Abstract
The naturalisation hypothesis has been gaining attention recently as a possible mechanism to explain variations in invasion success. It predicts that exotic genera with native representatives should be less successful because of an overlap in resource use and of the existence of common specialised enemies. In this study, we tested whether native congenerics have more negative impact on exotic species than heterogenerics by increasing the effects of soil pathogens. We sampled soil in populations of three exotic species (Epilobium ciliatum, Impatiens parviflora and Stenactis annua) at sites with and without respective congeneric species. This soil was used as an inoculum for cultivating the first plant cohort, which included exotics, as well as native congenerics and heterogenerics. The conditioned soil was subsequently used for cultivating the second cohort of plants (exotics only). We found no consistent impact of relatedness of conditioning species on exotic growth. Although soil conditioned by congeneric E. hirsutum had the largest reduction on the performance of E. ciliatum, the final biomass of S. annua was lowest when grown in soil conditioned by itself. There was no effect of stimulating species on the biomass of I. parviflora. In both experimental phases, performance of exotics was improved when cultivated with sterilised inoculua, indicating the dominance of soil generalist pathogens. However, the biomass of S. annua was increased most by congeneric-stimulated inoculum from congeneric sites, suggesting a possible role for specialised symbionts. Our results suggest that variations in invasion success of at least some exotics may be affected by species-specific interactions mediated by the soil biota.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, there is a strong interest in identifying the factors that determine which imported species are more likely to succeed and which of them will probably fail. Aside from other possible mechanisms, increasing attention is being paid to the effects of the phylogenetic relationships between exotics and colonised flora on invasion success. As previously proposed by Darwin (1859), this explanation assumes that exotic species with native representatives should be less successful than those that do not have relatives in the native flora. The reduced success of related exotics is explained by a larger overlap in resource use among close relatives and by their common specialised enemies. Both processes may increase the biotic resistance of colonised communities against invasion (Levine et al. 2004; Strauss et al. 2006).
Darwin’s naturalisation hypothesis is usually examined by linking the success of exotic species (e.g., expressed by their naturalisation probability or by the area colonised by them) to their phylogenetic similarity with the native flora. Such an approach was used by Strauss et al. (2006), who compared introduced invasive and non-invasive grasses in terms of their phylogenetic relatedness with native Californian grasses. Mack (1996), in his meta-analysis, investigated the relationship between the probability of naturalisation of introduced species and the presence of native congenerics in flora found in six U.S. states. In both of these studies, the invasiveness or naturalisation probability of introduced species was associated with their lesser phylogenetic similarity with native floras. On the other hand, Duncan and Williams (2002), who analysed introduced species in New Zealand, found a higher naturalisation rate in introduced genera containing native species. Lambdon and Hulme (2006) compiled presence/absence records of congenerics for all naturalised alien plant species found in islands of the Mediterranean basin and found no true influence from related natives on establishment success of alien species. Diez et al. (2008) found scale-dependent support for the naturalisation hypothesis in their study surveying data on naturalisation and abundance of exotic plants in the Auckland region: at a larger scale, the probability of naturalisation was positively correlated to the number of native species in a genus. Within habitats, however, exotic abundance was negatively related to native congeneric abundance.
Curiously, in spite of the increasing amount of research based on a correlative approach, there are very few studies that examined experimentally whether native related species reduce the performance of exotics in a new range more than non-related species (e.g., Dawson et al. 2009). This assumption was explored in studies whose main focus was not exotic species. Concerning competitive interactions, a recent meta-analysis by Cadotte et al. (2008) indicated more intense competition between closely related species, but a study by Cahill et al. (2008) did not. With respect to specialised enemies, more closely related plant taxa are more likely to share a more similar community of specialised herbivores or pathogenic fungi (Becerra and Venable 1999; Novotný et al. 2006; Gilbert and Webb 2007). Cross-infection of closely related species by pathogens was also used to explain the differences in seedling mortality in a tropical forest (Webb et al. 2006). Webb et al. (2006) showed that phylodiversity had a positive effect on seedling survival at a 36 m2 scale and a 4 m2 scale, indicating that the survival rate of seedlings is enhanced if heterospecific seedlings in a neighbourhood are not closely related.
Soil microorganisms are a significant group of biota with a regulatory impact on plants. They may influence plant performance directly, but very often, their impact can be modulated by conspecific or heterospecific plants. This impact has been shown repeatedly by plant-soil feedback experiments, a technique consisting of cultivating two “plant generations.” In this technique, the first plant generation is used to condition the soil, which is subsequently applied to the cultivation of the second plant generation. This technique is based on two assumptions (Kulmatiski et al. 2008); first, that plants cause species-specific changes to soil microbial communities; and second, that plants show species-specific responses (plant-soil feedback) to these changes.
It has been demonstrated that conditioning by conspecifics leads to a negative plant-soil feedback when compared to conditioning by heterospecifics (van der Putten et al. 1993; Klironomos 2002), which documents the accumulation of specialised pathogens during the first experimental phase. Negative plant-soil biota feedbacks appear to be predominant in natural systems; they serve as a frequency-dependent regulatory mechanism that helps to maintain plant species diversity according to the Janzen-Connell concept (Janzen 1970; Connell 1971; reviewed by Reinhart and Callaway 2006).
In exotic species, plant-soil feedbacks have been shown repeatedly to be less negative or even positive (Klironomos 2002; Callaway et al. 2004; van Grunsven et al. 2007). These effects can be explained by the absence of specialised soil biota with negative impacts and by stimulating generalist symbiotic biota. What remains untested, however, is whether native species that differ in their relatedness to exotics also differ in their influence on the soil microbial community and therefore in their effects on exotic plants (but see Brandt et al. 2009). Specifically, in accordance with the naturalisation hypothesis, we assumed that more negative plant-soil feedbacks in exotics will be found if the soil is preconditioned by congenerics compared to soil preconditioned by heterogenerics. To test this assumption, we carried out a plant-soil feedback experiment with three exotic species that have established themselves successfully in Central Europe, namely Epilobium ciliatum, Impatiens parviflora and Stenactis annua. Field surveys (Dostál, unpublished) have revealed that sites occupied by these exotics may differ in the phylogenetic structure of the plant community; specifically, exotic populations grow with or without the presence of congenerics. In this paper, we reported on the results of the effect of the soil microbial community harvested at sites with and without congenerics and on the relatedness of native plants used for the soil conditioning on the growth of exotics. We aimed to answer the following questions:
-
1)
Does an inoculum sampled at sites with congenerics have a more negative impact on exotic growth than an inoculum sampled at congeneric-free sites?
-
2)
Is exotic growth reduced more in soil conditioned by congenerics than by heterogeneric species? Do conditioning species and the origin of an inoculum interact to influence exotic growth?
-
3)
Using soil conditioned by exotics themselves, is their performance improved in comparison to their growth in soil conditioned by native species?
Methods
Species
We studied the effect of the soil microbial community on three exotics, specifically Epilobium ciliatum, Impatiens parviflora and Stenactis (Erigeron) annua. E. ciliatum (Onagraceae) is native to North America and Eastern Asia, and it was introduced to Central Europe in 1960 (Pyšek et al. 2002). It grows in a variety of habitats, including degraded grasslands, disturbed areas and roadsides. I. parviflora (Balsaminaceae) is native to Central Asia and was introduced to Central Europe in 1870 (Pyšek et al. 2002) where it is very common now (Sádlo et al. 2007). It grows mainly in the forest understory. S. annua (Asteraceae) is native to North America and was introduced to Central Europe in 1882 (Pyšek et al. 2002). It occupies disturbed habitats, degraded grasslands, rock outcrops and roadsides. S. annua is naturalised in the Czech Republic; the other two exotics are classified as invasive (Pyšek et al. 2002).
We were interested in how the effects of a soil microbial community are modulated by native species that differ in their relatedness to respective exotic species. Therefore, we used two natives, a congeneric and a heterogeneric, for each exotic species. All three species were observed to grow in an identical habitat type (Dostál, unpublished). In the case of Epilobium ciliatum, we used Epilobium hirsutum and Achillea millefolium (Asteraceae), for Impatiens parviflora, we used Impatiens noli-tangere and Galeopsis speciosa (Lamiaceae), and for Stenactis annua, we used Erigeron acer and Geum urbanum (Rosaceae).
Soil sampling
In May 2008, soil samples were collected at six sites for each exotic species, half of them in the presence of the exotic only and the other half in the presence of the exotic and its congeneric (see Table 1). The presence of heterogenerics were not considered in the soil sampling scheme. Only sites with exotic populations larger than 100 individuals and a minimum density of 1 plant/m2 were included; the same criteria were applied for congeneric populations. It was important to ensure that habitat types did not differ consistently for an environmental variable across site types in a way that could influence the composition of the soil microbial community. To test this assumption, we compared both habitat types in terms of species composition as a proxy for site environmental characteristics. There were no significant differences in species composition between both site types, as demonstrated by a Canonical Correspondence Analysis [F-ratio = 1.12, P = 0.172; number of permutations = 499 with block-constrained permutations (on exotic species)]. Therefore, we assumed that the habitat types did not differ consistently from each other in environmental characteristics.
At each site, we applied a systematic raster sampling to obtain soil samples. The soil was sampled at 40 locations along 5 transects, with a distance of 5 m between transects and between locations along the transects. At each location, a soil sample of 5 × 5 × 5 cm was taken, giving a total volume of approximately 5 L of soil substrate from each site. The soil was then transferred to the lab and sieved through a 5-mm mesh to remove stones and coarse roots. The soil was homogenised within the sites and used as an inoculum. Each pot (volume = 1 L) used in the first experimental phase (see below) received a mixture (in a ratio of 1:6) of soil substrate collected in the field and steam-sterilised river sand.
Soil conditioning (first experimental phase)
To analyse whether the origin of the soil inoculum (from habitats with and without a congeneric) could influence the growth of exotic species, each exotic species was grown in 30 pots (5 pots with soil from each source site, with one plant per pot) with an unsterilised inoculum. The same number of pots was prepared with an inoculum sterilised by gamma irradiation (45 kGray) that eliminated all soil biota (McNamara et al. 2003). To avoid the confounding effect of nutrient release after the sterilisation treatment (Troelstra et al. 2001; McNamara et al. 2003; van Grunsven et al. 2007), pots with sterilised and unsterilised inoculum were fertilised weekly by a 0.25-strength Hoagland nutrient solution (Hoagland and Arnon 1950).
In addition to exotics grown with an unsterilised inoculum that were used for conspecific soil conditioning, another two groups of plants were used for soil conditioning; these were congenerics and heterogenerics. These plants were processed using the same method as for the exotics. Each of the two native species associated with their respective exotic (see Species) was grown in 30 pots (5 pots per site of inoculum source), with one plant per pot, and an unsterilised inoculum that was mixed with steam-sterilised river sand (in a ratio of 1:6). The pots were fertilised weekly with 0.25-strength Hoagland nutrient solution. The plants were cultivated for 8 weeks from early June to early August 2008, when they were harvested and divided into groups of above- and below-ground biomass. After drying to a constant weight (at 70°C for 48 h), the biomass was weighed. Soil from each pot was kept separately in plastic bags in the fridge for 1 week at a temperature of 5°C.
Plant-soil feedback (second experimental phase)
In this experimental stage, a new cohort of the exotic plants was grown in soil conditioned by the conspecifics, congenerics and heterogenerics.
Plants of each exotic species were grown in pots with sterilised and unsterilised inoculua from the first experimental phase. The inoculum was obtained by mixing the substrates from the pots such that all combinations of conditioning species and source site were produced. Part of the inoculum was then sterilised by gamma irradiation (45 kGray). Each pot contained inoculum mixed with steam-sterilised river sand (in a ratio of 1:6) and one individual of each respective exotic. There were 180 pots in total for each exotic species (2 habitat types ×3 sites ×3 species used for conditioning ×2 sterilisation treatments ×5 plant replicates).
The plants were cultivated for a period of 10 weeks from mid-August until late October 2008. Throughout the experiments, the plants were fertilised weekly with 0.25-strength Hoagland nutrient solution. At the end of October, the plants were harvested and divided into above- and below-ground biomass. After drying to a constant weight (at 70°C for 48 h), the biomass was weighed.
During both of the experimental phases (soil conditioning and feedback), the pots were kept in a greenhouse and their position was completely randomised at the beginning of the experiment. The experimental plants were grown from seeds sown directly in pots or by planting the seedlings obtained by germination on sterilised sand. Seeds used in the experiment were harvested from multiple mother plants in 2007 at soil-sampling sites and then pooled. During the second experimental phase, 11 plants of E. ciliatum, 8 plants of I. parviflora and 11 plants of S. annua died before harvest and were excluded from the analyses.
Statistical analyses
For both experimental phases, we analysed the total biomass of the exotics as the response variable. The output of these analyses is described in the Results section. We also performed identical analyses with shoot and root biomass, and these can be found in the Supplementary Material section.
In the first experimental phase, mixed-effects ANOVAs were performed with the fixed factors being sterilisation, soil origin (two habitat types with and without congenerics) and their interaction. The sites were nested within soil origin and were entered as a random factor. Analyses were performed separately for each exotic species. The data were further merged and an analysis was done for all of the species together. In this case, the identity of the exotic species was included as an additional random factor.
In the second experimental phase, mixed-effects ANOVAs were performed with the fixed factors being sterilisation, soil origin (two habitat types with and without congenerics), conditioning species and their interactions. The sites were nested within soil origin and were entered as random factor. The analyses were performed separately for each exotic species. Further analysis was done with data merged from all species. In this case, the fixed factor “conditioning category” refers to one of three species categories used (conspecifics, congenerics and heterogenerics). Random factors in the overall analysis were the identity of exotic species, identity of conditioning species nested within a category and sites nested within soil origin.
Significant effects (in the case of fixed factors) were further examined by single-factor post-hoc comparisons (Student Newman-Keuls tests) to determine which specific treatment combinations were different from the others.
All analyses were done by STATISTICA 8.0, StatSoft Inc.
Results
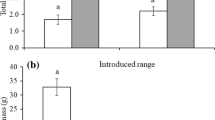
In the first experimental phase, sterilisation of the soil inoculum significantly improved the plant growth of all three species. In E. ciliatum, total biomass harvested from pots with sterilised inoculum after soil conditioning was 40% higher than biomass of plants from pots with unsterilised inoculum. In I. parviflora and S. annua, this difference was 62 and 22%, respectively (Table 2; Fig. 1). Within each species, the negative effect of the inoculum was independent of its origin; i.e., the presence of a congeneric at the site did not influence the impact of soil biota on exotic performance, nor was the interaction between soil origin and sterilisation significant. Aside from sterilisation treatment, growth also differed between individual species according to the overall test (Table 2). The pattern obtained for total biomass did not differ from the output of analyses that used root and shoot biomass as response variables. The only exception was shoot biomass of Stenactis annua, which was not influenced by sterilisation treatment (Supplementary Material, Table S1).
Total biomass (g; mean ± SE) of three exotics harvested after the first experimental phase. The plants were grown in pots with a soil inoculum originating from both site types, with a congeneric (SOILcongen+) and without (SOILcongen−). The inoculum was treated by sterilisation (ster+) or left untreated (ster−)
In the second experimental phase, inoculum sterilisation again improved plant performance. E. ciliatum, I. parviflora and S. annua plants grown in pots with sterilised inoculum were 51, 27 and 28% larger, respectively, than plants grown with unsterilised inoculum (Table 3; Fig. 2). In E. ciliatum and S. annua, total biomass depended on the conditioning species, and it interacted with sterilisation (in E. ciliatum this interaction was only marginally significant). In E. ciliatum, feedback was the most negative if the soil was conditioned by a congeneric. S. annua had the worst performance when it grew in soil that was conditioned by itself (Fig. 2). The significant effect of conditioning species was most likely due to changes in the soil microbial community and not due to allelopathy or nutritional changes. It was indicated by post-hoc comparisons showing that the identity of conditioning species induced differences in total biomass of target species, but this only occurred in plants grown with unsterilised inoculum (Fig. 2).
Total biomass (g; mean ± SE) of three exotics harvested after the second experimental phase. The plants were grown in pots with soil that was conditioned by conspecifics (self), heterogenerics (heterogen) and congenerics (congen) with an inoculum treated by sterilisation (ster+) or left untreated (ster−). Bars sharing the same letter are not significantly different (P > 0.05; S–N–K posthoc test)
Further, sterilisation × soil origin influenced the growth of S. annua that was most inhibited by unsterilised inoculum harvested at sites in the absence of congenerics (Table 3; Fig. 3a). There was a three-way interaction as the previous two factors interacted with conditioning species. If conditioned by itself and a congeneric using an inoculum from congeneric free-sites, growth was severely reduced by unsterilised inoculum. However, conditioning by a congeneric (but not by itself or a heterogeneric) and using the unsterilised inoculum from congeneric sites improved the performance of S. annua (Table 3; Fig. 3b).
a Total biomass (g; mean ± SE) of Stenactis annua harvested after the second experimental phase. Plants were grown in pots with a soil inoculum originating from both site types, with a congeneric (SOILcongen+) and without (SOILcongen−). The inoculum was treated by sterilisation (ster+) or left untreated (ster−). In B), identical results are further structured according to conditioning species used in the first experimental phase. The soil was stimulated by itself (self), congeneric Erigeron acer (congen) and heterogeneric Geum urbanum (heterogen). Bars sharing the same letter are not significantly different (P > 0.05; S–N–K post-hoc test)
Sterilisation treatment was the only significant fixed factor in the overall test. The effect of sterilisation × soil origin interaction was marginally significant and was influenced by the pattern found for S. annua. All three random factors were found to be significant (Table 3).
The pattern obtained for total biomass did not differ from the output of analyses that used root and shoot biomass as response variables. The only exception was the root biomass of Stenactis annua that was not influenced by the sterilisation treatment (Supplementary Material, Table S2).
Discussion
In this study, we demonstrated the negative effects of a soil microbial community on the growth of three exotic species in both experimental phases (soil conditioning and plant-soil feedback). This result indicates that soil pathogens’ effect on studied species dominated over the effect of soil microbial mutualists. Our results agree with the findings of Agrawal et al. (2005) and van Grunsven et al. (2007), whereas MacKay and Kotanen (2008) or Callaway et al. (2004) showed that symbionts were the dominant component of the soil microbiota. Unfortunately, we cannot say which species or even guilds of soil microbiota were responsible for the observed pattern. Despite improvements in molecular species identification techniques, the identities of soil microbiota that have significant impacts on plant performance in plant-soil feedback experiments remain mostly unknown (but see Packer and Clay 2000; Klironomos 2002).
We also found that exotics did not show less negative feedback when grown in their own soil in comparison to soil stimulated by other species. This contrasts with the findings of Klironomos (2002) or Callaway et al. (2004), who explained the better performance of exotics when grown in their own soil by claiming that they benefited from generalist soil microbes in their new environment when no longer in the presence of specialised enemies. Our results indicate that even if exotics leave behind some pathogens, they may encounter new enemies, and those are most likely generalists.
However, the results of our study do not support the assumptions of the naturalisation hypothesis. In the first experimental phase, whether the inoculum was harvested at sites with or without congenerics did not appear to affect exotic growth. In the latter experimental phase, we also did not prove the existence of a greater negative impact of soil conditioned by congenerics than of soil conditioned by other two plant categories. There was also a lack of evidence showing any interaction between the category of the conditioning species and soil origin.
Aside from other possible explanations, it is very likely that results of this study, namely those on relatedness of conditioning species, were determined by the power of the experimental design. First, at the individual species level, there were only three replicates per site category. However, this problem was mitigated when data were merged, increasing the total to nine replicates per site category. A much more problematic issue was the low number of replicates for each of three categories of conditioning species. Even after merging the data, each category was represented by only three species, which may be inadequate to prove or reject the naturalisation hypothesis.
Aside from the limitations of the rather low experimental power, there are other possible reasons that may explain the lack of a pattern of the effects of soil origin and relatedness of conditioning species. The lack of an effect of soil origin on plant growth during the first experimental phase could have occurred because the specific pathogens associated with congenerics existed in densities that were too low to show any significant effect. In contrast to previous studies that obtained the soil microbial community directly from the plant rhizosphere (e.g., Callaway et al. 2004; van Grunsven et al. 2007), we applied a rather conservative approach to search for habitat effects. We used similar method as MacKay and Kotanen (2008) did and sampled the soil irrespective of the position of congeneric individuals at the site to more realistically reflect the distribution of the exotic individuals and their interaction with soil biota. Consequently, the abundance of specific soil biota could be low.
The effect of soil origin × sterilisation appeared (marginally) significant in the second experimental phase because of the effect of this interaction on the total biomass of S. annua (Table 3; see also Fig. 3a). Therefore, we assume that microbes in this second phase were only abundant enough to influence the growth of plants of target species. Our findings are in accord with those of MacKay and Kotanen (2008), which showed that the impact of an inoculum on the growth of Ambrosia artemisifolia increased over several generations in plant-soil feedback experiments, suggesting accumulation of soil biota over time.
Surprisingly, the effect of soil origin on the growth of S. annua was in contrast to what we expected; a greater negative effect was shown for unsterilised inoculua sampled at congeneric-free sites than from sites where congenerics were present. The positive impact of congeneric Erigeron acer on S. annua was further shown by the significant effect of a three-way interaction between sterilisation, soil origin and stimulating species. Growth of this exotic was most improved when cultivated in soil with an unsterilised inoculum from congeneric-sites and stimulated by E. acer in the first experimental phase (Table 3; Fig. 3b). Although the naturalisation hypothesis predicts that related native species will produce specialised enemies that decrease the performance of exotics, in fact, they may be the hosts of specialised symbionts with positive effects on newcomers. A good example comes from Brown et al. (2002), who studied the interaction of an exotic plant, its native congeneric and shared pollinators. They found that invasive Lythrum salicaria may draw pollinators away from its native congeneric Lythrum alatum, suggesting that L. alatum (at its own expense) improves the performance of this exotic species.
The category of the conditioning species showed no effect in the overall analysis. At the level of individual exotic species, however, the identity of conditioning species influenced exotics’ growth. As discussed above, the performance of S. annua was improved by the congeneric E. acer. On the contrary, plants of E. ciliatum, performed worst when the soil was conditioned by the congeneric E. hirsutum. The response of I. parviflora was not influenced by conditioning species. Large variations of exotic species and their limited number, as well as a low number of conditioning species (see discussion above) precludes us from making a robust conclusion on the role of relatedness of native species on plant-soil feedback of exotics.
Nevertheless, significant differences of conditioning species on E. ciliatum and S. annua performance may indicate different levels of success of these exotics across invaded communities. Aside from differences in direct competition effects, variation in native species composition may change the proportions of soil mutualistic and pathogenic microorganisms and influence the performance of exotics indirectly. For I. parviflora, we found no variation in the feedback to stimulating species, and therefore, its success probably depends less on the native plant composition of the colonised communities. The question is then whether this finding can be linked with the fact that I. parviflora has become one the most widespread exotic species in Central Europe as documented by Sádlo et al. (2007).
References
Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W, Klironomos J (2005) Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology 86:2979–2989
Becerra JX, Venable DL (1999) Macroevolution of insect-plant associations: the relevance of host biogeography to host affiliation. Proc Natl Acad Sci USA 96:12626–12631
Brandt AJ, Seabloom EW, Hosseini PR (2009) Phylogeny and provenance affect plant–soil feedbacks in invaded California grasslands. Ecology 90:1063–1072
Brown BJ, Mitchell RJ, Graham SA (2002) Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology 83:2328–2336
Cadotte MW, Cardinale BJ, Oakley TH (2008) Evolutionary history and the effect of biodiversity on plant productivity. Proc Natl Acad Sci USA 105:17012–17017
Cahill JF, Kembel SW, Lamb EG, Keddy PA (2008) Does phylogenetic relatedness influence the strength of competition among vascular plants? Perspect Plant Ecol Evol Syst 10:41–50
Callaway RM, Thelen G, Rodriguez A, Holben WE (2004) Soil biota and exotic plant invasion. Nature 427:731–733
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forests. In: den Boer PJ, Gradwell GR (eds) Dynamics in populations. Center for Agricultural Publishing and Documentation, Wageningen, The Netherlands, pp 298–312
Darwin C (1859) On the origin of species by means of natural selection. Murray, London
Dawson W, Burslem DFRP, Hulme PE (2009) Herbivory is related to taxonomic isolation, but not to invasiveness of tropical alien plants. Divers Distrib 15:141–147
Diez JM, Sullivan JJ, Hulme PE, Edwards G, Duncan RP (2008) Darwin’s naturalization conundrum: dissecting taxonomic patterns of species invasions. Ecol Lett 11:674–681
Duncan RP, Williams PA (2002) Darwin’s naturalization hypothesis challenged. Nature 417:608–609
Gilbert GS, Webb CO (2007) Phylogenetic signal in plant pathogen-host range. Proc Nat Acad Sci USA 104:4979–4983
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn 347:1–32
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Kulmatiski A, Beard KH, Stevens JR, Cobbold SM (2008) Plant–soil feedbacks: a meta-analytical review. Ecol Lett 11:980–992
Lambdon PW, Hulme PE (2006) How strongly do interactions with closely-related native species influence plant invasions? Darwin’s naturalization hypothesis assessed on Mediterranean islands. J Biogeogr 33:1116–1125
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Mack RN (1996) Biotic barriers to plant naturalization. In: Moran VC, Hoffman JH (eds) Proceedings of the IX international symposium on biological control of weeds. University of Cape Town, South Africa, pp 39–46
MacKay J, Kotanen PM (2008) Local escape of an invasive plant, common ragweed (Ambrosia artemisiifolia L.), from above-ground and below-ground enemies in its native area. J Ecol 96:1152–1161
McNamara NP, Black HIJ, Beresford NA, Parekh NR (2003) Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl Soil Ecol 24:117–132
Novotný V, Drozd P, Miller SE, Kulan M, Janda M, Basset Y, Weiblen GD (2006) Why are there so many species of herbivorous insects in tropical rainforests? Science 313:1115–1118
Packer A, Clay K (2000) Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404:278–281
Pyšek P, Sádlo J, Mandák B (2002) Catalogue of alien plants of the Czech Republic. Preslia 74:97–186
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytol 170:445–457
Sádlo F, Chytrý M, Pyšek P (2007) Regional species pools of vascular plants in habitats of the Czech Republic. Preslia 79:303–321
Strauss SY, Webb CO, Salamin N (2006) Exotic taxa less related to native species are more invasive. Proc Nat Acad Sci USA 103:5841–5845
Troelstra SR, Wagenaar R, Smant W, Peters BAM (2001) Interpretation of bioassays in the study of interactions between soil organisms and plants: involvement of nutrient factors. New Phytol 150:697–706
van der Putten WH, Van Dijk C, Peters BAM (1993) Plant-specific soil-borne diseases contribute to succession in foredune communities. Nature 362:53–56
van Grunsven RHA, van der Putten WH, Bezemer TM, Tamis WLM, Berendse F, Veenendaal EM (2007) Reduced plant-soil feedback of plant species expanding their range as compared to natives. J Ecol 95:1050–1057
Webb CO, Gilbert GS, Donoghue MJ (2006) Phylodiversity-dependent seedling mortality, size structure, and disease in a Bornean rain forest. Ecology 87:S123–S131
Acknowledgments
We are grateful to Jana Trilčová and Věra Rydlová for their technical assistance in the greenhouse. This study was supported by grant GAAVČR no. KJB600050713, and partly by grant AVČR no. AV0Z60050516.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dostál, P., Palečková, M. Does relatedness of natives used for soil conditioning influence plant-soil feedback of exotics?. Biol Invasions 13, 331–340 (2011). https://doi.org/10.1007/s10530-010-9824-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9824-6