Abstract
We examined the effects of leaf herbivory by the dorcas gazelle, Gazella dorcas, on the compensatory growth of the geophyte Pancratium sickenbergeri (Amaryllidaceae) in the Negev desert, Israel. In three populations exposed to different levels of herbivory, we removed different amounts of photosynthetic leaf area from plants in five clipping treatments: 0, 25, 50%-dispersed over all leaves, 50%-entire area of half the leaves, 100%. The population with the lowest level of herbivory showed the lowest relative regrowth rate after clipping. In the population with a constantly high level of herbivory, plants in intermediate-clipping treatments overcompensated in leaf area after clipping. For all the populations, clipped plants produce more new leaves than unclipped plants. In the population with the highest level of herbivory, clipping treatments did not have a significant effect on the number of fruits per plant. In addition, we did not find a trade-off between investments in growth and reproduction in this population. Our results indicated that, in the desert lily, herbivores may select for plant mechanisms that compensate after damage as a tolerant strategy to maintain fitness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Above a certain threshold level of damage, plants have the ability to compensate at least partially for herbivory (Hendrix 1988; van der Meijden et al. 1988; Herms and Mattson 1992; Mauricio et al. 1993; van der Heyden and Stock 1996). The net effect of single or repeated grazing events on the cumulative growth of plants may be zero, negative, or positive, depending on the availability of leaf area, meristems, stored nutrients, and soil resources, and on the frequency and intensity of defoliation (Kulman 1971; Lee and Bazzaz 1980; Marquis 1984; Crawley 1985; Noy-Meir 1993). The ability to tolerate damage and then regrow requires a combination of normal growth processes, specialized structures, and physiological traits (Belsky 1986; Wandera et al. 1992). These structures and traits (such as thorns, tannins, and growth responses) have many functions, but they may have evolved, at least in part, in response to recurrent damage by herbivory.
Compensatory growth responses of plants after herbivore damage can alleviate the potential deleterious effects of herbivory (McNaughton 1983). However, whether this regrowth capacity (in the way of overcompensation) has a positive impact on fitness or not, and if it evolved as an antiherbivory strategy is a more controversial issue (McNaughton 1983; Belsky et al. 1993). Several studies assert that plants can benefit from being eaten because they respond by overcompensation, ultimately achieving greater fitness (McNaughton 1979a; Inouye 1982; Paige and Whitham 1987; Paige 1999). These researchers suggest that overcompensation may be an adaptation to a predictable risk of herbivory (McNaughton 1979b, 1986; Crawley 1987). This does not mean that herbivory maximizes plant fitness, but rather that the plant has the capacity to compensate for herbivory and may, at low levels of herbivory, overcompensate for damage so that fitness increases in comparison with ungrazed levels (McNaughton 1983). In contrast, other studies affirm that even though herbivores may benefit plants by reducing competition or removing senescent tissue, there is insufficient evidence for the theory that herbivory increases fitness of grazed plants (Belsky 1986; Veerkaar 1988). Regrowth is a generalized response by plants to all types of damage and is not merely an adaptation to herbivory (Belsky et al. 1993; Jaremo et al. 1996). There is no evidence or evolutionary mechanism to justify overcompensation caused by herbivory in this view (Belsky 1986; Verkaar 1988; Painter and Belsky 1993). If rapid regrowth following damage is an evolved response, it is more parsimonious to interpret it as a response to reduce the negative impact of damage (by recouping lost photosynthetic tissue) than as a strategy to increase fitness above ungrazed levels (Belsky et al. 1993).
The desert lily Pancratium sickenbergeri (Amaryllidaceae) is a geophyte commonly found in sand dunes of the Negev Desert. The dorcas gazelle (Gazelle dorcas) is the major herbivore on this lily (Ward and Saltz 1994; Saltz and Ward 2000) and may exert strong selection on the development of antiherbivore strategies. The desert lily appears to use a combination of defense mechanisms consisting of both avoidance and tolerance, depending on the phenological stage that is being eaten (Saltz and Ward 2000). The following defense mechanisms have been recorded: (1) Lilies that have deeper bulbs have more bulb remaining after herbivory, suggesting that there is selection pressure for downward growth. Lilies in areas of no herbivory do not grow as deep into the substrate as lilies in populations where there is herbivory (Ward and Saltz 1994). Growing deeper into the soil to minimize the effects of herbivory by gazelles in the summer months when they have no exposed supra-surface parts is an avoidance strategy. (2) Production of crystals of calcium oxalate in their leaves in the winter months is a resistance strategy (Ward et al. 1997, Ruiz et al. 2002a). (3) Compensatory growth of bulb, leaves, and flowers from the basal meristem after gazelle herbivory may be interpreted as a form of tolerance (Saltz and Ward 2000; Ruiz et al. 2002b, 2006a, b).
In this study, we wished to determine if the desert lily has evolved a compensatory growth strategy after damage as a tolerant strategy to minimize the effects of herbivory on fitness. Two specific questions are addressed: (1) Can the plants grow more (compensation) when different amounts of leaf tissue are removed? (2) What is the effect of this growth on fitness?
The desert lily possesses at least two traits that facilitate rapid regrowth: a storage organ and a dormant meristem in the basal part of the leaf (Belsky 1986; Tuomi et al. 1994), which suggests that this species has a potential capacity for compensation in response to herbivory (McNaughton 1983). However, if the plant grows in a low-resource environment, the capacity to regrow may be limited. Therefore, with increasing level of herbivory, the capacity for regrowth should decrease in plants from populations with low herbivory (genotype A). In populations with high herbivory (genotype B), the capacity for regrowth may remain unchanged or may increase as a result of selection by herbivory (Ruiz et al. 2006b). Consequently, fitness decreases for genotype A because the plant cannot replace the lost biomass, and this loss has a large cost that could affect the resources allocated to future reproduction. For genotype B, fitness may be constant under various levels of herbivory. However, if we assume that regrowth capacity has a cost, there should be a slight decline in fitness (although this will be a lower decline than for genotype A).
Material and methods
Study area
The study was conducted in different populations in two erosion cirques in the Negev Desert of Israel, Makhtesh Katan and Makhtesh Ramon. Makhtesh Katan is a small (30.6 km2), oval-shaped cirque surrounded by steep walls. This area is characterized by an arid to extremely arid climate, vegetation cover is scarce and confined to the stream channels. Makhtesh Ramon is an anticlinal 200 km2 erosion cirque on the southern boundary of the Negev highlands (see Saltz et al. 1999). Makhtesh Ramon and Makhtesh Katan are typified by low rainfall (40–90 mm per year) and a variety of soil substrates. The strong environmental changes (rainfall and temperature) are reflected in the vegetation (Ward et al. 1993; Ward and Olsvig-Whittaker 1993; Saltz et al. 1999).
In Makhtesh Ramon, we concentrated this study in Machmal valley. The predominantly western winds have created large sand deposits along the eastern walls of this valley (Ward et al. 1997). Loose (soft) sands can support dense populations of lilies, up to 2 m−2 and they are attraction points for the dorcas gazelles (Ward and Saltz 1994; Saltz and Ward 2000). Away from the dunes, the sands are more compact and mixed with loess. Compact sand habitats occur in the lower regions on the peripheries of the loose sand of Machmal valley (Ward and Saltz 1994). These compact sands are marginal habitats because of high water runoff, and characterized by lower densities (approx. 0.1 m−2) of smaller lilies with shallower bulbs, and fewer leaves and flowers (Saltz and Ward 2000; Ruiz et al. 2006a). We can classify the different lily populations into those enduring high and low herbivory with a high degree of certainty based on 10 years of observations (see Ward and Saltz 1994; Ward et al. 1997; Saltz and Ward 2000). Machmal dune lilies suffer a higher level of herbivory than Machmal compact sand. In Makhtesh Katan, the population of lilies grows in a small sandy valley and is exposed to a very low level of herbivory.
Study system
In the desert lily, leaves appear on the surface after the winter rains in late November and December (i.e., after the flowers have wilted) and may remain green until late spring, depending on rainfall and temperature. In spring, all the leaves dry up and fall off, leaving no aboveground material (Saltz and Ward 2000). During winter, gazelles eat the leaf tip (±1 cm) of the desert lily, and up to 100% of the plants may be affected. The tip is the only part of the leaf not defended by needle-like raphides of calcium oxalate (Ward et al. 1997). The behavioral avoidance by the gazelle of calcium oxalate in the leaves of Pancratium sickenbergeri suggests that the chemical is an effective deterrent to this herbivore (Ward and Saltz 1994; Ward et al. 1997; Saltz and Ward 2000; Ruiz et al. 2002a). Lilies in habitats with high levels of herbivory produce more calcium oxalate crystals in their leaves than plants in populations with little herbivory (Ward et al. 1997; Ruiz et al. 2002a). Lily leaves have a basal meristem (Bold et al. 1987) and, therefore, the growing point of the leaves is not affected by gazelle herbivory (Ward and Saltz 1994). In the summer, gazelles dig for underground parts of lilies and may consume all or part of the bulb, which contains most of the plant’s volume (Ward and Saltz 1994). During this time, 50–88% of the lilies have their underground parts partially consumed and up to 5–10% are completely consumed (i.e., the bulb is removed). The greatest impact the gazelles have on the lily populations in sand dunes is the consumption of flowers. A lily flower on this sand dune has less than a 0.0001 probability of surviving to the seed-producing stage (Saltz and Ward 2000).
The dorcas gazelle is a small herbivore native to the deserts of southern Israel and the Middle East (Lawes and Nanni 1993). Gazelles show a number of behavioral characteristics that are consistent with a long period of coadaptation with the lily. They concentrate their activity in areas of high lily density, take the biggest plants with the most leaves in the winter, avoid those parts of the leaf defended by calcium oxalate, and in the summer dig for those plants that maximize the benefit:cost ratio of foraging (Ward and Saltz 1994; Ward et al. 2000). Gazelles prefer to feed on the lilies in loose (soft) sands. As sand compaction increases, the proportion of lilies that is dug up by gazelles decreases (Ward and Saltz 1994). Gazelles also dig deeper in the loose (soft) sand, removing more of the bulb of each plant. In addition, in the compacted-sand areas, the probability of a flowering lily reaching the seed-producing stage is 0.026, which is considerably higher than in the loose sands (1:30,000—Saltz and Ward 2000; Ruiz et al. 2006c). Thus, although compact sands provide a poorer growing substrate, the level of herbivory is less. The differences in gazelle foraging behavior among substrate types are presumably due to a higher energetic cost of digging in compact sands and/or to the lower density of lilies.
Clipping experiment
To determine the regrowth capacity of the plant in response to leaf herbivory, we conducted a field clipping experiment during the winter of 1998 in three populations with different levels of herbivory: one in Makhtesh Katan and two in Makhtesh Ramon (Machmal soft sand and Machmal hard sand). The former population has the lowest level of herbivory while Machmal hard sand has a low level and Machmal soft sand has the highest level of herbivory. For this experiment, we selected 50 adult plants for each of the following leaf clipping treatments of available photosynthetic area: (1) 0% (controls), (2) 25% (removal of the tips of the leaves), (3) 50%-dispersed (we removed half of each leaf), (4) 50%-concentrated (we entirely removed half of the leaves of the plant), (5) 100% (removal of all the leaves). Treatment (2) simulated natural herbivory. In (3) and (4) the same amount of photosynthetic area was removed but in a distinct spatial pattern that can affect the allocation of resources in a different way. Our prediction regarding this effect would be that, in unbranched plants such as these, there would be no significant difference because the degree of vascular integration should be high (Watson and Casper 1984). Treatment (5) was an extreme treatment to assess the total potential for regrowth. All plants were clipped in December. No plants had been eaten prior to clipping. The plants were not protected from consumption. We remeasured the length of the leaves one month after removal and determined the Relative Growth Rate (RGR) per month.
Statistical analyses
We recorded Relative Growth Rate (RGR) as follows:
where L0 is the initial length of the leaves (cm) per plant and L1 = final length of the leaves per plant after one month. Additionally, we counted the number of new leaves that were produced per plant. After testing for normality, we log10-transformed the data. We analyzed the data for RGR using an analysis of covariance (ANCOVA), with leaf width as a covariate indicating plant age (developmental effect; Saltz and Ward 2000). Also, we used an analysis of variance (ANCOVA) to determine the effects of clipping and RGR on the production of new leaves per plant. To check the cost of regrowth and reproduction, we determined the effects of leaf removal and RGR on production of fruits, in the following fall. We ran ANOVA and a linear regression model to analyze the data, as well as appropriate Scheffe post hoc tests, using SYSTAT 7 (Wilkinson 1997).
Results
There was a significant difference among the clipping treatments in the growth response of the leaves. The analysis of covariance for log10 RGR, with log10 leaf width as a covariate, showed a significant effect of population, treatment, and a significant interaction between these two factors (Table 1). In the population with the lowest level of herbivory (Makhtesh Katan), RGR was lowest, and the plants overcompensated only at the lowest level of clipping (25%) (Fig. 1). In the population with an intermediate level of herbivory, which grows in hard sand (Machmal hard), RGR was highest. In the population with the highest level of herbivory, the population in soft sand (Machmal soft), RGR was lower, but the plants overcompensated at low and intermediate damage levels (25 and 50%-concentrated) (Fig. 1).
Mean ± S.E. log Relative Growth Rate (RGR) of leaves of experimentally clipped plants in three populations with different levels of herbivory. Hard = hard sand, Soft = soft sand. Machmal soft has a high level of gazelle herbivory, Machmal compact has an intermediate to low level of herbivory, and Katan has a very low level of herbivory. RGR is based on elongation measurements
In addition, we found that there were differences in the production of new leaves per plant in response to clipping. The analysis of covariance for the number of new leaves showed a significant effect of treatment and log10 RGR, and a significant interaction between these two factors, but there was no significant effect of population (Table 2). For all the populations, the plants that were clipped produced more new leaves than the control treatment (Fig. 2).
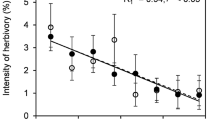
In terms of the costs in current fitness, the ANCOVA showed that in the population with the highest level of herbivory (Machmal soft) there was no significant effect of clipping treatments on the number of fruits per plant (F = 1.12; P = 0.34), but there was a significant effect of the Relative Growth Rate (RGR) on the number of fruits per plant (F = 7.23; P = 0.008). Moreover, there was a weak positive relationship between log10 RGR and number of fruits (r = 0.31; F = 4.08; P = 0.05; df = 38) (Fig. 3), suggesting that there is no trade-off between investment in growth and investment in reproduction.
Discussion
Overcompensation is more easily explained as normal plant growth that occurs under highly favorable environmental conditions than as a special heritable trait or suite of traits associated with herbivory (Belsky et al. 1993). However, in the desert lily, which grows in environments with limited resources, we found that the plant can compensate or overcompensate in biomass at certain levels of clipping (Ruiz et al. 2002a, b). Moreover, responses to herbivory were in accordance with the level of herbivory experienced in each population. That is, lilies in the population that experiences the lowest level of leaf herbivory showed the lowest regrowth capacity, although lilies experiencing an intermediate level of herbivory (Machmal hard) had a higher level of regrowth than lilies experiencing the highest level of herbivory (Machmal soft). The reason for the last-mentioned difference is not well understood although it may be related to higher soil water retention.
In this study, the population of lilies growing in compact sand showed a higher relative growth rate (RGR) than the population in soft sand, presumably because plants in compact sand retain water longer into the growing season. This is the same pattern shown by Saltz and Ward (2000) and Ruiz et al. (2006b). We found that the greatest regrowth response was the extreme treatment (100%), which is similar to the results found by van der Heyden and Stock (1996) for a shrub Osteospermum sinuatum in a semi-arid region where compensatory mechanisms were more evident at intense browsing levels. For example, in van der Heyden and Stock’s (1996) study, these mechanisms included improved plant water balance and more efficient measures of carbon acquisition and nitrogen allocation. Thus, given the longer availability of water in the soil, lilies in compact sand may have more efficient physiological mechanisms and higher potential for growth and for replacement of the photosynthetic area after damage than lilies in soft sand.
Additionally, the production of new leaves in the desert lily was higher in clipped treatments than in the control. However, we did not find significant differences between populations. If the functional lifespan of the leaves is short, as in the desert lily (and in other hysteranthous geophytes—Dafni et al. 1981), there are repeated annual costs of producing new leaves that affect the carbon budget of the plant (Larcher 1975). Thus, production of new leaves may be mainly a response following damage that involves different patterns of allocation of resources and activation of dormant buds. According to Tuomi et al. (1994), selection for bud dormancy requires both that the risk of herbivory is high and that herbivores remove a large fraction of active meristems per plant. In the desert lily, the second condition is not met, because the gazelles do not remove the basal meristem. Hence, herbivores may not exert strong pressure on this trait.
We found a significant effect of dispersion of clipping on the production of new leaves. Leaves that were clipped by 50% on half the leaves (concentrated) showed a greater negative effect than clipping by 50% on all the leaves (dispersed). The distribution of vascular connections can affect the distribution of nutrients, sugars, wound-induced signals, hormones, and other chemicals (Watson and Casper 1984). A number of authors have examined this effect and have found that concentrated effects are more detrimental than dispersed effects (Lowman 1982; Marshall 1989; Marquis 1992; Price and Hutchings 1992; Mauricio et al. 1993), although some others have shown no clear effect (e.g., Garrish and Lee 1989; Shea and Watson 1989; Avila-Sakar et al. 2003; Avila-Sakar and Stephenson 2006). Where an effect has been found, it has been ascribed to sectoriality. That is, the branching patterns of the plant facilitate the degree of integration that occurs (Watson and Casper 1984). Since our plants are geophytes, no pattern of response would be predicted because there are no branches and so there should be a high degree of vascular integration and resource sharing, yet we found a highly significant difference between dispersed and concentrated clipping. The reason for this difference is not known.
We did not find a significant negative effect of clipping on the production of fruits (the relationship was positive). In other words, plants that were clipped did not produce more fruits than the control; they did not overcompensate in number of fruits or show greater fitness (see Ward and Saltz 1994 and Saltz and Ward 2000 for explanation). Thus, this result contradicts the idea of “herbivore optimization” (Owen and Wiegert 1976; Owen 1980; McNaughton 1979a, b, 1983; Paige and Whitham 1987; Paige 1992; Vail 1992), but it is in agreement with the idea that plant regrowth is a response to reduce the negative impact of damage rather than a strategy to increase fitness above ungrazed levels (Belsky et al. 1993).
If regrowth capacity is a tolerant strategy, selection is expected to act against tolerance at reduced levels of herbivory and favor tolerance at elevated levels of herbivory (Tiffin and Rausher 1999). The high level of tolerance to defoliation shown by the desert lily in a harsh environment can in part be interpreted as having been selected for by a history of intense herbivore pressure.
References
Avila-Sakar G, Leist LL, Stephenson AG (2003) Effects of the spatial pattern of leaf damage on growth and reproduction: nodes and branches. J Ecol 91:867–879
Avila-Sakar G, Stephenson AG (2006) Effects of the spatial pattern of leaf damage on growth and reproduction: whole plants. Int J Plant Sci 167:1021–1028
Belsky AJ (1986) Does herbivory benefit plants? A review of the evidence. Am Nat 127:870–892
Belsky AJ, Carson WP, Jensen CL, Fox GA (1993) Overcompensation by plants: herbivore optimization or red herring? Evol Ecol 7:109–121
Bold H, Alexopoulos CJ, Delevoryas T (1987) Morphology of plants and fungi. Harper & Row, New York
Crawley MJ (1985) Reduction of oak fecundity by low density herbivore populations. Nature 314:163–164
Crawley MJ (1987) Benevolent herbivores? Trends Ecol Evol 2:167–168
Dafni A, Shmida A, Avishai M (1981) Leafless autumnal-flowering geophytes in the Mediterranean region—Phytogeographical, ecological and evolutionary aspects. Plant Syst Evol 137:181–193
Garrish RS, Lee TD (1989) Physiological integration in Cassia fasciculata Michx. inflorescence removal and defoliation experiments. Oecologia 81:279–284
Hendrix SD (1988) Herbivory and its impact on plant reproduction. In: Lovett J, Lovett L (eds) Plant reproductive ecology: patterns and strategies. Oxford Univ. Press, Oxford, pp 246–263
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Inouye DW (1982) The consequences of herbivory: a mixed blessing for Jurines mollis (Asteraceae). Oikos 39:269–272
Jaremo J, Nilsson P, Tuomi J (1996) Plant compensatory growth: herbivory or competition? Oikos 77:238–247
Kulman HM (1971) Effects of insect defoliation on growth and mortality of trees. Annu Rev Entomol 16:289–324
Larcher W (1975) Physiological plant ecology. Springer-Verlag, Berlin
Lawes MJ, Nanni RF (1993) The density, habitat use and social organization of the dorcas gazelles (Gazella dorcas) in the Makhtesh Ramon, Negev desert, Israel. J Arid Environ 24:177–196
Lee TD, Bazzaz FA (1980) Effects of defoliation and competition on growth and reproduction in the annual plant Abutilon theophrasti. J Ecol 68:813–821
Lowman MD (1982) Effects of different rates and methods of leaf area removal on rain forest seedlings of coachwood. Austr J Bot 30:477–483
Marquis RJ (1984) Leaf herbivores decrease fitness of a tropical plant. Science 226:537–539
Marquis RJ (1992) A bite is a bite is a bite? Constraints on response to folivory in Piper arieianum (Piperaceae). Ecology 73:143–152
Marshall DL (1989) Integration of response to defoliation within plants of two species of Sesbania. Funct Ecol 3:207–214
Mauricio R, Bowers MD, Bazzaz FA (1993) Pattern of leaf damage affects fitness of the annual plant Raphanus sativus (Brassicaceae). Ecology 74:2066–2071
McNaughton SJ (1979a) Grazing as optimization process: grass-ungulate relationships in the Serengeti. Am Nat 113:691–703
McNaughton SJ (1979b) Grassland herbivore dynamics. In: Sinclair ARE, Norton-Griffiths M (eds) Serengeti: dynamics of an ecosystems. Chicago Univ. Press, Chicago, pp 46–81
McNaughton SJ (1983) Compensatory plant growth as a response to herbivory. Oikos 40:329–336
McNaughton SJ (1986) On plants and herbivores. Am Nat 128:765–770
Noy-Meir I (1993) Compensating growth of grazed plants and its relevance to the use of rangelands. Ecol Appl 3:32–34
Owen DF (1980) How plants may benefit from the animals that eat them. Oikos 35:230–235
Owen DF, Wiegert RG (1976) Do consumers maximizes plant fitness? Oikos 27:488–492
Paige KN (1992) Overcompensation in response to mammalian herbivory: from mutualistic to antagonistic interaction. Ecology 73:2076–2085
Paige KN (1999) Regrowth following ungulate herbivory in Ipomopsis aggregata: geographic evidence for overcompensation. Oecologia 118:316–323
Paige KN, Whitham TG (1987) Overcompensation in response to mammalian herbivory: the advantage of being eaten. Am Nat 129:407–416
Painter EL, Belsky AJ (1993) Application of herbivore optimization theory to rangelands of the western United States. Ecol Appl 3:2–9
Price EAC, Hutchings MJ (1992) Studies of growth in the clonal herb Glechoma hederacea. II. The effects of selective defoliation. J Ecol 80:39–47
Ruiz N, Ward D, Saltz D (2002a) Crystals of calcium oxalate in leaves: constitutive or induced defense? Funct Ecol 16:99–105
Ruiz N, Ward D, Saltz D (2002b) Responses of Pancratium sickenbergeri to simulated bulb herbivory: combining defense and tolerance strategies. J Ecol 90:472–479
Ruiz N, Saltz D, Ward D (2006a) The effects of herbivory and resource variability on the production of a second inflorescence by the desert lily, Pancratium sickenbergeri. Plant Ecol 186:47–55
Ruiz N, Ward D, Saltz D (2006b) Population differentiation and the effects of herbivory and sand compaction on the subterranean growth of a desert lily. J Hered 97:409–416
Ruiz N, Saltz D, Ward D (2006c) Signal selection in a desert lily Pancratium sickenbergeri. Evol Ecol Res 8:1461–1474
Saltz D, Ward D (2000) Responding to a three-pronged attack: desert lilies subject to herbivory by dorcas gazelles. Plant Ecol 148:127–138
Saltz D, Schmidt H, Rowen M, Karnieli A, Ward D, Schmidt I (1999) Assessing grazing impacts by remote sensing in hyper-arid environments. J Range Manage 52:500–507
Shea MM, Watson MA (1989) Patterns of leaf and flower removal: their effect on fruit growth in Chamaenerion angustifolium (fireweed). Amer J Bot 76:884–890
Tiffin P, Rausher MD (1999) Genetic constraints and selection acting on tolerance to herbivory in the common morning glory Ipomoea purpurea. Am Nat 154:700–716
Tuomi J, Nilsson P, Astrom M (1994) Plant compensatory responses-bud dormancy as an adaptation to herbivory. Ecology 75:1429–1436
Vail SG (1992) Selection for overcompensatory plant responses to herbivory: a mechanism for the evolution of plant-herbivore mutualism. Am Nat 139:1–8
Van Der Heyden F, Stock WD (1996) Regrowth of a semiarid shrub following simulated browsing: the role of reserve carbon. Funct Ecol 10:647–653
Van Der Meijden E, Wijn M, Verkaar HJ (1988) Defence and regrowth, alternative plant strategies in the struggle against herbivores. Oikos 51:355–363
Verkaar HJ (1988) Are defoliators beneficial for their host plant in terrestrial ecosystems—a review? Acta Bot Neerl 37:137–152
Wandera JL, Richards JH, Muller RJ (1992) The relationship between relative growth rate, meristematic potential and compensatory growth of semiarid-land shrubs. Oecologia 90:391–398
Ward D, Olsvig-Whittaker L (1993) Plant species diversity at the junction of two desert biogeographic zones. Biodivers Lett 1:172–185
Ward D, Saltz D (1994) Foraging at different spatial scales: dorcas gazelles foraging for lilies in the Negev desert. Ecology 75:48–58
Ward D, Olsvig-Whittaker L, Lawes MJ (1993) Vegetation-environment relationships in a Negev desert erosion cirque. J Veg Sci 4:83–94
Ward D, Spiegel M, Saltz D (1997) Gazelle herbivory and interpopulation differences in calcium oxalate content of leaves of a desert lily. J Chem Ecol 23:333–346
Ward D, Saltz D, Olsvig-Whittaker L (2000) Distinguishing signal from noise: long-term studies of vegetation in Makhtesh Ramon erosion cirque, Negev desert, Israel. Plant Ecol 150:27–36
Watson MA, Casper BB (1984) Morphogenetic constraints on patterns of carbon distribution in plants. Ann Rev Ecol Syst 15:233–258
Wilkinson L (1997) Systat: the system for statistics. SYSTAT, Inc., Evanson, IL
Acknowledgments
We thank Iris Musli, Ofrit Gilan, and Betina Berendock for assistance in the field. We thank the anonymous reviewers for their insights. This study was supported by the Israel Science Foundation. This is publication number 587 of the Mitrani Department for Desert Ecology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz-R, N., Ward, D. & Saltz, D. Leaf compensatory growth as a tolerance strategy to resist herbivory in Pancratium sickenbergeri . Plant Ecol 198, 19–26 (2008). https://doi.org/10.1007/s11258-007-9381-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-007-9381-y