Abstract
Invasive alien organisms can impact adversely on indigenous biodiversity, while riparian invasive alien trees (IATs), through shading of the habitat, can be a key threat to stream invertebrates. We ask here whether stream fauna can recover when the key threat of riparian IATs is removed. Specifically, we address whether IAT invasion, and subsequent IAT removal, changes benthic macroinvertebrate and adult dragonfly assemblages, for the worse or for the better respectively. Natural riparian zones were controls. There were statistically significant differences between stream reaches with natural, IAT-infested and IAT-cleared riparian vegetation types, based on several metrics: immature macroinvertebrate taxon richness, average score per macroinvertebrate taxon (ASPT), a macroinvertebrate subset (Ephemeroptera, Plecoptera, Trichoptera and Odonata larvae; EPTO), and adult dragonfly species richness. Reaches with natural vegetation, or cleared of IATs, supported greater relative diversity of macroinvertebrates than reaches shaded by dense IATs. Greatest macroinvertebrate ASPT and EPTO were in reaches bordered by natural vegetation and those bordered by vegetation cleared of IATs, and the lowest where the riparian corridor was IATs. Highest number of adult dragonflies species was along streams cleared of dense IATs. Overall, results showed that removal of a highly invasive, dense canopy of alien trees enables recovery of aquatic biodiversity. As benthic macroinvertebrate scores and adult dragonfly species richness are correlated and additive, their combined use is recommended for river condition assessments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive alien organisms are a major threat to biodiversity, and have caused species extinctions (Tallamy 2004; Clavero and Garcia-Berthou 2005). Invasive alien trees are among these threatening organisms (Latimer et al. 2004), and are an extinction threat to over 1,000 plant and animal species (Calder 1999), including those in riparian zones and grasslands (Water Research Commission 2001). Riparian invasive alien trees (IATs) compete with indigenous plant species for water, space, sunlight, and other resources, so reducing structural diversity of indigenous vegetation, as well as changing the functioning of the ecosystem. This, in turn, influences the number and type of animal species that can be supported by that vegetation (Tallamy 2004). Reversing the impact of IATs would therefore likely be of major benefit for biodiversity recovery.
In response to the IAT problem, South Africa has developed a national-scale of IAT removal programme, aiming in part to restore indigenous biodiversity (Zimmermann et al. 2004), as nearly one tenth of the surface area of South Africa is infested with alien plants (Water Research Commission 2001). IATs can form dense stands along river banks and change local ecology (Richardson and Van Wilgen 2004), while their removal increases, among other things, stream flow (Dye and Jarmain 2004), and enables recovery of population levels of certain rare and threatened dragonfly species (Samways and Taylor 2004; Samways et al. 2005).
Benthic macroinvertebrate assemblages are sensitive to changes in water conditions caused by various anthropogenic impacts (Resh and Jackson 1993; Norris and Thoms 1999; Hawkins and Norris 2000). Macroinvertebrate assessments are usually conducted only at family or other higher taxonomic level. Changes in finer, species-level composition of some invertebrate communities in response to invasion by, and clearing of, IATs may not be detected when using these higher levels of taxonomic resolution. Furthermore, it is often relatively difficult to identify the young stages of aquatic insect species. Thus ideally, species-level, finer-resolution data should supplement the higher-level taxonomic surrogates.
Adult dragonfly species are highly sensitive to the quality of local riparian vegetation type (Chovanec and Waringer 2001; Schindler et al. 2003; D’Amico et al. 2004; Hofmann and Mason 2005; Butler and deMaynadier 2008), and can be used to supplement macroinvertebrate assemblages for evaluating the local success of IAT removal (Smith et al. 2007; Samways and Sharratt 2010; Simaika and Samways 2009). Adult male dragonflies respond to habitat quality by recognizing some intrinsic characters that correlate with relatively high-quality sites (Orians and Wittenberger 1991). Macroinvertebrate assemblages identified at a higher taxonomic level (low resolution) may be sensitive enough for comparing different rivers, but are less sensitive for specific sites, while dragonflies are sensitive to both (Smith et al. 2007). Presence of a wide range of indigenous dragonflies is an indication of a healthy system, and adults react rapidly to changes in physical conditions (Samways 1989; Samways and Steytler 1996) and have been used as potential indicators at the species level (e.g. Clark and Samways 1996; Bulánková 1997; Stewart and Samways 1998; Hawking and New 1999).
The aim of this large-scale restoration study was to assess the degree of recovery of benthic macroinvertebrate higher taxa and adult dragonfly species assemblages at sites heavily invaded by riparian IATs, and to compare these sites with those cleared of riparian IATs, using natural riparian vegetation sites as controls. This enabled us to ascertain the extent to which removal of IATs promotes recovery of the local stream fauna. The null hypothesis was that natural vegetation, alien vegetation (IAT sites) and cleared-of-aliens sites had similar assemblages, with rejection of the null hypothesis when there were significant differences at P < 0.05. The value of using species-level adult dragonfly assessments alongside higher taxon macroinvertebrate assessments of riparian condition was also determined.
Methods
Study area and sites
Invasive alien trees (IATs) are being removed on a national scale in South Africa, and offers an opportunity for undertaking a landscape study with considerable practical application. The study area was in the upper reaches of the Luvuvhu River catchment in the Soutpansberg mountains, Limpopo Province, South Africa, which are highly infested with IATs. Three sites were identified: Piesanghoek, Shefeera, and Entabeni, covering a total of three streams, each of which had native, alien (IATs) and cleared IAT riparian vegetation zones (Table 1). All SUs were selected from the headwaters, where there was minimal anthropogenic impact than the foothills that could have influenced the results. Each sampling unit (SU) was categorized here as (1) ‘natural’, (2) ‘alien’ (i.e. IATs), or (3) ‘cleared’ (i.e. cleared of IATs). Spatial replication was 23 SUs in each of the natural and alien vegetation types, and 25 SUs in cleared vegetation. Three types of riparian treatment were studied at each stream: (1) natural riparian vegetation, (2) dense alien riparian vegetation, mostly Solanum mauritianum Scop. (bugweed), Acacia mearnsii De Wild. (black wattle), Pinus patula Schiede ex Schlecht and Cham. (patula pine), Ceasalpinia decapitala (Roth) Alston (mauritius thorn), and Eucalyptus gomphocephala A. DC. (bluegum tree), and (3) completely cleared of dense IATs. Natural riparian vegetation is defined here as riparian vegetation with cover less (mostly far less) than 15% IATs, while ‘alien riparian vegetation’ was cover greater than 75% IATs. Cleared riparian vegetation was where the IATs were manually removed on a large, commercial scale (totaling hundreds of ha in the study area, with many hundreds of ha still awaiting removal) at least 1 year prior to sampling.
Each SU was a 10 m stretch of a stream with the 2 m wide strip of vegetation on both banks, with an absolute 50 m minimum distance between SUs. As point sampling of invertebrates was involved (see below), these SUs were considered sufficiently independent for statistical analysis. All types of microhabitats (riffles, run, aquatic macrophytes, etc.) were included in each SU to minimize variation between the sites and to cover many macroinvertebrates with different substrate preference (Ulfstrand 1967; Hildrew and Townsend 1976; Dickens and Graham 2002).
Sampling
Benthic macroinvertebrates
Benthic macroinvertebrates were assessed using the protocol of Dickens and Graham (2002), where high sensitivity scores are allocated to the most sensitive taxa, and lowest scores to those which are least susceptible. The sum of the individual scores is the macroinvertebrate score, which is divided by the number of sampled taxa to give the standardized measure of river health, the average score per taxon (ASPT) (Dickens and Graham 2002).
Benthic macroinvertebrates (including dragonfly larvae) were sampled once at each SU during August–October 2004, when there were high population levels of these aquatic stages. All available microhabitats in each SU were sampled, including stones, both in and out of current, vegetation (marginal and aquatic vegetation), gravel, sand and mud. Stones were kicked systematically across the SU continuously for five min. All microhabitats available within a SU were combined to produce a composite sample per SU. The collecting-net was a 950 μm mesh kick net on a 30 cm square frame, which was placed below the water level, moving it backwards and forwards. In lentic habitats, marginal vegetation, gravel and mud were swept. Loose substrata were agitated for 30 s, dislodging benthic macroinvertebrates into the net. Netted samples were washed and tipped into a sorting tray, and all specimens, large and small, identified to higher taxon (usually family), and sampled abundances recorded by counting individuals. Sampling was sequentially from the lowest sites (downstream) up to the highest sites, avoiding errors associated with benthic macroinvertebrate drift (Dickens and Graham 2002). The final macroinvertebrate score was the sum of sensitivity scores for benthic macroinvertebrates. Number of taxa and ASPT (i.e. macroinvertebrate score/number of taxa) were also obtained.
Previously available data on benthic macroinvertebrate taxon richness and ASPT were obtained from Diedericks (2002), using the same methodology as described in Dickens and Graham (2002) and sites, and were incorporated into this study to record any improvement in benthic macroinvertebrate diversity over the years after clearing of IATs. Diedericks (2002) data were collected in 2001 and 2002 from stream cleared of riparian IATs (Shefeera and Piesanghoek) in 1999, and the follow-up clearings were made yearly.
Adult dragonflies
The same SUs for benthic macroinvertebrates were used for adult dragonfly sampling. These adult individuals were recorded using close-focus binoculars, and a butterfly net used to collect voucher specimens. This method has been used elsewhere to study dragonflies (Suh and Samways 2001; Marden and Cobb 2004) and produced reliable results. Sampling effort was a 30 min observation period in each SU, during December 2004–January 2005, the peak season for adult dragonflies. Observation was from 1000 h to 1500 h only on sunny, windless days, to ensure maximum dragonfly activity. Only male individuals were counted and matched to the particular riparian vegetation type (Clark and Samways 1996; Suh and Samways 2001; Marden and Cobb 2004), because most of the time females are not associated with water (Samways et al. 1996; Foster and Soluk 2004). Dragonfly individuals are highly site-faithful between days: 32.5% of the time males returned to the same oviposition site, and 62.3% of the time males returned to within 3 m of their previous site (Switzer 1997). Unaided visual scanning is 100% accurate for the dragonfly sub-order Anisoptera and 80% accurate for Zygoptera (Moore 1991) and even more accurate for Zygoptera when using close-focus binoculars.
Environmental variables associated with the three types of riparian vegetation
Physical/chemical environmental variables (EVs) (dissolved oxygen, pH, water temperature, electrical conductivity) were measured simultaneously at each sampling unit (SU) using a YSI 556 Multi Probe System. Measured habitat EVs at each SU were: vegetation type (natural, alien or cleared), canopy cover, and number of years since first clearing (where IATs had been cleared).
Two measurements were taken of each EV in each SU, one at the beginning of the SU and one at the end. In total, eight EVs were measured: elevation (in m above sea level), temperature (10 cm under the water surface), pH, conductivity, dissolved oxygen, flow (categorized into three classes: (1) riffles (very fast-flowing, broken water on the surface), (2) runs (flows with no broken water) and, (3) pools (water flows slowly)), shade (canopy cover; the estimated percentage cover over each SU, to the nearest 10%), and microhabitat (categorized into six categories according particle size (Dickens and Graham 2002): (1) silt (<0.06 mm), (2) sand (0.06–2 mm), (3) gravel (2–20 mm), (4) stones (2–30 mm), (5) boulders (>30 cm), (6) bedrock (slabs of rock)).
Data analyses
Data collected from all streams were pooled for each of the three riparian (natural, alien, or cleared) vegetation types for the comparison of aquatic macroinvertebrate and adult dragonfly assemblages. Macroinvertebrate taxon richness was determined, then the ASPT for each SU (see above) (Dickens and Graham 2002). Analysis of variance (ANOVA), after log transformation of raw data, using SPSS 15.0 for Windows (SPSS Inc. 2006) was used to compare natural, alien, and cleared riparian vegetation types in terms of macroinvertebrate taxon richness in various SUs. Multiple comparisons for benthic macroinvertebrates and adult dragonfly species using Tukey HSD tests were also done. Canonical Correspondence Analysis (CCA) and the Monte Carlo permutation test (499 permutations) was used to analyze the macroinvetebrate assemblages and to relate the assemblages to EVs, using the software CANOCO (Ter Braak and Šmilauer 2002).
Average adult dragonfly species richness and abundance per SU and per riparian vegetation type were derived and used for further analyses. Principal component analysis (PCA) included in PRIMER 5 for Windows (Clarke and Warwick 2001), using Bray-Curtis similarity measures was used to find out whether adult dragonflies from different riparian vegetation types fall into a distinct group or not. An ordination plot of adult dragonflies in all the SUs from natural, alien and cleared riparian vegetation type was created.
Additional analysis, to obtain a temporal perspective, was done using three macroinvertebrate data sets, in the years 2001 and 2002 (Diedericks 2002), and 2004 (current study). These data were collected from SUs bordered by riparian vegetation cleared of dense IATs. These IATs were cleared initially during 1999, and almost yearly after that. One-way ANOVA’s were performed using SPSS 15.0 for Windows to ascertain whether there was any significant recovery of macroinvertebrates, specifically taxon richness and ASPT following IAT clearing over the years. Multiple comparisons using Tukey HSD was carried out to determine any significant change in macroinvertebrate assemblages over the years.
To ascertain whether adult dragonfly species richness varied in the same direction to the same extent as the ASPT scores, a linear regression analysis was carried out on all the SUs, and accounting for all three vegetation types.
Results
Benthic macroinvertebrates
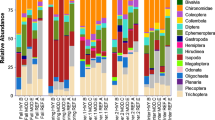
Highest average scores for macroinvertebrates (taxon richness, ASPT and EPTO) across all SUs were in natural vegetation and in cleared SUs, and the lowest in IAT SUs (Fig. 1) sites. Overall, these differences were statistically significant (Table 2). However, there was no statistical significant difference between different riparian vegetation types in terms of SASS5 total scores (F = 2.497, P = 0.090).
Selected analysis of variance (ANOVA) of benthic macroinvertebrate taxon richness; benthic macroinvertebrate average score per taxon (ASPT); Ephemeroptera, Plecoptera, Trichoptera and Odonata larvae (EPTO) taxon richness, and adult dragonfly species richness among natural, invasive alien trees (IATs) and cleared IAT riparian vegetation types. The mean difference among riparian vegetation types is significant at the P = .05 level. Standard error bars are displayed. Differing letters above bars show significant differences
When the earlier findings of Diedericks (2002) were compared with our results, there was a significant difference in benthic macroinvertebrate taxon richness (df = 72, F = 39.972, P = 0.000), and ASPT (df = 72, F = 98.336, P = 0.000), between 2001 and 2004. Over the years 2001, 2002 and 2004, there was a significant increase in taxon richness and ASPT (Fig. 2). The stream bordered by cleared riparian vegetation had an average ASPT of 4.9 in 2001, which improved to 5.1 and 6.9 in 2002 and 2004 respectively (Fig. 2). An average of 5.5 families per SU, was recorded in 2001, while 7.5 and 10.6 were recorded in 2002 and 2004 respectively. Total SASS5 score was 80 in 2001, 145 in 2002 and 227 in 2004. Some of the most sensitive families, Oligoneuridae, Heptageniidae, Helodidae and Perlidae, were absent in 2001 and 2002, but present during 2004 (see Magoba 2005 for details). Although, the results indicated significant recovery of macroinvertebrate taxon richness from 2002, there was no significant change in stream conditions as indicated by ASPT during 2002, but had improved significantly by 2004 (Fig. 2).
Selected analysis of variance (ANOVA) of average benthic macroinvertebrate taxa, and benthic macroinvertebrate average score per taxon (ASPT), for the years 2001, 2002 and 2004 recorded from sites cleared of invasive alien trees (IATs) in 1999. Columns represent average values per vegetation type and standard error bars are displayed
Benthic macroinvertebrate assemblages and environmental variables
Monte Carlo tests resulted in statistically significant axes for macroinvertebrate taxa (F = 1. 51, P = 0.028). Benthic macroinvertebrate-EV correlation in natural vegetation was high, with shade accounting for most of the variation in benthic macroinvertebrates (F = 3.21, P = 0.002). Measured EVs accounted for 43% of variability in macroinvertebrate assemblages (Fig. 3). Similar analysis for alien vegetation showed that benthic macroinvertebrate-EV correlations were non-significant (F = 1.272, P = 0.058), while for cleared sites, they were highly significant (F = 1.734, P = 0.002), with the measured EVs accounting for 77.4% of the variation. Microhabitats accounted for most of the variation (F = 3.073, P = 0.01; 25%), followed by shade cover (F = 1.683, P = 0.036; 15%). Dissolved oxygen, flow, elevation, temperature and conductivity had no significant effect in cleared areas. CCA of EPTO taxa in all three vegetation types showed that the measured EVs (Fig. 3) were highly significant (F = 2.614, P = 0.002), accounting for 54% of the variability.
Canonical Correspondence Analysis of macroinvertebrate assemblages with environmental variables in natural vegetation. Macroinvertebrates code used for analysis are Oligochaeta: Oli; Potamonautidae: Pot; Perlidae: Per; Baetidae: Bae; Caenidae: Cae; Heptageniidae: Hep; Leptophlebiidae: Lep; Oligoneuridae: Oligo; Tricorythidae: Tri; Chlorocyphidae: Chl; Synlestidae: Syn; Coenagrionidae: Coe; Lestidae: Les; Platycnemidae: Pla; Protoneuridae: Pro; Aeshnidae: Aes; Gomphidae: Gom; Libellulidae: Lib; Corixidae: Cor; Gerridae: Ger; Veliidae: Vel; Hydropsychidae: Hyd; Leptoceridae: Lep; Dytiscidae: Dyt; Elmidae: Elm; Gyrinidae: Gyr; Helodidae: Hel; Psephenidae: Pse; Athericidae: Ath; Chironomidae: Chi; Tabanidae: Tab; Tipulidae: Tip; Ancylidae: Anc
The mean stream temperature in natural vegetation during the study period was 14.3°C (daily ranges 3.2–23°C), 18.1°C under IATs (daily ranges 7.4–27.2°C) and 20°C in cleared vegetation (daily ranges 6.9–31.7°C).
Adult dragonfly species richness
A total of 1,506 adult dragonfly individuals in 33 species were recorded across all 71 SUs combined. The average species richness per SU associated with natural riparian vegetation SUs was eight, with alien riparian vegetation it was nine, and with cleared riparian vegetation it was 17. Aeshna subpupillata was recorded only in natural vegetation, whereas Orthetrum trinacria, Trithemis dorsalis and T. stictica were localized in association with IATs. Of the total of 28 adult dragonfly species recorded in cleared riparian vegetation, twelve were recorded only in association with cleared riparian vegetation.
Adult dragonfly responses to vegetation type
Different dragonfly species responded differently to each of the three riparian vegetation types. Some species, such as Orthetrum julia falsum, Africallagma glaucum and Elattoneura glauca, were remarkably tolerant of riparian vegetation type, being common across all three (natural, alien and cleared). In contrast, other adult dragonfly species were very sensitive to vegetation type, with Allocnemis leucosticta being abundant only in highly shaded natural vegetation yet absent in sunlit, cleared conditions. Other species, such as Nesciothemis farinosa, O. abbotti and Crocothemis erythraea, were locally abundant in the sunlit conditions where riparian vegetation had been cleared, but scarce or absent in alien or naturally-shaded conditions.
ANOVA indicated highly significant differences (F = 48.635, P = 0.000) among riparian vegetation types in terms of adult dragonfly species richness (Fig. 1; Table 2). Principal component analysis (PCA) ordination of Bray-Curtis similarity showed overall separate clustering of dragonfly species according to natural, alien and cleared IAT riparian vegetation, although with some overlap (Fig. 4). When this was examined in more detail, there was significant differences in adult dragonfly species richness in natural versus cleared riparian vegetation (F = 6.368, P = 0.015), and in alien versus cleared riparian vegetation (F = 13.24, P = 0.001), but not between natural and IAT riparian vegetation (F = 0.113, P = 0.738) (Fig. 1).
Principal component analysis (PCA) ordination of Bray-Curtis similarity between adult dragonflies from 71 sampling units (SUs) from different vegetation types (natural, alien and cleared), in which the placement of SUs represent the similarity in terms of adult dragonfly assemblage composition across riparian vegetation types
Relationship between adult dragonfly species richness and benthic macroinvertebrate taxa richness
Across all 71 SUs, adult dragonfly species richness was significantly positively related to ASPT (r 2 = 0.3044, df = 68, P < 0.005) (Fig. 5).
Discussion
Macroinvertebrate assemblages under riparian invasive alien trees
Local disturbance can lead to a change in composition of aquatic invertebrate communities (Merz and Ochikubo Chan 2005; Sarriquet et al. 2007). Alien streamside vegetation threatens aquatic macroinvertebrates by altering water supplies and sedimentation (Wittenberg and Cock 2001; Hirji et al. 2002). It is therefore not surprising that sites here with riparian invasive alien trees (IATs) supported a lower Average Score Per Taxon (ASPT) of macroinvertebrates than did those with natural riparian vegetation (Fig. 2). Nevertheless, some taxa survived perfectly well (e.g. Gyrinidae) and others even benefited (e.g. Dixidae and Simuliidae), apparently in preference for shady conditions provided by the alien trees at this location, other stream conditions being equal (Magoba 2005). In contrast, ASPT scores were remarkably improved where IATs were removed, although the ASPT scores did not overall match those of natural vegetation (Fig. 2). However, the results in Fig. 1, showing differences between cleared and natural vegetation, suggest that cleared site assemblages were closely approaching those of natural sites. Generally, the most sensitive taxa were the EPTO (larvae of Ephemeroptera, Plecoptera, Trichoptera, and Odonata), with higher average taxon richness associated with natural than with alien riparian vegetation, but like ASPT, were showing good recovery in all cleared areas. The low ASPT associated with IATs was due to loss of sensitive taxa, especially Tricorythidae, Perlidae, Psephenidae, Protoneuridae and Oligoneuridae (Magoba 2005).
Historical recovery of macroinvertebrates after removal of invasive alien trees
Invasion of riparian zones by exotic trees changes the characteristics of invertebrate habitats through indigenous plant replacement (Tallamy 2004), riverbank destabilization, and eradication of pool-riffle sequences, together with a reduction of the substrate mosaic heterogeneity (Boon 1988). In short, there is homogenization of aquatic habitats and hence the associated communities. Indeed, we show here that alien riparian vegetation type was poor in macroinvertebrate taxon richness. Yet, macroinvertebrate taxon richness significantly improved within a 2-year period after alien tree clearing. But river quality, as measured by ASPT, took more time to fully recover, and significantly improved only 5 years after the initial removal of the alien trees. The lowest number of aquatic macroinvertebrate taxa was recorded in 2001, when the stream was first beginning to recover following IAT removal in 1999. Low macroinvertebrate scores in 2001 may be attributed to the former alien infestation. In 2001, macroinvertebrates reflected impoverished stream conditions (very low ASPT). By 2002 and then 2004, it appears that the impact of IATs had subsided, resulting in a significant improvement in the aquatic macroinvertebrate assemblage.
The recovery was remarkable, and seems to be partly due to the fact that macroinvertebrates in the region are intrinsically adaptable to major disturbances, including floods in the same overall region, where macroinvertebrate recovery occurred within 1 year (Samways 1989). Thus, the floods in 2000 were not considered to have significantly influenced aquatic macroinvertebrate assemblage during the study period (i.e. 18 months later). In summary, clearing of IATs and subsequent follow-ups were beneficial to the aquatic macroinvertebrate assemblage, with the final results almost comparable to those associated with stream reaches bordered by natural riparian vegetation.
Environmental variables and benthic macroinvertebrates
Environmental variables, especially riparian vegetation type and available microhabitats, had a significant effect on the macroinvertebrate assemblage, a well-known phenomenon (Norris and Thoms 1999). Availability of microhabitats can be important for macroinvertebrate diversity, especially in savanna habitats like these here, where environmental conditions are unpredictable and extreme (Stewart and Samways 1998).
Different benthic macroinvertebrates show preferences for different microhabitats, especially in natural vegetation, where in our study here, this EV was highly significant. The heterogeneity of microhabitats is among the most important factors controlling the macroinvertebrate assemblage (Sheldon 1986; Townsend and Hildrew 1994), because it increases the diversity of resources such as substratum characteristics (Culp et al. 1983; Rempel et al. 2000), hydraulic conditions (Quinn and Hickey 1994) and food availability (Hawkins et al. 1982; Dobson and Hildrew 1992) and hence macroinvertebrate diversity. Clark and Samways (1996) showed that natural sites with an abundance of macrophytes had significantly higher species abundance by providing good oviposition sites for adult dragonflies as well as perching sites, especially for the smaller Zygoptera. It follows that natural vegetation increases benthic macroinvertebrate diversity, but conversely when this natural vegetation is replaced by a dense IAT canopy, there is a drop in macroinvertebrate diversity.
Adult dragonfly species composition and relative abundance
Dragonfly species are generally very sensitive to riparian vegetation type (Buchwald 1992; Chwala and Waringer 1996; Samways and Steytler 1996; Rith-Najarian 1998; Samways and Taylor 2004; Foote and Hornung 2005; Samways 2007; Samways and Sharratt 2010), with the highest species richness and density here being associated with cleared IAT vegetation. Interestingly, almost an equal number of dragonfly species was recorded in association with both natural vegetation and IATs. Shady conditions reduced relative abundance, whether the riparian vegetation was natural or alien, confirming the results of Kinvig and Samways (2000).
Adult dragonfly assemblages have the potential to indicate water quality, with some species being more sensitive than others (Magoba 2005; Samways and Sharratt 2010). Multispecies assemblages here were good ecological indicators of natural or alien riparian vegetation. N. farinosa, C. erythraea and O. abbotti benefited from clearing of vegetation, while O. trinacria, T. dorsalis and T. stictica were highly tolerant of IAT impact.
Environmental variables and adult dragonfly species
Like the benthic macroinvertebrate scores, adult dragonfly scores were depressed under the alien tree canopy. Yet the cleared vegetation, with its reduced shade and moderately high temperature, was associated with the highest average adult dragonfly species richness, which was almost double that associated with natural vegetation and IATs. This contrasted with the benthic macroinvertebrate scores, which were highest in natural vegetation. The reason for this is that most local dragonfly species are highly sensitive to shade (Samways 2007), with adult assemblages at alien tree sites often mirroring those at shady natural sites (Kinvig and Samways 2000). Yet, when alien trees are removed there is a surge in the sun-loving dragonfly assemblage, which decreases again as natural, bushy vegetation re-establishes (Samways and Sharratt 2010).
Correlation between macroinvertebrate scores and adult dragonflies
We found here that the ASPT is the best measure for comparing the status of different parts of the rivers, supporting earlier findings (Armitage et al. 1983; Wright et al. 1984; Metcalfe 1989; Resh and Jackson 1993; Dickens and Graham 2002; Dinakaran and Anbalagan 2007). ASPT and adult dragonfly species richness, responded similarly to riparian vegetation type and can be considered mutually supporting metrics (Fig. 5), although the adult dragonflies were much more responsive to the sunlit conditions that were provided when alien trees were removed. The combined use of ASPT and adult dragonfly species richness composition (especially integrated into the Dragonfly Biotic Index; Simaika and Samways 2008, 2009) is recommended for measuring impact of invasive alien plants on stream faunas is justified, confirming Smith et al. (2007).
We also found that the EPTO taxa group alone was a sensitive indicator, with the results showing a significant difference between stretches of rivers with different riparian vegetation conditions compared to the use of overall macroinvertebrate taxon richness. These findings confirm others, including those of Dinakaran and Anbalagan (2007), where aquatic macroinvertebrates, especially those belonging to the EPT taxa, were highly sensitive to habitat changes in the south Western Ghats.
Implications for practice
We showed here that removal of riparian invasive alien trees (IATs) is a very positive management activity for recovery of aquatic biodiversity. The spatial variation in local richness of stream macroinvertebrates is affected by IAT disturbances, and recovery and maintenance of intact riparian vegetation is significant for the conservation of stream invertebrate faunas. Although macroinvertebrate assemblages started to respond remarkably quickly to removal of dense stands of IATs, full recovery of the assemblage took a few years. In summary, use of EPTO taxon richness and ASPT, with adult dragonfly species richness as an important supporting metric at the species level, is recommended in river condition assessments.
References
Armitage PD, Moss D, Wright JF, Furse MT (1983) The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res 17:333–347
Boon PJ (1988) The impact of river regulation on invertebrate communities in the UK. Reg Rivers Res Manag 2:389–409
Buchwald R (1992) Vegetation and dragonfly fauna - characteristics and examples of biocenological field studies. Vegetatio 101:99–107
Bulánková E (1997) Dragonflies (Odonata) as bioindicators of environmental quality. Biologia 52:177–180
Butler RG, deMaynadier PG (2008) The significance of littoral and shoreline habitat integrity to the conservation of lacustrine damselflies (Odonata). J Insect Conserv 12:23–36
Calder IR (1999) The blue revolution: land use and integrated water resources management. Earthscan, London
Chovanec A, Waringer J (2001) Ecological integrity of river-floodplain systems-assessment by dragonfly surveys (Insecta: Odonata). Reg Rivers Res Manag 17:493–507
Chwala E, Waringer J (1996) Association patterns and habitat selection of dragonflies (Insecta: Odonata) at different types of Danubian backwaters at Vienna, Austria. Arch Hydrobiol Suppl Large Rivers 115:45–60
Clark TE, Samways MJ (1996) Dragonflies (Odonata) as indicators of biotope quality in the Kruger National Park, South Africa. J Appl Ecol 33:1001–1012
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Clavero M, Garcia-Berthou E (2005) Invasive species are a leading cause of animal extinctions. Trends Ecol Evol 20:110
Culp JM, Walde SJ, Davies RW (1983) Relative importance of substrate particle size and detritus to stream benthic macroinvertebrate microdistribution. Can J Fish Aquat Sci 40:1568–1574
D’Amico FD, Darblade S, Avignon S, Blanc-Manel S, Ormerod SJ (2004) Odonates as indicators of shallow lake restoration by liming: comparing adult and larval responses. Restor Ecol 12:439–446
Dickens CWS, Graham PM (2002) The South African scoring system (SASS) version 5 rapid bioassessment system for rivers. Afr J Aquatc Sci 27:1–10
Diedericks GJ (2002) Assessment of stream conditions draining Mondi and SAFCOL commercial tree plantations in the Limpopo catchment, using SASS5. Internal report for Mondi Forests and SAFCOL, South Africa
Dinakaran S, Anbalagan S (2007) Anthropogenic impacts on aquatic insects in six streams of south Western Ghats. J Insect Sci 7:37
Dobson M, Hildrew AG (1992) A test of resource limitation among shredding detritivores in low order streams in southern England. J Anim Ecol 61:69–77
Dye P, Jarmain C (2004) Water use by black wattle (Acacia mearnsii): implications for link between removal of invading trees and catchment streamflow response. Sth Afr J Sci 100:40–44
Foote AL, Hornung CLR (2005) Odonates as biological indicators of grazing effects on Canadian prairie wetlands. Ecol Entomol 30:273–283
Foster SE, Soluk DA (2004) Evaluating exuvia collection as a management tool for the Federally endangered Hine’s emerald dragonfly, Somatochlora hineana Williamson (Odonata: Cordulidae). Biol Conserv 118:15–20
Hawking JH, New TR (1999) The distribution patterns of dragonflies (Insecta: Odonata) along the Kiewa River, and their relevance in conservation assessment. Hydrobiologia 392:249–260
Hawkins CP, Norris RH (2000) Performance of different landscape classifications for aquatic bioassessment: introduction to the series. J North Am Benthol Soc 16:728–749
Hawkins CPM, Murphy ML, Anderson NH (1982) Effects of canopy, substrate composition, and gradients on the structure of macroinvertebrate communities in Cascade Range streams of Oregon. Ecology 62:387–397
Hildrew AG, Townsend CR (1976) The distribution of two predators and their prey in an iron rich stream. J Anim Ecol 45:41–57
Hirji R, Johnson P, Maro P, Matiza Chiuta T (2002) Defining and mainstreaming environmental sustainability in water resources in southern Africa. SADC, IUCN, SARDC, World Bank, Maseru
Hofmann TA, Mason CF (2005) Habitat characteristics and the distribution of Odonata in a lowland river catchment in eastern England. Hydrobiologia 539:137–147
Inc SPSS (2006) SPSS version 15.0 for windows. SPSS Inc, Chicago
Kinvig R, Samways MJ (2000) Conserving dragonflies along streams running through commercial foestry. Odonatologica 29:195–208
Latimer AM, Silander JA, Gelfand AE, Rebelo AG, Richardson DM (2004) Quantifying threats to biodiversity from invasive alien plants and other factors: a case study from the Cape Floristic Region. Sth Afr J Sci 100:81–86
Magoba RNN (2005) Effect of invasion and clearing of alien riparian vegetation on benthic macroinvertebrate and adult Odonata assemblages in Soutpansberg rivers. MSc Thesis, University of Stellenbosch, South Africa
Marden JH, Cobb JR (2004) Territorial and mating success of dragonflies that vary in muscle power output and presence of gregarine gut parasites. Anim Behav 68:857–865
Merz JE, Ochikubo Chan LK (2005) Effects of gravel augmentation on macroinvertebrate assemblages in a regulated California river. River Res Applic 21:61–74
Metcalfe JL (1989) Biological water quality assessment of running waters based on macroinvertebrate communities: history and present status in Europe. Environ Pollut 60:101–139
Moore NW (1991) The development of dragonfly communities and the consequences of territorial behaviour: a 27-year study of small ponds at Woodwalton Fen, Cambridgeshire, United Kingdom. Odonatologica 20:203–231
Norris RH, Thoms MC (1999) What is river health? Freshw Biol 41:197–209
Orians GH, Wittenberger JF (1991) Spatial and temporal scales in habitat selection. Am Nat 137:S29–S49
Quinn JM, Hickey CW (1994) Hydraulic parameters and benthic invertebrate distributions in two gravel-bed New Zealand rivers. Freshw Biol 32:489–500
Rempel LL, Richardson JS, Healey MC (2000) Macroinvertebrate community structure along gradients of hydraulic and sedimentary conditions in large gravel-bed river. Freshw Biol 45:57–73
Resh VH, Jackson JK (1993) Rapid assessment approaches to biomonitoring using benthic macroinvertebrates. In: Rosenberg DM, Resh VH (eds) Freshwater biomonitoring and benthic macroinvertebrates. Chapman and Hall, New York
Richardson DM, Van Wilgen BW (2004) Invasive alien plants in South Africa: how well do we understand the ecological impacts? Sth Afr J Sci 100:45–52
Rith-Najarian JC (1998) The influence of forest vegetation variables on the distribution and diversity of dragonflies in a northern Minnesota forest landscape: a preliminary study (Anisoptera). Odonatologica 27:335–351
Samways MJ (1989) Insect conservation and the disturbance landscape. Agric Ecosyst Environ 27:183–194
Samways MJ (2007) Threat levels to odonate assemblages from invasive alien tree canopies. In: Cordero Rivera A (ed) Forests and dragonflies. Pensoft, Sophia, pp 209–224
Samways MJ, Sharratt NJ (2010) Recovery of endemic dragonflies after removal of invasive alien trees. Conserv Biol 24:267–277
Samways MJ, Steytler NS (1996) Dragonfly (Odonata) distribution patterns in urban and forest landscapes, and recommendations for riparian management. Biol Conserv 78:279–288
Samways MJ, Taylor S (2004) Impacts of invasive alien plants on red-listed South African dragonflies (Odonata). Sth Afr J Sci 100:78–80
Samways MJ, Caldwell PM, Osborn R (1996) Spatial patterns of dragonflies (Odonata) as indicators for design of a conservation pond. Odonatologica 25:157–166
Samways MJ, Taylor S, Tarboton W (2005) Extinction reprieve following alien removal. Conserv Biol 19:1329–1330
Sarriquet PE, Bordenave P, Marmonier P (2007) Effects of bottom sediment restoration on interstitial habitat characteristics and benthic macroinvertebrate assemblages in a headwater stream. River Res Applic 23:815–828
Schindler M, Fesl C, Chovanec A (2003) Dragonfly associations (Insecta: Odonata) in relation to habitat variables: a multivariate approach. Hydrobiologia 497:169–180
Sheldon AL (1986) Species diversity and longitudinal succession in stream fishes. Ecology 49:193–198
Simaika JP, Samways MJ (2008) Reserve selection using red listed taxa in three global biodiversity hotspots: dragonflies in South Africa. Biol Conserv 142:638–651
Simaika JP, Samways MJ (2009) An easy-to-use index of ecological integrity for prioritizing freshwater sites and for assessing habitat quality. Biodivers Conserv 18:1171–1185
Smith J, Samways MJ, Taylor S (2007) Assessing riparian quality using two complementary sets of bioindicators. Biodivers Conserv 16:2695–2713
Stewart DAB, Samways MJ (1998) Conserving dragonfly (Odonata) assemblages relative to river dynamics in an African savanna game reserve. Conserv Biol 12:683–692
Suh AN, Samways MJ (2001) Development of a dragonfly awareness trail in an African botanical garden. Biol Conserv 100:345–353
Switzer PV (1997) Factors affecting site fidelity in a territorial animal, Perithemis tenera. Anim Behav 53:865–877
Tallamy DW (2004) Do alien plants reduce insect biomass? Conserv Biol 18:1689–1692
Ter Braak CJF, Šmilauer P (2002) CANOCO reference manual and user’s guide to Conoco for Windows: software for Canonical Community Ordination (version 4.5). Microcomputer Power, Ithaca, NY
Townsend CR, Hildrew AG (1994) Species traits in relation to a habitat templet for river systems. Freshw Biol 31:265–275
Ulfstrand S (1967) Microdistribution of benthic species (Ephemeroptera, Plecoptera, Trichoptera, Diptera: Simuliidae) in Lapland streams. Oikos 18:293–310
Water Research Commission (2001) State of rivers report: Crocodile, Sabie, Sand, and Olifants river systems. RSA, WRC Report No.TT 147/01
Wittenberg R, Cock MJW (eds) (2001) Invasive alien species: a toolkit of best prevention and management practices. CAB International, Wallington
Wright JF, Moss D, Armitage PD, Furse MT (1984) A preliminary classification of running-water sites in Great Britain based on macro-invertebrate species and the prediction of community type using environmental data. Freshw Biol 14:221–256
Zimmermann HG, Moran VC, Hoffmann JH (2004) Biological control in the management of invasive alien plants in South Africa, and the role of the Working for Water Programme. Sth Afr J Sci 100:34–40
Acknowledgments
We thank Steven Lumber Mills and Komatiland Forest for allowing access to their plantations, S. Taylor for logistic support, and G. Diedericks for access to his internal report. We especially thank R. Madzivhe, M. Tshikalange, G. Maluleke and L. Magoba for assistance in the field. Financial support was from the Working for Water Programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magoba, R.N., Samways, M.J. Recovery of benthic macroinvertebrate and adult dragonfly assemblages in response to large scale removal of riparian invasive alien trees. J Insect Conserv 14, 627–636 (2010). https://doi.org/10.1007/s10841-010-9291-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-010-9291-5