Abstract
Both invasive alien trees and agricultural conversion have major impacts on biodiversity. We studied here the comparative impact of these two types of land transformation on a wide range of surface-active arthropod species using pitfall traps, with evergreen sclerophyllous natural vegetation (fynbos) as the control. The study was in the Cape Floristic Region, a global biodiversity hotspot, where alien trees are of major concern and where vineyards replace natural fynbos vegetation. Surface-active arthropods were selected as they are species rich, relatively immobile, and occur in high abundance. We hypothesized that the impact of the two types of land cover transformation would produce similar qualitative and quantitative effects on the arthropods. We also compared the results in the transformed and natural areas with those in areas cleared of alien trees. Arthropod species richness in cleared areas was higher than in vineyards and more similar to that in natural fynbos, while alien trees had the lowest. Overall abundance scores were highest in cleared areas, closely followed by fynbos, then vineyards and lowest in alien trees. Several species were restricted to each vegetation type, including alien trees. In terms of assemblage composition, all vegetation types were significantly different, although fynbos and vineyards grouped, suggesting that vineyards have less impact on the arthropod community than do alien trees. When rare species were excluded, vineyards and cleared sites grouped, indicating some recovery but only involving those species that were common and habitat tolerant. Our results suggest that vineyards retain a greater complement of indigenous species than alien trees, but that clearing of these aliens soon encourages establishment of indigenous species. Although there were significant differences in soil moisture and litter depth within and between vegetation types, we did not record them as significantly affecting species richness or abundance, even in alien vegetation, an encouraging sign for restoration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasion by alien tree species is a global environmental problem (Mack et al. 2000; Richardson and Pyšek 2006), affecting movement patterns of animals, including insects (Wood and Samways 1991), and threatening their habitats (Armstrong and van Hensbergen 1996; Samways et al. 1996; Richardson and van Wilgen 2004; Samways and Taylor 2004). However, different ecosystems vary considerably in their susceptibility to invasion (Chytrý et al. 2008), with the impacts of alien tree species in natural systems being dependent on invader attributes and on characteristics of the invaded community (Mason and French, 2008). Invasive alien trees (IATs) are widespread in the Cape Floristic Region (CFR), South Africa, and their success attributed to their good colonizing abilities, especially in disturbed areas (Holmes and Richardson 1999). In many parts of the world, alien trees are the main component of commercial forestry, with these trees often spreading from planting sites (Simberloff et al. 2010). IATs such as Pinus, Acacia and Eucalyptus species are of major commercial importance in South Africa, but they are also a threat to water supplies and biodiversity (Wittenberg and Cock 2001; Le Maitre et al. 2004), especially in the CFR (Macdonald and Richardson 1986).
Another impact on natural systems is conversion to agriculture, which changes ecosystem composition and function (Donald and Evans 2006), and its biodiversity (Turin and den Boer 1988; Newton 2004; Gaigher and Samways 2010). Agriculture is one of the most significant human-induced disturbances that threatens terrestrial biodiversity (Sala et al. 2000; Tilman et al. 2001), affecting the availability of suitable terrestrial habitats (Feber et al. 1996; Warren et al. 1997; Jeanneret et al. 2003; Kleijn and van Langevelde 2006). For example, vegetation structure is important to some arthropods because it can affect their ability to thermoregulate and reproduce (Holl 1996). However, vegetation loss for agricultural purposes does not threaten all arthropods equally (Fleishman et al. 1999), with some relatively small-scale agriculture maintaining open, early seral biotopes favoured by some arthropod species (Shreeve and Mason 1980; Sibatani 1980).
The regional focus for this study is the CFR, a global biodiversity hotspot (Mittermeier et al. 2005) and a world centre of plant diversity and endemism (Linder 2005; Procheş and Cowling 2006), with 67% of plant species endemic to the region (Linder 2005). It is also an important area for many rare and endemic arthropod species (Johnson 1992; Picker and Samways 1996; Wright and Samways 1998; Giliomee 2003; Procheş and Cowling 2006; Procheş et al. 2009). Of significance here is that both invasive alien trees (IATs) and agriculture, especially vineyards, have an impact on this biodiversity (Rouget et al. 2003). Yet there is little knowledge on the comparative impact, or footprint, of these two types of human-induced land transformations on this biodiversity, so we investigate here the comparative impact of IATs and vineyards on soil-surface arthropod diversity, and compare it with patches where IATs had been removed. We chose this group of arthropods as it is species-rich, occurs in high abundance, and most species are relatively immobile (therefore allowing spatially-explicit interpretation of the arthropod data). We hypothesized that the quantitative and qualitative adverse footprint in its entirety (interior and edge) of IATs and vineyards on arthropod diversity is the same, as they are both, at least to the human eye, major transformations of landscape matrix at the spatial scale of the patch.
Study area and methods
Study sites
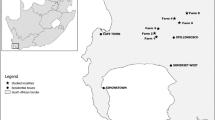
Sampling was in three nature reserves and seven wine estates within the CFR (Table 1). At each of these ten localities, transects were established, so that the focal land cover/land use types (hereafter referred to as ‘vegetation type’) were adjacent to each other. In total, there were 36 transects, each 256 m in length. Half of the transect, i.e. 128 m, was on either side of the boundary of land use/land cover types, with the exception of two transects (128 m in length) that were established across native vegetation and small IAT fragments (Table 1). Vegetation type was in four categories: natural fynbos (evergreen schlerophyllous shrublands characterized by graminoids of the Restionaceae, and shrubs of the Ericaceae and Proteaceae), IATs, cleared of invasive alien trees (CIATs), and vineyards. These resulted in six different pairs of vegetation types (Table 1).
Natural fynbos was relatively untransformed by human activity and selected from the nature reserve and the wine farms with less than 10% alien tree vegetation. Natural fynbos was predominantly mountain fynbos, with common plant species being geophytes Watsonia borbonica, Cyphia phyteuma and Chasmanthe aethiopica; herbs Gymnodiscus capillaris and Dimorphotheca pluvialis; shrubs Aspalathus forbesii, A. aspalathoides, Lebeckia sepiaria, Lotononis prostrata, Hymenolepis crithmoides, Protea compacta, P. repens, P. neriifolia, and Salix species, as well as various ericas. IATs was considered a vegetation type with more than 90% alien trees, mainly Acacia mearnsii, A. longifolia, A. saligna, Hakea sericea, H. drupacea, Pinus Pinaster, P. radiata, Eucalyptus lehmannii, E. diversicolor and Populus trees, with an understorey of grasses and forbs. Since 2000, farmers together with government authorities started clearing these invasive alien trees, with follow-up clearing undertaken at least every 3 years. Physical or manual and chemical control methods were applied during IAT clearing. In essence, IATs were cut and herbicides applied to the remaining mainstem. Only organic vineyards were considered. Organic vineyard management involves no application of artificial fertilizers as the soils are relatively fertile through permanent cover crop (i.e. wheat), and only apply pesticides when absolutely necessary. One application of chemical agent chlorpyrifos was applied during early August to control mealybugs which are the vectors of a viral disease of the vines.
The 9800 ha Jonkershoek nature reserve comprises the Jonkershoek mountains and portions of the upper Jonkershoek valley where large Radiata pine (Pinus radiata) plantations are a distinctive feature bordering fynbos vegetation. This reserve was considered due to the presence of mountain fynbos adjacent to invasive alien trees (i.e. Pinus and Hakea spp.). The 286 ha Helderberg nature reserve has mountain fynbos dominated mainly by protea species. This site was chosen for its IATs adjacent to a site cleared of IATs (i.e. Pinus spp.). The Hottentots Holland nature reserve is 42 000 ha and comprises the Hottentots Holland mountains with the presence of pristine mountain fynbos adjacent to IATs (i.e. P. radiata).
Sampling
Sampling of the arthropods was on three occasions (August-October 2006, May–July 2007 and November 2007–January 2008), when soil surface characteristics (i.e. soil moisture with radioactive moisture-density gauge (Troxler 3411-B) and leaf litter depth by inserting a steel rod, 4 mm in diameter, into the leaf-litter until the harder soil layer was reached) were also measured. Pitfall traps were used to sample arthropods as this method is particularly good for comparative studies of soil-surface active arthropods (Samways et al. 2010). The 256 m transects consisted of a trap-set of two individual pitfall traps, 1 m apart, placed at log 2 intervals: 2, 4, 8, 16, 32, 64 and 128 m on either side of the boundary between two adjoining vegetation types to ensure all species across the whole vegetation type were accounted for. The reason for this layout of traps was because the ‘footprint’ of any one patch has both interior and edge, with a cross over at approximately 30 m (Samways and Moore 1991; J.S. Pryke unpublished data). The aim was to give approximate equal weighting to both interior and edge, while at the same time having a sampling programme that was practical in terms of time, hence the log 2 intervals. This is a study of a land mosaic consisting of patchwork of land use types, making a reference site of a small-sized patch of fynbos being more appropriate than an extensive nature reserve so as to compare different types of patches of comparative size. Furthermore, beta diversity is very high in extensive natural habitats and we did not want to invoke another variable into the data. It was not the intention here to unpack all the complex details of edge effects for the different taxa but rather to compare patches in their entirety in a variety of landscape contexts (see Wiens et al. 1993). However, two transects, between IATs and fynbos, were each four traps short, owing to unavailability of extensive sites (Table 1). The total was 1000 pitfall traps (two per set, fourteen sets per transect, six transects per vegetation type pair and six vegetation pairs from four vegetation types, minus eight traps) (Table 1).
Pitfall traps for sampling arthropods were 500 ml plastic honey jars, each containing a replaceable paper cups, 8 cm diameter, 12 cm deep. Each trap was one-third filled with 70% ethylene glycol. Traps remained closed during non-sampling periods, and opened for five consecutive days without rain (Borgelt and New 2006). Samples then were washed in water, and transferred to 70% ethanol.
Sampling in vineyards was under vine rows to minimize impact of disturbance by farm activity. All pitfall traps were established three months prior initial sampling to eliminate ‘digging effects’. Arthropod samples from each trap set were combined, resulting in one sample per sampling station (i.e. 1000 pitfall traps gave 500 samples per sampling period, making 1 500 samples over the three sampling periods).
Arthropods were identified to species, or where this was not possible, assigned to morphospecies. Voucher specimens are in the Entomology Museum, Stellenbosch University, although spiders are in the National Collection of Arachnida, National Museum, Pretoria. Identification was by keys and expert opinion.
Data analyses
Species accumulation curves, using EstimateS version 8.0.0 with samples randomized 50 times (Colwell 2006), were produced for all vegetation types separately, and for all combined. Non-parametric species estimators were used to provide the best overall arthropod species estimates for all vegetation types (Hortal et al. 2006). The incidence based Coverage Estimator (ICE) is a robust and accurate estimator of species richness (Chazdon et al. 1998), whereas Chao2 and Jackknife estimators provide the least biased estimates should insufficient sampling be an issue (Colwell and Coddington 1994), and were calculated here using EstimateS (Colwell 2006) for all vegetation types separately and for all combined.
As arthropod species richness was normally distributed and variance homogeneous, one-way analysis of variance (ANOVA) was performed on species richness data. However, log transformations were used for abundance data. Multiple comparisons of the means were made using Bonferroni methodology (Legendre and Legendre 1998). ANOVA was used to test for differences between means of populations.
ANOVAs were performed on the selected environmental variables in the different vegetation types using SPSS v17 software (SPSS Inc. 2006), and significance level was set at a P-value > 0.05. Where the ANOVAs were not significant, the analysis was terminated. However, where the result was significant, it was investigated further using multi-comparison tests.
Classification trees, using CHAID growth limits (SPSS Inc. 2006) determined the relationship between the vegetation types in terms of species richness and abundance relative to environmental variables. Significance level for splitting nodes and merging categories was 0.05, and the significance values were adjusted using Bonferroni methodology. Correlations between species richness and abundance with environmental variables for all vegetation types, separately and collectively, were calculated using Spearman’s Rank Order Coefficient, since the data were not normally distributed.
Multivariate analysis, using Primer Ver. 5 (Clarke and Gorley 2001), was used to detect trends and to explore the differences in arthropod assemblages between different vegetation types. Bray-Curtis similarity coefficients were used to derive similarity matrices of arthropod data, then clustering dendrograms and non-metric, multi-dimensional scaling (NMDS) ordination plots to detect trends in similarity. The main advantage of NMDS is its greater ability to represent complex relations accurately in low-dimensional space (Clarke and Warwick 2001). Joint absences in the NMDS were ignored to emphasize similarity in common or rare species, comparing only percentage composition (Clarke and Warwick 2001). The dendrograms were group-average linking on Bray-Curtis species similarities from standardized abundance data.
Principal Components Analysis (PCA) was used to determine whether arthropods from the different vegetation types fell into distinct groups (Clarke and Warwick 2001). PCA considers a different starting point by making different assumptions about the definition of (dis)similarity of samples being compared. Rare species were excluded from the PCA, so that the species retained were more comparable with the number of samples (Clarke and Warwick 2001). Distances between vegetation types on the ordination attempt to match the corresponding dissimilarities in arthropod assemblage composition i.e. similar vegetation types would have very similar arthropod assemblages, while vegetation types that are dissimilar would have few species in common, or the same species at very different levels of abundance. However, PCA can produce inconclusive results, so the data were further analyzed using cluster analysis for determining how the vegetation types varied, and how they grouped in terms of their arthropod assemblages. Cluster analysis produces results that broadly agree with PCA (Clarke and Warwick 2001), and usually it is much easier to observe grouping in cluster analysis than in PCA. Nevertheless, it may be easier to understand what the groupings indicate if they have been produced by PCA. Therefore, using a combination of cluster analysis and PCA is an effective approach.
Analysis of similarity (ANOSIM) tests the hypothesis that there are no assemblage differences between groups of samples specified, here vegetation type (Clarke and Warwick 2001). R is approximately zero if the null hypothesis is true, indicating that similarities between and within vegetation types will be the same on average (Clarke and Warwick 2001).
Because of the large number of species sampled, a more automatic, analytical procedure for identifying influential species (typifying species) was carried out.
Results
Species richness and abundance
In total, 25 225 individuals were sampled, and allocated to 198 species, 106 families and 24 orders (Electronic Supporting Table 1). Species accumulation curves for the four vegetation types, fynbos, IATs, CIATs and vineyards, although flattening, did not quite reach an asymptote, despite the large number of individuals sampled. Nevertheless, an overall species accumulation curve combining all vegetation types did reach an asymptote after counting over 400 samples and 20 000 individuals, the latter of which was exceeded here.
CIATs and fynbos supported highest and next highest mean species richness and abundance respectively (Table 2). Vineyards and particularly IATs had the least number of species and lowest overall abundance (Table 2). ANOVA among the four vegetation types showed significant differences in arthropod species richness (df = 509, F = 41.65, P < 0.001) and abundance (df = 509, F = 62.59, P < 0.001). Nested ANOVAs for arthropod species richness among all four vegetation types revealed significant differences between all vegetation pairs except fynbos and CIATs (Table 3). For abundance, all pairs of vegetation types were significantly different, except vineyards and IATs (Table 3).
Of the sampled arthropods, 77 species were restricted to 1–3 of the four possible vegetation types, with some being restricted to only one vegetation type: fynbos (6 spp.); IATs (4 spp.); CIATs (3 spp.) and vineyards (5 spp.) (Electronic Supporting Table 2).
The classification tree for vegetation based on arthropod species richness indicated some similarity between fynbos and CIATs (Fig. 1). In turn, IATs and vineyards were significantly different from each other in species richness, and both were not comparable to either fynbos or CIATs (Fig. 1). Classification of different vegetation types in terms of arthropod abundance gave three different nodes (Fig. 2). IATs and vineyards clustered together and had significantly lower species abundance than fynbos or CIATs.
Arthropod assemblage composition
Results on assemblage composition, where both species and their abundances are considered together, gave a different arrangement of vegetation types compared to the results from species richness and abundance above. Cluster analysis showed that fynbos and vineyards were most similar, followed by IATs and then CIATs (Fig. 3). NMDS gave similar results. In short, while IATs and vineyards were species poor and low in abundance, when it came to the species characterizing the assemblages, vineyards and fynbos were similar, and CIATs still had an arthropod legacy of the IATs, even though there was an increase in species richness and abundance. However, when vegetation types were grouped by assemblage composition, there were statistically significant differences between these vegetation types (R = 0.149, P = 0.001) using ANOSIM. Yet PCA, which excluded the rare species, gave a slightly different picture from the cluster analysis, with IATs and fynbos as outliers, and vineyards and CIATs close to each (Fig. 4).
Selected environmental variables influencing arthropod biodiversity
Vineyards supported the lowest percentage soil moisture followed by CIATs, whereas fynbos had the highest (Table 4). Greatest leaf litter depth was in IATs, while vineyards had a very shallow leaf litter (Table 4). ANOVA of fynbos, IATs, CIATs, and vineyards showed that there were significant differences among (df = 3, F = 5.81, P < 0.01), and within (df = 509, F = 5.81, P < 0.01) sites in terms of percentage soil moisture. There were also significant differences, among fynbos, IATs, CIATs, and vineyards in terms of leaf litter depth (df = 3, F = 296.6, P < 0.0001). Vineyards had significantly different soil moisture content compared to fynbos and IATs, but comparable with CIATs (Table 5).
Overall, there were no significant correlations between percentage soil moisture and species richness (Spearman’s Coefficient = 0.05; P < 0.919, n = 510), as well as abundance (Spearman’s Coefficient = 0.061; P < 0.171, n = 510). Moreover, there were no significant correlations between leaf litter depth and species richness (Spearman’s Coefficient = −0.20; P = 0.651, n = 510), nor abundance (Spearman’s Coefficient = −0.033; P < 0.454, n = 510).
Species typifying vegetation types
The similarity between all pairs of sites in fynbos was made up mainly of contributions from eight species including Camponotus sp.2, Linepithema humile and Gryllus bimaculatus with a cumulative contribution of over 60% of the total similarity of 16.32 (Table 6). These arthropod species can be considered as typical of associated vegetation types, although L. humile is alien (Table 6). The lower ratio of their contribution to the SD was an indication that the species were inconsistently present in large numbers.
Some species such as L. humile were typical of different vegetation types (i.e. fynbos, CIATs, and vineyards), an indication of its strong adaptation towards different vegetation conditions (Table 6). Nevertheless, alien L. humile was not typical of IATs, suggesting that this species had a preference of various vegetation types but not IATs. An overlap of typifying arthropod species may also indicate that some vegetation types have similar resources required by arthropods and that not all arthropod species are vegetation specific.
Discussion
Species richness and abundance in the different vegetation types
As none of the species accumulation curves in the various individual vegetation types reached an asymptote, species estimates were underestimates. Nevertheless, the overall species accumulation curve did reach an asymptote, indicating that the sample size (> 25 000 individuals) was enough to estimate surface-active arthropod species richness using both number of samples or individuals.
We found that the overall results were categorical, with all species estimators showing highest species richness in fynbos, with cleared areas and vineyards having the next highest estimates. Lowest species richness was in invaded areas, supporting other findings that invasion of fynbos by alien trees is highly impoverishing in the CFR (Ratsirarson et al. 2002; Pryke and Samways 2009). In terms of overall abundance, invaded areas were much poorer than vineyards, indicating greater impact of alien trees over that of vineyards. Fynbos had very high arthropod abundance, the highest of all the vegetation types, even though this footprint study was highly conservative with similar weight being given to edge species as to interior species. Yet species richness of the cleared areas was close to that of fynbos, showing that clearing of alien trees increases species richness, an encouraging sign for restoration. However, we must not over-simplify here, because as pointed out by Holl (1996) and Brändle et al. (2000), the restoration process can be very dynamic, with different species and population abundance being favoured at different times during the recovery process.
Arthropods restricted to particular vegetation types
All vegetation types had some unique arthropod species, with only five being unique to natural vegetation. This figure would in all likelihood be higher if extensive natural reference sites had been used, but the aim here was to compare patches of comparative size. In turn, four species were unique to vineyards, and three to each in invaded and cleared areas. However, as species richness was lowest in invaded areas, this tantamounts to the largest number of absentees compared to all the other vegetation types. This is not surprising because alien trees can impoverish the local terrestrial fauna even over a few metres (Samways et al. 1996). However, some species such as the thomisid spider Ozyptila sp., the chrysidid hymenopteran Spintharina sp., the millipede Centrobolus sp.2 and the army ant Dorylus helvolus were only in invaded areas, indicating that although the majority of arthropods cannot tolerate alien trees, some thrive under such conditions, as is the case with dragonflies under alien trees on Mayotte Island (Samways 2003). Presumably, in the wild, they are restricted to heavily shaded, highly local habitats, which prevail in the sheltered ravines of the Cape Fold Mountains (Pryke and Samways 2008). In contrast, certain species such as the calliphorid fly Chrysomya chloropyga and cicadellid hemipteran Ciphalilus sp. preferred vegetation types other than IATs.
Effectiveness of clearing of alien trees varies with density of the original plant infestation, species type, and time that the site has been invaded (Holmes and Richardson 1999). Removal of the dense stands here led to the remarkable recovery of certain arthropod species (e.g. the cantharid beetle Cantharis sp., the chrysomelid beetle Leptinotarsa sp., C. chloropyga, the reduviid hemipteran Ectrichodia crux, the nemopterdid neuropteran Laurhervasia setacea and the ant Componotus maculatus), as is the case with aquatic arthropods when IATs are removed from river banks (Magoba and Samways 2010).
We also found the alien ant Linepithema humile to be common in fynbos, which may seem to contradict earlier findings where it is usually associated with disturbed areas (Suarez et al. 1998; Suarez et al. 2000; Holway et al. 2002; Carpintero et al. 2005; Ward 2005). This suggests that while the fynbos may appear relatively undisturbed from a vegetation point of view, the context of the fynbos patches (i.e. adjacent to very disturbed patches) has an influence, which would reconcile the two sets of findings. This was confirmed by the fact that the ant was common in vineyards, and from these agricultural areas it is apparently able to penetrate the adjacent fynbos.
Arthropod assemblage composition
The four vegetation types had distinct arthropod assemblages, based on cluster, NMDS and ANOSIM analyses. Even though fynbos was much more species rich than vineyards, their assemblages nevertheless grouped close together, suggesting that the conversion to vineyards was much more faithful in maintaining indigenous ecological integrity than was conversion caused by alien trees. Why this is so is not clear but shading might play a role, because shade from alien trees is the key factor affecting some aerial insects in the same area (Samways and Sharratt 2010). However, as the arthropods here are living in, and dependent on, the litter and soil, there is likely to be a whole host of chemical and physical factors, not measured here, playing a role at various spatial scales from a meter or two to several hundreds of kilometres.
The invaded and cleared areas also grouped together in the multivariate analyses, indicating, not surprisingly, that the recovery process follows a trajectory based on the existing fauna in the invaded areas. Interestingly, when rare species were excluded from the analyses in the PCA, fynbos and cleared areas were no longer comparable in terms of assemblage composition, indicating that the arthropod recovery in the cleared areas was mostly the result of re-establishment by common species, including some that appeared very opportunistic, such as the termite Microhodotermes viator. Then, as vineyards and cleared areas had a similar species composition, it is likely that both vegetation types supported species that preferred more open areas rather than dense and shady fynbos vegetation, but nevertheless re-inforces the fact that vineyards have less impact on species composition than do alien trees.
Arthropod species and environmental variables
Soil under natural fynbos, alien trees, and in cleared areas had comparable percentage soil moisture, which appeared to be a result of comparable ground cover. In contrast, vineyards with their bare surface between the vine rows, had lower percentage soil moisture than fynbos or cleared areas, with resultant lower arthropod abundance than even in invaded areas. However, soil moisture did not statistically correlate with arthropod species richness or abundance. On the other hand, alien tree areas had significantly deeper leaf litter compared to either natural vegetation or cleared areas, possibly due to lack of decomposers necessary to reduce the leaf litter. Nevertheless, there was no significant correlation between leaf litter and arthropod species richness and abundance, despite vineyards having the lowest. Overall, these results, being not very categorical, are encouraging for restoration, as soil conditions under both vineyards and alien trees are not sufficiently adverse to be of concern, at least in terms of the arthropods we studied.
Conclusions
Our results emphasize that the impoverishing effect of a particular type of agriculture (vineyards) on indigenous surface-active arthropods is not as great as that of invasive alien trees in patches of comparative size. Furthermore, the remnant assemblage in vineyards is more of a subset of that in natural fynbos than is the case with alien trees. This suggests that conversion of vineyards to more biodiversity friendly farming methods, as outlined by Gaigher and Samways (2010), has a good base on which to work. In turn, clearing of alien trees will continue to benefit biodiversity recovery, but it will take time for the original set of species to return, as it is only the common, and presumably more habitat-tolerant, species which readily recover. This re-establishment of the original set of arthropod species would be promoted by clearing alien trees in close proximity to large patches of natural fynbos, to enable easy movement and recolonization of the areas being restored.
References
Armstrong AJ, Van Hensbergen HJ (1996) Impacts of afforestation with pines on assemblages of native biota in South Africa. South Afr For J 175:35–42
Borgelt A, New TR (2006) Pitfall trapping for ants (Hymenoptera, Formicidae) in mesic Australia: what is the best trapping period? J Insect Conserv 10:75–77
Brändle M, Durka W, Altmoos M (2000) Diversity of surface dwelling beetle assemblages in open-cast lignite mines in central Germany. Biodivers Conserv 9:1297–1311
Carpintero S, Reyes-López J, Arias de Reyna L (2005) Impact of Argentine Ants (Linepithema humile) on an arboreal ant community in Donana National Park, Spain. Biodivers Conserv 14:151–163
Chazdon RL, Colwell RK, Denslow JS, Guariguata MR (1998) Species richness of woody regeneration in primary and secondary rainforests of northeastern Costa Rica. In: Dallmeier F, Comiskey JA (eds) Forest biodiversity research, monitoring and modeling: conceptual background and old World case studies. Pathenon, Paris
Chytrý M, Maskell LC, Pino J, Pyšek P, Vilà M, Font X, Smart SM (2008) Habitat invasions by alien plants: a quantitative comparison among Mediterranean, subcontinental and oceanic regions of Europe. J Appl Ecol 45:448–458
Clarke KR, Gorley RN (2001) Primer Version 5. Primer-E, Plymouth
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth
Colwell RK (2006) EstimateS 8.0.0. http://viceroy.eeb.uconn.edu/EstimateS
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Phil Trans Roy Soc 345:101–118
Donald PF, Evans AD (2006) Habitat connectivity and matrix restoration: the wider implications of agri-environment schemes. J Appl Ecol 43:209–218
Feber RE, Smith H, MacDonald DW (1996) The effects on butterfly abundance of the management of uncropped edges of arable fields. J Appl Ecol 33:1191–1205
Fleishman E, Austin GT, Brussard PF, Murphy DD (1999) A comparison of butterfly communities in native and agricultural riparian habitats in the great basin. Biol Conserv 89:209–218
Gaigher R, Samways MJ (2010) Surface-active arthropods in organic vineyards, integrated vineyards and natural habitat in the Cape Floristic Region. J Insect Conserv 14:595–605
Giliomee JH (2003) Insect diversity in the Cape Floristic Region. Afr J Ecol 41:237–244
Holl KD (1996) The effect of coal surface mine reclamation on diurnal lepidopteran conservation. J Appl Ecol 33:225–236
Holmes PM, Richardson DM (1999) Protocols for restoration based on recruitment dynamics, community structure, and ecosystem function: perspectives from South African fynbos. Rest Ecol 7:215–230
Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–233
Hortal J, Borges PAV, Gaspar C (2006) Evaluating the performance of species richness estimators: sensitivity to sample grain size. J Anim Ecol 75:274–287
Jeanneret P, Schüpbach B, Pfiffner L, Walter T (2003) Arthropod reaction to landscape and habitat features in agricultural landscapes. Land Ecol 18:253–263
Johnson SD (1992) Plant–animal relationships. In: Cowling RM (ed) The ecology of fynbos: nutrients, fire and diversity. Oxford University Press, Cape Town, pp 175–205
Kleijn D, van Langevelde F (2006) Interaction effects of landscape context and habitat quality on flower visiting insects in agricultural landscapes. Basic Appl Ecol 7:201–214
Le Maitre DC, Richardson DM, Chapman RA (2004) Alien plant invasions in South Africa: driving forces and the human dimension. South Afr J Sci 100:103–112
Legendre P, Legendre L (1998) Numerical ecology: developing in environmental Modelling 20. Elsevier, Amsterdam
Linder HP (2005) Evolution of diversity: the Cape flora. Trends Plant Sci 10:536–541
Macdonald IAW, Richardson DM (1986) Alien species in terrestrial ecosystems of the fynbos biome. In: Macdonald IAW, Kruger FJ, Ferrar AA (eds) The ecology and management of biological invasions in southern Africa. Oxford University Press, Cape Town, pp 77–91
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Magoba RN, Samways MJ (2010) Recovery of benthic macroinvertebrate and adult dragonfly assemblages in response to large scale removal of riparian invasive alien trees. J Insect Conserv 14:627–636
Mason TJ, French K (2008) Impacts of a woody invader vary in different vegetation communities. Divers Distrib 14:829–838
Mittermeier RA, Gil PR, Hoffman M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, da Fonseca GAB (2005) Hotspots revisited: earth’s biologically richest and most endangered terrestrial ecoregions. Conservation International and Agrupacion Sierra Madre, Monterrey, Cemex
Newton I (2004) The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation action. Ibis 146:579–600
Picker MD, Samways MJ (1996) Faunal diversity and endemicity of the Cape Peninsula, South Africa—a first assessment. Biodivers Conserv 5:591–606
Procheş Ş, Cowling RM (2006) Insect diversity in Cape fynbos and neighbouring South African vegetation. Global Ecol Biogeogr 15:445–451
Procheş Ş, Forest F, Veldtman R, Chown SL, Cowling RM, Johnson SD, Richardson DM, Savolainen V (2009) Dissecting the plant–insect diversity relationship in the Cape. Mol Phyl Evol 51:94–99
Pryke JS, Samways MJ (2008) Conservation of invertebrate biodiversity on a mountain in a global biodiversity hotspot, Cape Floral Region. Biodivers Conserv 17:3027–3043
Pryke JS, Samways MJ (2009) Recovery of invertebrate diversity in a rehabilitated city landscape mosaic in the heart of a biodiversity hotspot. Land Urb Plan 93:54–62
Ratsirarson H, Robertson HG, Picker MD, van Noort S (2002) Indigenous forests versus exotic eucalypt and pine plantations: a comparison of leaf-litter invertebrate communities. Afr Entomol 10:93–99
Richardson DM, Pyšek P (2006) Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog Phys Geogr 30:409–431
Richardson DM, van Wilgen BW (2004) Invasive alien plants in South Africa: how well do we understand the ecological impacts? South Afr J Sci 100:45–52
Rouget M, Cowling RM, Pressey RL, Richardson DM (2003) Identifying spatial components of ecological and evolutionary processes for regional conservation planning in the Cape Floristic Region, South Africa. Divers Distrib 9:191–210
Sala OE, Chapin FS III, Armesto JJ, Berlow R, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge D, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Samways MJ (2003) Threats to the tropical island dragonfly fauna (Odonata) of Mayotte, Comoro archipelago. Biodivers Conserv 12:1785–1792
Samways MJ, Moore SD (1991) Influence of exotic conifer patches on grasshopper (Orthoptera) assemblages in a grassland matrix at a recreational resort, Natal, South Africa. Biol Conserv 57:205–219
Samways MJ, Sharratt NJ (2010) Recovery of endemic dragonflies after removal of invasive alien trees. Conserv Biol 24:267–277
Samways MJ, Taylor S (2004) Impacts of invasive alien plants on Red-Listed South African dragonflies (Odonata). South Afr J Sci 100:78–80
Samways MJ, Caldwell PM, Osborn RM (1996) Ground-living invertebrate assemblages in native, planted and invasive vegetation in South Africa. Agric Ecosyst Environ 59:19–32
Samways MJ, McGeoch MA, New TR (2010) Insect conservation: a handbook of approaches and methods (techniques in ecology and conservation). Oxford University Press, Oxford
Shreeve TG, Mason CF (1980) The number of butterfly species in woodlands. Oecologia 45:414–418
Sibatani A (1980) Wing homoeosis in Lepidoptera: a survey. Dev Biol 79:1–18
Simberloff D, Nuñez MA, Ledgard NJ, Pauchard A, Richardson DM, Sarasola M, van Wilgen BW, Zalba SM, Zenni RD, Bustamante R, Peña E, Ziller SR (2010) Sprea and impact of introduced conifers in South America. Austral Ecol 35:489–504
SPSS Inc. (2006) SPSS version 15.0 for windows. SPSS Inc., Chicago
Suarez AV, Bolger DT, Case TJ (1998) Effects of fragmentation and invasion on native ant communities in coastal southern California. Ecology 79:2041–2056
Suarez AV, Richmond JQ, Case TJ (2000) Prey selection in horned lizards following the invasion of Argentine ants in southern California. Ecol Appl 10:711–725
Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D (2001) Forecasting agriculturally driven global environment change. Science 292:281–284
Turin H, den Boer PJ (1988) Changes in the distribution of carabid beetles in the Netherlands since 1880. II. Isolation of habitats and long-term time trends in the occurrence of carabid species with different powers of dispersal (Coleoptera, Carabidae). Biol Conserv 44:179–200
Ward P (2005) A synoptic review of the ants of California (Hymenoptera: Formicidae). Zootaxa 936:1–63
Warren MS, Barnett LK, Gibbons DW, Avery MI (1997) Assessing national conservation priorities: an improved Red List of British butterflies. Biol Conserv 82:317–328
Wiens JA, Stenseth NC, Horne B, van Ims RA (1993) Ecological mechanisms in landscape ecology. Oikos 66:369–380
Wittenberg R, Cock MJW (eds) (2001) Invasive alien species: a toolkit of best prevention and management practices. CAB International, Wallingford, UK
Wood PA, Samways MJ (1991) Landscape element pattern and continuity of butterfly flight paths in an ecologically landscaped botanic garden, Natal, South Africa. Biodivers Conserv 58:149–166
Wright MG, Samways MJ (1998) Insect species richness tracking plant species richness in a diverse flora: gall-insects in the Cape Floristic Region, South Africa. Oecologia 115:427–433
Acknowledgments
Financial support was from the Centre for Invasion Biology and the Andrew Mellon Foundation. Rejoyce Gavhi, Rozwivhona Magoba, Mbula Tshikalange, Adam Johnson, Tshilidzi Muofhe and Sne Mchunu kindly assisted in the field. Ansie Dipenaar identified spiders, and Henk Geertsema assisted with other identifications. We also appreciate the very constructive criticisms of two anonymous referees which greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Magoba, R.N., Samways, M.J. Comparative footprint of alien, agricultural and restored vegetation on surface-active arthropods. Biol Invasions 14, 165–177 (2012). https://doi.org/10.1007/s10530-011-9994-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-9994-x