native plant species b Tanacetum vulgare and c Melilotus albus grown either with their own soil biota (‘home’) or with soil biota from other species (‘foreign’) and with or without snail addition (n = 9 without snails, n = 6 with snails). ‘Suburban’ and ‘urban’ indicate the origin of the soil inocula that were used for the experiment. P values for main and interaction effects are derived from linear mixed-effects models
Exotic S. canadensis treated with inocula from ‘suburban’ sites produced more aboveground biomass (Fig. 2a; Table 1) than plants inoculated with biota from ‘urban’ sites, while there was no difference in total biomass (Fig. 1). There were no effects of the soil history (‘home’ vs. ‘foreign’), snail addition or interaction effects between the three factors on the growth of S. canadensis (Fig. 2a; Table 1).
There were also no significant effects of the habitat type, the soil biota history or snail addition on the growth of the native legume M. albus (Fig. 2c, Table 1). In general, total biomass production of the legume M. albus was 32.4 and 28.2 % higher than total biomass production of S. canadensis and T. vulgare, respectively (F = 27.20, P < 0.001, Fig. 1).
Herbivore performance
At the end of the experiment 141 of 144 snails were recovered alive. Snail biomass on S. canadensis, T. vulgare and M. albus plants increased on average by 0.53 % (SE = 1.41), 7.84 % (SE = 2.32) and 2.89 % (SE = 1.67), respectively. Neither effects of the habitat type or soil history were detected on the weight gain of the snails.
Drivers of the soil feedback effect
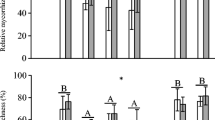
There were no significant effects of the habitat type, the soil biota history or snail addition on the colonization rates of the different AMF structures (hyphae, arbuscules and vesicles) of T. vulgare plant roots (Table 2). However, the root colonization by DSE was significantly higher when plants were inoculated with their own soil biota community than when grown with the community of S. canadensis (Fig. 3, Table 2). Linear regression analysis indicated that there was a significant negative relationship between DSE colonization and shoot growth (P < 0.01, R2 = 0.17) and total plant growth (P < 0.01, R2 = 0.12), but no relationship between DSE colonization and root growth (P > 0.1, R2 = 0.01).
Effects of soil history (‘home’ and ‘foreign’) on the dark-septate endophyte (DSE) colonization of T. vulgare plants grown in soil either with ‘suburban’ soil biota inoculation or ‘urban’ soil biota inoculation (mean ± SE, n = 15 per bar). The P value for the soil history effect is derived from linear mixed-effects models
There were no significant main effects on the total numbers of nematodes in soils from the T. vulgare pots at the end of the experiment. However, there was a significant three-way interaction between habitat type, history and snails on total nematode numbers, and a significant interaction between habitat type and history on the abundance of plant feeding nematodes (Table 3). When plants were inoculated with soil biota from the ‘suburban’ sites there were more plant feeding nematodes in ‘home’ soil compared to the ‘foreign’ soil at the end of the experiment (344 ± 98 individuals/100 g soil in ‘home’ compared to 150 ± 33 in ‘foreign’ soil). With inoculation of ‘urban’ site soil biota the numbers of plant feeders did not differ significantly between ‘home’ and ‘foreign’ soil (on average 206 ± 35 individuals/100 g soil).
Also snail addition to T. vulgare plants significantly affected the abundance of plant feeding nematodes in the soil (Table 3). When plants encountered snails the abundance of plant feeding nematodes was lower (215 ± 45 individuals/100 g soil) compared to the soil of plants without snails (282 ± 50 individuals/100 g soil).
Discussion
Despite the growing number of studies that highlight the importance of a combined aboveground-belowground perspective on plant performance, little is known about how plant-soil feedback affects plant-herbivore interactions above the ground and the invasion of an exotic plant into a native plant community. We found that soil from the root zone of the dominant invader S. canadensis facilitates the growth of a native competitor plant species, which is a type of feedback that has been described as heterospecific positive feedback (Perkins and Nowak 2013). However, the feedback effect from the belowground compartment had no effect on the herbivore interaction in the aboveground compartment. Concerning the question which soil biota fraction may be responsible for the plant-soil feedback effect, we found that the not well studied DSE might carry over that part. In the following paragraphs the aforementioned results will be discussed in detail.
Soil feedback and plant growth
The native plant species T. vulgare had a negative growth response to its own soil biota community, while the exotic S. canadensis showed a slightly positive soil feedback, though not significant. These results agree with the general findings of other studies (Klironomos 2002; Callaway et al. 2004; van Grunsven et al. Van Grunsven 2007; Morriën et al. 2011) which suggest that exotic plant species tend to exhibit a positive or a less negative feedback than native plant species. The better performance of T. vulgare with soil biota that were conditioned by S. canadensis compared to growth with its own soil biota community suggests that a native plant species may benefit from the presence of a dominant exotic invader. Personal observations of plant species abundance and spatial occurrence on our study sites are consistent with our experimental findings. The multi-stemmed clonal colonies of both species occur frequently in immediate vicinity to each other and collectively cover a high percentage of area on abandoned and/or disturbed urban fields.
Potential facilitative effects mediated by changes in the soil biota community among an exotic and a co-occurring native plant species are rarely reported. A meta-analysis by Suding et al. (2013) showed that the growth responses of native species were often greater in soil conditioned by native species than in soil conditioned by exotic species. Further, the majority of studies on S. canadensis deal with its direct and indirect negative impacts on other plants (Meiners et al. 2002; de Groot et al. 2007), soil microbial communities (Zhang et al. 2009, Zhang et al. 2010), pollinators (De Groot et al. 2007; Moroń et al. 2009), soil nematodes (Xu et al. 2011), and generalist predators like carabid beetles (De Groot et al. 2007). Positive aspects related with the integration of S. canadensis to the Middle European flora may be present as well, but are sparsely reported. It is discussed, for example, whether its opulent flowerage in late season increases the availability of nectar for honey bees in autumn (Kowarik 2003).
The lesser magnitude of the feedback effect strengths detected in our study compared to others could be attributed to differences in the methods used. Generally the designs of plant-soil feedback experiments can be distinguished by whether the whole soil or a soil inoculum is sterilized and plants grow thus with or without living soil biota (‘sterilization approach’), or whether plants grow in ‘home’ or ‘foreign’ soils with living soil biota derived from other plant species (‘conditioning approach’). Comparative studies showed that the ‘sterilization approach’ produces bigger effect sizes and is more likely to yield significant results (Kulmatiski and Kardol 2008; Brinkman et al. 2010), but the ecological relevance is in our opinion less clear. Also the treatment and origin of the inoculum seems to influence the experimental outcome: A meta-analytical review by Kulmatiski et al. (2008) documented that studies using soils conditioned under controlled conditions showed stronger feedback effects than studies using field-collected soils. Abiotic conditions are likely to be different in the greenhouse compared to natural field conditions, which might lead to exaggerated feedback effects of soil biota on plant growth, but may not reflect effect strengths appearing in the field (Brinkman et al. 2010).
Influence of soil feedback on aboveground herbivore interaction
We further were interested if plant-soil feedbacks may cascade up to higher trophic levels above the ground. However, soil history did not affect the impact of snails on plant growth for all three plant species. Thus, plant tolerance or resistance to aboveground herbivores was not modified by feedback effects caused by soil organisms. As far as we are aware there are only two studies that have investigated the individual and combined effects of belowground organisms on plant growth and foliage-grazing snails (Thompson et al. 1993; Wurst and Rillig 2011). Both studies focused on effects of individual soil biota fractions (earthworms and earthworms plus AMF in case of Thompson et al. 1993 and Wurst and Rillig 2011, respectively) on the productivity, structure and diversity of experimental grassland communities which makes the comparability to the present study that focuses on plant-soil feedback effects on individual plant species quite low.
Consistent with the results of the present study, Morriën et al. (2011), who investigated the impact of plant-soil feedbacks on aboveground insect herbivores (aphids and locusts), found no correlation between the strength of soil feedback and the effect size of insect shoot herbivory using 15 plant species in a greenhouse experiment. The authors argued that the lack of any correlation may have resulted from the independence and contrasting effects of the two main plant defence pathways involved, i.e. the jasmonic acid pathway and the salicylic acid pathway (Beckers and Spoel 2006). In our study, the non-significant interaction between soil history and snail herbivory could also have been resulted from the relative low effect size of the plant soil feedback effects compared to other feedback studies (see discussion above). Although effects of belowground organisms on aboveground herbivores are well documented, the soil history did not modify plant tolerance or resistance to aboveground herbivores in the two studies on consequences of plant soil feedbacks on higher trophic levels (our study and Morriën et al. 2011). This inconsistency may be due to the fact that the soil feedback approach investigates interactions of the soil biota community as a whole and does not focus on individual components of the soil biota community separately. The presence of soil biota fractions with different ecological roles (i.e. antagonism or mutualism) and their interactions among each other may outweigh their individual effects in the complex entity of a soil community (Ladygina et al. 2010; Wurst et al. 2012).
Drivers of the soil feedback effect
In this study we aimed to investigate which soil biota fraction may have been a causal driver of the detected feedback effect. We suspected a group supposed to be symbiotic (AMF), another one known to be pathogenic and/or parasitic (plant feeding nematodes), and a third one (DSE) with so far rather unclear consequences on plant performance. Callaway et al. (2004) and MacKay and Kotanen (2008) showed that symbionts were the dominant drivers of plant-soil feedback, Agrawal et al. (2005), (Van Grunsven 2007) and Dostál and Palečková (2011) suggested in contrast that pathogens might play the most important role. Klironomos (2002), who studied effects of both symbionts and pathogens in a series of experiments, found that plants may benefit from positive feedback with symbiotic AMF, which may be counteracted by a negative feedback with soil pathogens. Since the root colonization of T. vulgare by AMF did not differ between the ‘home’ and ‘foreign’ treatment in our experiment, we have no indication that AMF played a major role for the observed feedback effect, because AMF root colonization and plant growth responsiveness are often positively related in non-leguminous plant species (Wilson and Hartnett 1998). In contrast to the study by Klironomos (2002), we used the whole soil biota community and not isolates of AMF and pathogens; therefore a potential positive effect of AMF might have been counteracted by other soil biota present in our set-up. Further, effects of AMF on plant performance depend on the specific combination of AMF and plant species (Klironomos 2003) and on the abiotic soil context in which the plant-AMF interactions take place (Hoeksema et al. 2010).
In terms of soil nematode abundance and community composition, we also have no indication that nematodes were the responsible driver for the detected feedback effect on T. vulgare. No significant differences in total nematode numbers and numbers within the four functional nematode groups (plant feeders, fungal feeders, bacterial feeders, predators) between the ‘home’ and ‘foreign’ treatment were detected at the end of our experiment. However, in agroecosystems, the accumulation of plant species-specific soil nematodes is a well-known phenomenon and it is a crucial factor for the implementation of crop rotation in agricultural practices (Agrios 2005). In natural systems, negative plant-soil feedback effects associated with nematode accumulation have been described for sand dune communities (van der Stoel et al. 2002). However, these effects were only detected in absence of other soil organisms, i.e. not in natural soil with a variety of interacting organisms. The above-mentioned results suggest that soil nematodes may have only minor impacts on the development of plant-soil feedback effects in natural soils, but existing data is still insufficient to draw proper conclusions.
Based on the results of our study we suggest that the worse performance of T. vulgare in its own soil compared to S. canadensis soil may be caused by DSE. The colonization by this group of ascomycetous anamorphic fungi, that colonize root tissues intracellularly and intercellularly (Jumpponen 2001), was significantly higher in ‘home’ soil compared to ‘foreign’ soil at the end of our experiment which reflected the observed soil feedback effect on shoot biomass. Consistently, data of an experimental greenhouse study by Camenzind (2010) showed that the individual root colonization of ten naturally derived DSE isolates negatively affected growth of T. vulgare in four cases and neutrally in the other six cases. The higher root colonization rates by DSE of T. vulgare grown with ‘home’ soil biota can either indicate that the density of these fungi is suppressed in the root zone of S. canadensis or enhanced in root zone soil of T.vulgare. The first assumption is supported by the findings of Zhang et al. (2009) which show that S. canadensis can suppress soil borne pathogenic fungi (Pythium ultimum and Rhizoctonia solani) through exudation of allelochemicals. The second assumption—which predicts an accumulation of pathogens in the rhizosphere of T. vulgare—is also possible but less likely, because two other independent plant-soil feedback studies (Petermann et al. 2008 and a yet unpublished study by ourselves) whose set of plant species also contained T. vulgare documented a positive soil feedback for this species. In both experiments the ‘foreign’ treatment soil of T. vulgare was influenced by other plant species than S. canadensis.
However, it is necessary to notice that it is still poorly understood what ecological role DSE play and to which extent they interact with plants and other plant colonizing fungi like AMF and fine endophytes (Jumpponen 2001; Postma et al. 2007). Because their impacts on plant performance are highly variable—even differing between different fungal strains and isolates—it is assumed that they stretch a continuum from mutualism to antagonism (Tellenbach et al. 2011). Future research on this relatively unknown root colonizing fungi should include questions about their role in plant-soil feedback, plant invasions, and also in plant community composition.
Herbivore effects on plant growth and soil biota
Interestingly, snail addition tended to increase shoot biomass production of T. vulgare (significantly) and M. albus (marginally significantly) when plants were growing with the S. canadensis soil community, but only when the soil was inoculated with biota collected from ‘urban’ sites. Compensatory growth following herbivory is often reported as an indicator of tolerance and plant response after tissue damage (Noy-Meir 1993; Wise and Abrahamson 2007; Ruiz-R et al. 2007). The direction and magnitude of the net effect of grazing on plant growth is assumed to depend on conditions such as amount of green leaf area, number of meristems, amount of stored nutrients and assimilates, availability of soil resources, length of growing season, and frequency and intensity of defoliation (Noy-Meir 1993). The results of our study indicate that soil biota history may be added to this list of influential factors. Soil biota history dependent compensatory shoot growth in response to snail herbivory was detectable in T. vulgare and M. albus with inoculation from the ‘urban’ sites and in S. canadensis independent of soil origin, respectively.
We found that when T. vulgare encountered aboveground snail attack the abundance of root feeding nematodes in the soil was lower at the end of the experiment compared to plants without snails. Responses of soil nematode abundances to aboveground herbivory have been also described by Fu et al. (2001) in agroecosystems. They found that numbers of bacterivorous and fungivorous nematodes were affected by aboveground feeding by grasshoppers, but not the numbers of root feeding nematodes as in our experiment.
Influence of habitat type
Although soil history did not affect snail herbivory, our results show that soil biota communities from different habitat types may differently influence plant interactions with aboveground herbivores. Snails positively affected T. vulgare plants growing with soil inocula from ‘urban’ sites, but had negative effects on shoot biomass when the plants were growing with soil biota from ‘suburban’ sites. This and the enhanced shoot growth of S. canadensis with soil communities from ‘suburban’ sites indicate that the soil biota communities from ‘urban’ and ‘suburban’ sites differed in their abundance and/or composition. It is also possible that the abiotic soil properties of the field-collected inocula had an effect in our experiment, although we added it to the same background soil to correct for differences in abiotic conditions. The C:N ratio did not differ between the root zone samples of the different plant species but it differed significantly between the two habitat types. The ‘suburban’ soil inocula had a lower C:N ratio as the ‘urban’ soil inocula, which might explain the higher shoot growth of S. canadensis with ‘suburban’ soil inoculation due to higher availability of nitrogen. However, there was no main effect of the habitat type on the other two plant species. The interaction of habitat type and snail herbivory on shoots of T. vulgare promotes the hypothesis that compositions and/or abundances of soil biota differed between the two habitat types. Bezemer et al. (2006) showed that effects of soil biota communities on feedback effects are not only plant species-specific but also may vary in different soils.
Conclusion
Our plant-soil feedback study found evidence for the impact of the history of the soil biota community on the biomass production of one out of three experimental plants. However, we could not show that this effect cascades up to another trophic level by affecting plant interactions with aboveground herbivores. Nevertheless, our study revealed two interesting issues that should be taken into account by future research: On the one hand we found that a native plant species (T. vulgare) may benefit from the presence of a dominant co-occurring exotic invader (S. canadensis) mediated by changes in the soil biota community. On the other hand our study puts a relatively unknown group of soil organisms—the DSE—in perspective to be a potential driver for plant-soil feedback effects, and thus also for plant competition and community composition.
References
Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W, Klironomos JN (2005) Enemy release? An experiment with congeneric plant pairs and diverse above-and belowground enemies. Ecology 86:2979–2989. doi:10.1890/05-0219
Agrios GN (2005) Plant Pathology. Academic Press, Fifth Edition
Bardgett RD, Mawdsley JL, Edwards S, Hobbs PJ, Rodwell JS, Davies WJ (1999) Plant species and nitrogen effects on soil biological properties of temperate upland grasslands. Funct Ecol 13:650–660. doi:10.1046/j.1365-2435.1999.00362.x
Beckers GJM, Spoel SH (2006) Fine-tuning plant defence signalling: salicylate versus Jasmonate. Plant Biol 8:1–10. doi:10.1055/s-2005-872705
Belnap J, Phillips S (2001) Soil biota in an ungrazed grassland: response to annual grass (Bromus tectorum) invasion. Ecol Appl 11:1261–1275. doi:10.1890/1051-0761(2001)011[1261:SBIAUG]2.0.CO;2
Bezemer TM, Lawson CS, Hedlund K, Edwards AR, Brook AJ, Igual JM, Mortimer SR, Van der Putten WH (2006) Plant species and functional group effects on abiotic and microbial soil properties and plant-soil feedback responses in two grasslands. J Ecol 94:893–904. doi:10.1111/j.1365-2745.2006.01158.x
Bezemer TM, Van der Putten WH, Martens H, Van de Voorde TFJ, Mulder PPJ, Kostenko O (2013) Above- and below-ground herbivory effects on below-ground plant-fungus interactions and plant-soil feedback responses. J Ecol 101:325–333. doi:10.1111/1365-2745.12045
Brinkman EP, Van der Putten WH, Bakker E-J, Verhoeven KJF (2010) Plant-soil feedback: experimental approaches, statistical analyses and ecological interpretations. J Ecol 98:1063–1073. doi:10.1111/j.1365-2745.2010.01695.x
Callaway R, Thelen G, Rodriguez A, Holben W (2004) Soil biota and exotic plant invasion. Nature 427:731–733. doi:10.1038/nature02322
Camenzind T (2010) The ecology and physiology of root-colonising pathogenic fungi. Freie Universität Berlin, Diploma thesis
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40. doi:10.1111/j.1472-4642.2008.00521.x
De Groot M, Kleijn D, Jogan N (2007) Species groups occupying different trophic levels respond differently to the invasion of semi-natural vegetation by Solidago canadensis. Biol Conserv 136:612–617. doi:10.1016/j.biocon.2007.01.005
Dostál P, Palečková M (2011) Does relatedness of natives used for soil conditioning influence plant-soil feedback of exotics? Biol Invasions 13:10. doi:10.1007/s10530-010-9824-6
Engelkes T, Morriën E, Verhoeven KJF, Bezemer TM, Biere A, Harvey JA, McIntyre LM, Tamis WLM, Van der Putten WH (2008) Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 456:946–948. doi:10.1038/nature07474
Flegg J (1967) Extraction of Xiphinema and Longidorus species from soil by a modification of Cobb’s decanting and sieving technique. Ann Appl Biol 60:429–437. doi:10.1111/j.1744-7348.1967.tb04497.x
Fu S, Kisselle KW, Coleman DC, Hendrix PF, Crossley D (2001) Short-term impacts of aboveground herbivory (grasshopper) on the abundance and 14C activity of soil nematodes in conventional tillage and no-till agroecosytems. Soil Biol Biochem 33:1253–1258. doi:10.1016/S0038-0717(01)00031-1
Hartley SE, Gange AC (2009) Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu Rev Entomol 54:323–342. doi:10.1146/annurev.ento.54.110807.090614
Harvey JA, Bukovinszky T, Van der Putten WH (2010) Interactions between invasive plants and insect herbivores: a plea for a multitrophic perspective. Biol Conserv 143:2251–2259. doi:10.1016/j.biocon.2010.03.004
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407. doi:10.1111/j.1461-0248.2009.01430.x
Hulme PE (1996) Herbivores and the performance of grassland plants: a comparison of arthropod, mollusc and rodent herbivory. J Ecol 84:43. doi:10.2307/2261698
Jumpponen A (2001) Dark septate endophytes—are they mycorrhizal? Mycorrhiza 11:207–211. doi:10.1007/s005720100112
Jumpponen A, Trappe J (1998) Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol 140:295–310. doi:10.1046/j.1469-8137.1998.00265.x
Kaplan I, Sardanelli S, Denno R (2009) Field evidence for indirect interactions between foliar-feeding insect and root-feeding nematode communities on Nicotiana tabacum. Ecol Entomol 34:262–270. doi:10.1111/j.1365-2311.2008.01062.x
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70. doi:10.1038/417067a
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301. doi:10.1890/02-0413
Kourtev P, Ehrenfeld J, Häggblom M (2002) Exotic plant species alter the microbial community structure and function in the soil. Ecology 83:3152–3166. doi:10.1890/0012-9658(2002)083[3152:EPSATM]2.0.CO;2
Kowarik I (2003) Biologische Invasionen: Neophyten und Neozoen in Mitteleuropa. Ulmer (Eugen)
Kulmatiski A, Kardol P (2008) Getting plant-soil feedbacks out of the greenhouse: experimental and conceptual approaches. InL: Lüttge U, Beyschlag W, and Murata J, (eds.). Progress in Botany 69:449–472
Kulmatiski A, Beard KH, Stevens JR, Cobbold SM (2008) Plant-soil feedbacks: a meta-analytical review. Ecol Lett 11:980–992. doi:10.1111/j.1461-0248.2008.01209.x
Ladygina N, Henry F, Kant MR, Koller R, Reidinger S, Rodriguez A, Saj S, Sonnemann I, Witt C, Wurst S (2010) Additive and interactive effects of functionally dissimilar soil organisms on a grassland plant community. Soil Biol Biochem 42:2266–2275. doi:10.1016/j.soilbio.2010.08.027
MacKay J, Kotanen PM (2008) Local escape of an invasive plant, common ragweed (Ambrosia artemisiifolia L.), from above-ground and below-ground enemies in its native area. J Ecol 96:1152–1161. doi:10.1111/j.1365-2745.2008.01426.x
Marrs RH, Gough MW, Griffiths M (1991) Soil chemistry and leaching losses of nutrients from semi-natural grassland and arable soils on three contrasting parent materials. Biol Conserv 57:257–271. doi:10.1016/0006-3207(91)90072-H
Marschner P, Crowley D, Yang CH (2004) Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 261:199–208. doi:10.1023/B:PLSO.0000035569.80747.c5
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. doi:10.1111/j.1469-8137.1990.tb00476.x
Meiners SJ, Pickett STA, Cadenasso ML (2002) Exotic plant invasions over 40 years of old field successions: community patterns and associations. Ecography 25:215–223. doi:10.1034/j.1600-0587.2002.250209.x
Moroń D, Lenda M, Skórka P, Szentgyörgyi H, Settele J, Woyciechowski M (2009) Wild pollinator communities are negatively affected by invasion of alien goldenrods in grassland landscapes. Biol Conserv 142:1322–1332. doi:10.1016/j.biocon.2008.12.036
Morriën E, Engelkes T, Van der Putten WH (2011) Additive effects of aboveground polyphagous herbivores and soil feedback in native and range-expanding exotic plants. Ecology 92:1344–1352. doi:10.1890/10-1937.1
Newsham KK (2011) A meta-analysis of plant responses to dark septate root endophytes. New Phytol 190:783–793. doi:10.1111/j.1469-8137.2010.03611.x
Nijjer S, Rogers WE, Siemann E (2007) Negative plant-soil feedbacks may limit persistence of an invasive tree due to rapid accumulation of soil pathogens. Proc R Soc B Biol Sci 274:2621–2627. doi:10.1098/rspb 2007.0804
Noy-Meir I (1993) Compensating growth of grazed plants and its relevance to the use of rangelands. Ecol Appl 3:32–34. doi:10.2307/1941787
Perkins LB, Nowak RS (2013) Native and non-native grasses generate common types of plant-soil feedbacks by altering soil nutrients and microbial communities. Oikos 122:199–208. doi:10.1111/j.1600-0706.2012.20592.x
Petermann J, Fergus A, Turnbull L, Schmid B (2008) Janzen-Connell effects are widespread and strong enough to maintain diversity in grasslands. Ecology 89:2399–2406. doi:10.1890/07-2056.1
Phillips J, Hayman D (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Postma JWM, Olsson PA, Falkengren-Grerup U (2007) Root colonisation by arbuscular mycorrhizal, fine endophytic and dark septate fungi across a pH gradient in acid beech forests. Soil Biol Biochem 39:400–408. doi:10.1016/j.soilbio.2006.08.007
R Development Core Team (2011) R: A language and environment for statistical computing
Rillig MC, Allen MF, Klironomos JN, Chiariello NR, Field CB (1998) Plant species-specific changes in root-inhabiting fungi in a California annual grassland: responses to elevated CO 2 and nutrients. Oecologia 113:252–259. doi:10.1007/s004420050376
Ruiz-R N, Ward D, Saltz D (2007) Leaf compensatory growth as a tolerance strategy to resist herbivory in Pancratium sickenbergeri. Plant Ecol 198:19–26. doi:10.1007/s11258-007-9381-y
Soler R, Bezemer TM, Van der Putten WH, Vet LEM, Harvey JA (2005) Root herbivore effects on above-ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. J Anim Ecol 74:1121–1130. doi:10.1111/j.1365-2656.2005.01006.x
Suding KN, Stanley Harpole W, Fukami T, Kulmatiski A, MacDougall AS, Stein C, Van der Putten WH (2013) Consequences of plant-soil feedbacks in invasion. J Ecol 101:298–308. doi:10.1111/1365-2745.12057
Tellenbach C, Grünig CR, Sieber TN (2011) Negative effects on survival and performance of Norway spruce seedlings colonized by dark septate root endophytes are primarily isolate-dependent. Environ Microbiol 13:2508–2517. doi:10.1111/j.1462-2920.2011.02523.x
Thompson L, Thomas CD, Radley JMA, Williamson S, Lawton JH (1993) The effect of earthworms and snails in a simple plant community. Oecologia 95:171–178. doi:10.1007/BF00323487
Turkington R, Cavers P, Rempel E (1978) The biology of Canadian weeds: 29. Melilotus alba Desr. and M. officinalis (L.) Lam. Can J Plant Sci 58:523–537. doi:10.4141/cjps78-078
Van Dam NM, Raaijmakers CE, Van der Putten WH (2005) Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra. Entomol Exp Appl 115:161–170. doi:10.1111/j.1570-7458.2005.00241.x
Van der Putten WH, Vet LEM, Harvey JA, Wäckers FL (2001) Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol Evol 16:547–554. doi:10.1016/S0169-5347(01)02265-0
Van der Stoel CD, Van der Putten WH, Duyts H (2002) Development of a negative plant-soil feedback in the expansion zone of the clonal grass Ammophila arenaria following root formation and nematode colonization. J Ecol 90:978–988. doi:10.1046/j.1365-2745.2002.00727.x
Van Grunsven R (2007) Reduced plant–soil feedback of plant species expanding their range as compared to natives. J Ecol 95:1050–1057. doi:10.1111/j.1365-2745.2007.01282.x
Vilà M, Corbin JD, Dukes JS, Pino J, Smith SD, Canadell JG, Pataki DE, Pitelka LF (2007) Terrestrial ecosystems in a changing world. In: Canadell JG, Pataki DE, and Pitelka LF (eds) Springer Berlin, Heidelberg
Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am Sci 84:468–478
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, Van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633. doi:10.1126/science.1094875
Werner PA, Gross RS, Bradbry IK (1980) The biology of Canadian weeds.: 45. Solidago canadensis L. Can J Plant Sci 60:1393–1409. doi:10.4141/cjps80-194
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738
Wise MJ, Abrahamson WG (2007) Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. Am Nat 169:443–454. doi:10.1086/512044
Wolfe B, Klironomos JN (2005) Breaking new ground: soil communities and exotic plant invasion. Bioscience 55:477. doi:10.1641/0006-3568(2005)055[0477:BNGSCA]2.0.CO;2
Wurst S, Rillig MC (2011) Additive effects of functionally dissimilar above- and belowground organisms on a grassland plant community. J Plant Ecol 4:221–227. doi:10.1093/jpe/rtr012
Wurst S, Van der Putten WH (2007) Root herbivore identity matters in plant-mediated interactions between root and shoot herbivores. Basic Appl Ecol 8:491–499. doi:10.1016/j.baae.2006.09.015
Wurst S, Gebhardt K, Rillig MC (2011) Independent effects of arbuscular mycorrhiza and earthworms on plant diversity and newcomer plant establishment. J Veg Sci 22:1021–1030. doi:10.1111/j.1654-1103.2011.01321.x
Wurst S, De Deyn G, Owen K (2012) Soil biodiversity and functions. In: Wall DH (ed) Soil Ecology and Ecosystem Services. Oxford University Press, UK
Xu X, Wang Y, Lu Q, Lin Z, Chen H (2011) 加拿大一枝黄花入侵对杭州湾地区 土壤线虫群落的影响. 生物多样性 19:519–527. doi:10.3724/SP.J.1003.2011.09048
Yeates GW, Bongers T, De Goede RG, Freckman DW, Georgieva SS (1993) Feeding habits in soil nematode families and genera-an outline for soil ecologists. J Nematol 25:315–331
Zhang S, Jin Y, Tang J, Chen X (2009) The invasive plant Solidago canadensis L. suppresses local soil pathogens through allelopathy. Appl Soil Ecol 41:215–222. doi:10.1016/j.apsoil.2008.11.002
Zhang Q, Yang R, Tang J, Yang H, Hu S, Chen X (2010) Positive feedback between mycorrhizal fungi and plants influences plant invasion success and resistance to invasion. PLoS One 5:e12380. doi:10.1371/journal.pone.0012380
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schittko, C., Wurst, S. Above- and belowground effects of plant-soil feedback from exotic Solidago canadensis on native Tanacetum vulgare . Biol Invasions 16, 1465–1479 (2014). https://doi.org/10.1007/s10530-013-0584-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0584-y