Abstract
Frequent bark beetle outbreaks cause biome-scale impacts in boreal and temperate forests worldwide. Despite frequent interceptions at ports of entry, the most aggressive bark beetle species of Ips and Dendroctonus in North America and Eurasia have failed to establish outside their original home continents. Our experiments showed that Ips typographus can breed in six North American spruce species: Engelmann spruce, white spruce¸ Sitka spruce, Lutz spruce, black spruce and red spruce. This suggests that differences between the Eurasian historical host and North American spruce species are not an insurmountable barrier to establishment of this tree-killing species in North America. However, slightly diminished quality of offspring beetles emerged from the North American spruces could reduce the chance of establishment through an Allee effect. The probabilistic nature of invasion dynamics suggests that successful establishments can occur when the import practice allows frequent arrivals of non-indigenous bark beetles (increased propagule load). Model simulations of hypothetical interactions of Dendroctonus rufipennis and I. typographus indicated that inter-species facilitations could result in more frequent and severe outbreaks than those caused by I. typographus alone. The potential effects of such new dynamics on coniferous ecosystems may be dramatic and extensive, including major shifts in forest structure and species composition, increased carbon emissions and stream flow, direct and indirect impacts on wildlife and invertebrate communities, and loss of biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions represent a threat to the world native plant biota, including the coniferous forests that cover at least 31% of global forested areas. In northern forest ecosystems, coniferous forests constitute a large portion of the terrestrial land, e.g. 43.0% in Fennoscandia, 24.7% in Russia, and 16.7% in Canada (Global Forest Resources Assessment 2010). These proportions increase substantially if mixed conifer stands are also considered (e.g. 34% in Canada).
Thousands of insect species are associated with coniferous forest ecosystems although relatively few of them can impact ecosystem structure and function. For example, bark and ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) total more than 5,800 species worldwide (Wood and Bright 1992); however, only few of them, mostly in the genera of Dendroctonus, Ips and Scolytus, are considered economically important species. Some Dendroctonus and Ips species are considered among the most destructive insect species in temperate and boreal conifer forests and may virtually kill all host trees over extensive areas during periods of outbreak (Christiansen and Bakke 1988; Økland and Bjørnstad 2006; Raffa et al. 2008).
Different species of bark beetles are responsible for large outbreaks in different continents. For example, only a handful of bark beetles undergo large-scale outbreaks on conifers in North America, such as Dendroctonus ponderosae Hopkins (mountain pine beetle), D. frontalis Zimmermann (southern pine beetle), D. rufipennis (Kirby) (North American spruce beetle), D. pseudotsugae Hopkins (Douglas fir beetle), and Ips perturbatus (Eichhoff) (Northern spruce engraver). None of these species are naturally found in Eurasia. Eurasian spruce bark beetle, Ips typographus (L), is the most damaging bark beetle species in Eurasia (Wermelinger 2004) and considered a great risk to invade North America, where it may cause extensive ecological and economic impact as a result of mortality to native spruce species (Haack 2006).
Increasing global trade of timber and frequent use of wood packaging materials like pallets, crating, and dunnage, have provided important pathways for the introduction of bark and ambrosia beetles worldwide (Haack 2001, 2006; Brockerhoff et al. 2006; Piel et al. 2008; Skarpaas and Økland 2009). For example, in New Zealand more than 1,500 interceptions of 103 bark and ambrosia beetle species were recorded at the border from 1950 to 2000, including bark beetles classified as high-risk species such as D. ponderosae and I. typographus (Brockerhoff et al. 2006). Likewise, in the USA, 6,825 interception records of alien bark beetles, including some of the high risk species, were reported at ports of entry for the period of 1985–2000 (Haack 2001).
Some bark beetle species have successfully invaded new geographical ranges and extended their host range. For instance, the red turpentine beetle, Dendroctonus valens LeConte, native to North America was introduced into China around 1985 and, since then, has caused extensive mortality on the native pine species, such as Pinus tabuliformis Carrière and other pine species in north central China (Li et al. 2001; Yan et al. 2005). In contrast, others have been intercepted repeatedly without becoming established, despite the presence of suitable host plants and climate (Haack 2001, 2006; Brockerhoff et al. 2006; Liebhold and Tobin 2008). For example, I. typographus has been intercepted in the USA 253 times from 1985 to 2005 (Haack 2006). Considering this intense propagule pressure and the presence of many suitable host trees, it is puzzling that this species has never become established in North America. This could be a failure of detection in which beetles may be present at lower levels than the detection threshold (Skarpaas and Økland 2009). Likewise, under suitable conditions, they may establish populations in surrounding forests, but may occur at levels below the threshold for successful attack on living trees, i.e. on timber or already dying trees. A clear understanding of why invading insect populations sometimes fail or succeed to establish is of considerable interest to the development of strategies for managing and preventing biological invasions.
Our main questions are about the invasion likelihood and what we should expect if any of the tree-killing bark beetle species mentioned above establish and survive in other continents. Predicting the effects of non-indigenous invasive species is difficult, but careful consideration of their potential impact is important in the development of pre-emptive management priorities for areas with particularly high risk of beetle establishment. Several syntheses already exist for invasive species in general (e.g. Williamson 1996; Pimentel 2002; Kenis et al. 2009), but such studies are urgently needed for eruptive bark beetles that may have widespread effects on forest ecosystem processes.
In this paper we focus on the likelihood of bark beetle invasions, the outcome of potential interaction between non-indigenous invasive and native species of tree-killing bark beetles and review on the ecosystem effects that may occur due to such interactions in coniferous forest ecosystems. We (1) present results from an experiment on six North American spruce species to give insight in the role of host tree species in the potential establishment of I. typographus in North America, (2) present simulation results of interaction effects given that a non-indigenous tree-killing bark beetle is successfully established in the habitat of a native tree-killing bark beetle, and (3) utilize our results as an integrated part of an extended discussion of invasion likelihood and the potential outcome of inter-species interactions between two outbreak species on different landscape level processes.
Methods
Colonization and breeding experiments of Ips typographus
We performed colonization and breeding experiments to test the suitability of spruce species for the potential establishment of I. typographus in North America, using six potential spruce host species on this continent compared to the major native host of the beetle in Eurasia, Norway spruce (Picea abies (L.) Karsten, 1881). We were particularly interested in determining if I. typographus can colonize and produce brood on the North American spruce species. Because field experiments with live I. typographus in North America were not possible, we utilized planted North American spruces in Scandinavia. As these exotic spruce species are not systematically planted in large numbers in Scandinavia, we found it necessary to divide our experiments on two areas where some of the North American spruces are planted together with Norway spruce, Ås in Norway and Tönnersjöheden in Sweden. While our experiments were restricted to a few trees, other factors were kept as similar as possible, including stand, growing conditions, soil, size of tree and phloem thickness. Therefore, we could only look for striking differences that could indicate insurmountable establishment barriers due to tree species, while detailed analyses of differences due to factors within and between forest stands could not be addressed.
In field experiments in Norway (59°40′N, 10°46′E), we utilized three North American spruce species, red spruce (Picea rubens Sargent 1898), Engelmann spruce (P. engelmanii Parry ex Engelmann 1863) and Lutz spruce (P. sitchensis × P. glauca) growing together with Norway spruce in an arboretum with a natural I. typographus population. We were allowed to fell one tree and perform attack experiments on additional five standing trees of each species, using trees that had about the same size (diameter at breast height 19.7 ± 3.2 cm), phloem thickness (0.41 ± 0.07 cm) and bark structure (all smooth-barked, Engelmann spruce with some scales). All trees grew at about similar densities and conditions, standing at most 300 meter apart.
On 26th May 2008, at the onset of I. typographus’ flight period, we randomly selected five trees per species within the stands (to avoid edge effects). As the population levels of I. typographus was well below the outbreak threshold during the course of the experiment, initial attraction of bark beetles was stimulated by attaching a pheromone dispenser (Ipslure®, www.kjemikonsult.no) 2 m above ground on each tree for 3 days. Then, all baits were removed to determine if the beetles were able to sustain colonization without the synthetic pheromone during the short flight period. Fifteen days after removing the baits (June 10, 2008), the outer bark was carefully shaved away around two randomly selected beetle entrance holes per tree (ten attacks per tree species) and the length of maternal egg galleries (i.e. from the nuptial chamber to the end of egg gallery) was measured.
We also cut ten 50 cm long stem sections from each of the tree species in the arboretum (May 26, 2008). The trees had approximately similar size (diameter at breast height 18.5 ± 1.6 cm), similar phloem thickness (about 0.4 cm) and growing conditions as described above. The resulting 40 bolts were randomly distributed on a nearby recent clear-cut spruce area in Såner (59°32′N, 10°47′E) with 10–15 m distance between bolts. The replicates of ten bolts from each species accounted for a possible variation in placement and attraction within the clear-cut area. Each bolt was baited with synthetic aggregation pheromone of I. typographus, which was removed after 7 days when all bolts had some level of beetle colonization. The bolts were left in the field for additional 7 days for beetle colonization, and then brought to an insectary in Ås. After counting the number of entrance holes per m2 (surface area = mean of circumferences at the ends of the bolt × length of the bolt) all bolts were individually placed in emergence bags with collection bottles. The bottles were emptied weekly and the reared beetles were counted and weighed (dry weight of ten offspring beetles from each bolt collected on 4th August 2008).
The field experiment in Sweden (56°41′N, 4°57′E) included three North American spruce species, black spruce (Picea mariana (Miller) Britton Sterns, & Poggenburg 1888), white spruce (Picea glauca (Moench) Voss 1907) and Sitka spruce (Picea sitchensis (Bongard) Carrière 1855) spruce, together with Norway spruce. Eight 80–100 cm long stem sections were cut from each species on 8th May 2009. The sections were cut from two trees in each species, four sections per tree. All trees were of similar size (about 20 cm diameter at breast height) and phloem thickness (about 0.4 cm). The bark was generally smooth, Sitka and black spruce having some scales. All trees were growing in about similar densities and conditions within forests of the same area at Tönnersjöheden. All bolts were laid out in a randomized block design at a nearby storm-felling site and baited with pheromone dispensers. On 1st July 2009, six logs of each tree species were moved to the local research station and hung up in emergence bags with collection bottles, while two bolts of each species remained in the storm-felling site. The bottles were emptied weekly and reared beetles were counted and weighed (dry weight of all offspring beetles from each bolt on 12th December 2009). On 8–9th September 2009, maternal gallery lengths were measured from on a 15 × 40 cm area in the middle of each bolt.
Data for all spruce species were compared to Norway spruce (control species), using two-sample t tests with separate variances and the Welch approximation to the degrees of freedom, using the ‘‘R’’ statistical and programming environment (R Development Core Team 2009).
Simulation of interaction effects between non-indigenous and native tree-killing bark beetles
While the field experiments above address the possibilities for colonization and breeding, the effects of subsequent interactions between non-indigenous and native tree-killing bark beetles on large-scale outbreak dynamics cannot be tested by field experiments. We therefore used model simulations to explore the possible outcomes for two examples species that are aggressive bark beetles on spruce in their home ranges, I. typographus and D. rufipennis. For the native species, we utilized a resource-based Gompertz model to characterize the population dynamics of I. typographus in Scandinavia, including a full parameterization based on a comprehensive literature (detailed descriptions are given in Økland and Bjørnstad 2006). The resource base of the model consists of two components: wind-felled trees and living trees that are susceptible to beetle attacks. A key property of this model is the existence of a population threshold for starting tree-killing and outbreaks. The model reproduces the general behaviour of the bark beetle outbreak dynamics reasonably well, and the results are consistent with historical outbreak periods in Scandinavia (Økland and Bjørnstad 2006). This model base was extended (Økland et al. 2009) to a two-species interaction model by including a second species (D. rufipennis) on the same resources using a Lotka-Volterra type of interaction term (\( \alpha \)):
where N t is the population density in year t, a is the Gompertz growth rate, and K t is the resource base, and the subscripts i and j denote species i and j, respectively. The net interaction effect of species j on species i (ΔR i,j,t ) was defined as the difference between the per capita growth rate with an interaction effect (\( R_{i,j,t} = N_{i,j,t} /N_{i,j,t - 1} - 1 \)) and the growth rate without an interaction effect (\( R_{i,t} = N_{i,t} /N_{i,j,t - 1} - 1 \)). As a positive ΔR i,j,t implies a facilitation between the two species (a mutualism where the species “help” each other to exceed the threshold at which they get access to an extended common resource by killing trees), the percentage of years in the time series with a positive ΔR i,j,t was used as a measure of how often this facilitation occurs.
Due to uncertainties about biological parameters for a non-indigenous species (D. rufipennis) that is not established and observed under new system conditions, we varied parameters assumed to be of importance. Using the parameterized model framework, we explored in detail how the frequency of positive interactions varied as a function of the aggressiveness of the invasive species (D. rufipennis). It is assumed that the non-indigenous invasive species has to coexist with the native I. typographus, and that a significant overlap between the species ensures optimal frequency of positive interactions. We varied the relative aggressiveness of the invasive species with an index ranging from 0 (minimum aggressiveness) to 1 (maximum aggressiveness) (according to a beetle density threshold needed to kill trees), while the native species was set at maximum aggressiveness. In each time step, a total beetle density weighted by aggressiveness is compared to the threshold density of beetles needed to kill living trees. The simulations were repeated 100 times at each parameter combination, and a smoothed trend was calculated for each species using the smooth.spline function. All simulations and calculations were performed in the ‘‘R’’ statistical and programming environment (R_Development_Core_Team 2009).
Results
Colonization and breeding experiments of Ips typographus
I. typographus can colonize all six species of North American spruce (Tables 1, 2, Fig. 1). Only Engelmann spruce had lower densities of beetle attacks (entrance holes m−2) than Norway spruce (t = 4.27, df = 14.36, P = 0.0007) (Table 1). For the remaining trees, differences were not statistically significant. Beetles attacked live trees of all four spruce species in the experiment in Norway, and no significant differences in mean length of maternal galleries were observed during the initial period (Table 1).
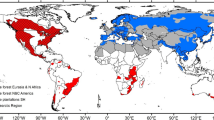
Geographical range of the major host spruce species for the tree-killing bark beetle species, Ips typographus, Dendroctonus rufipennis and Ips pertubatus. Major hosts of I. typographus: Norway spruce and Siberian spruce. Exact border between these spruce species is not drawn on the map due to hybridization zone, but is assumed to be to the east of the Ural Mountains (Tollefsrud et al. 2009; M. Tollefsrud pers. comm.). Other hosts of I. typographus with limited distributions in Eurasia are not included in the map (e.g., states around the Black Sea, Ukraine, Far East of Russia, parts of China, Japan, both Korean states). Hosts of D. rufipennis: Engelmann spruce, white spruce¸ Sitka spruce, black spruce, blue spruce, and red spruce. Hosts of I. pertubatus: white spruce and Sitka spruce. Both D. rufipennis and I. pertubatus utilize Lutz spruce covered by the distribution areas of white spruce and Sitka spruce on the map
All the measured variables indicate that I. typographus can breed and develop successfully in the tested North American spruce species (Tables 1 and 2). Fully developed maternal galleries were found in all spruce species during inspection of bark samples in the fall (Table 2). Beetle offspring had a complete development on all species and the beetle production was fairly high for all of the North American spruces. In the experiment in Norway, the mean number of offspring per pair of parents for Engelmann spruce was higher (t = −3.56, df = 12.92, P = 0.004) relative to that on Norway spruce, whereas for red spruce it was lower (t = 2.95, df = 17.73, P = 0.009) (Table 1). Offspring production did not differ significantly between the spruce species in the experiment in Sweden (Table 2).
The offspring quality (dry weight per beetle) was comparable to Norway spruce for all of the North American spruce species; however, the mean weights were smaller in Sitka spruce (t = 5.16, df = 7.39, P-value = 0.001), Lutz spruce (t = 5.008, df = 14.83, P = 0.0002), red spruce (t = 2.34, df = 12.25, P = 0.037) and Engelmann spruce (t = 2.63, df = 17.49, P = 0.017) than the mean weights of beetles emerged from Norway spruce. White spruce and black spruce did not produce significantly lighter offspring than Norway spruce (Table 2).
Simulation of interaction effects between non-indigenous and native tree-killing bark beetles
The net inter-species interaction (ΔR i,j,t ) between I. typographus and D. rufipennis was found to be highly dynamic and may alternate in time between positive and negative values. The frequency of net positive interactions varied with interaction strengths and the relative aggressiveness of the species, while net positive interactions did not occur if the productivity and aggressiveness of D. rufipennis should be very low as an invasive species under new conditions. However, the interaction effects could be significant without a high productivity and aggressiveness of D. rufipennis. Frequent facilitations for both species occur even if D. rufipennis should happen to be much less aggressive than I. typographus (Fig. 2). For I. typographus, the presence of the invasive can yield facilitations in the majority of years (>50%) when the relative aggressiveness of D. rufipennis (compared to I. typographus) is higher than 0.5, and facilitations would occur in about 75% of years for relative aggressiveness higher than 0.8. The facilitation effect for the invasive species (D. rufipennis) is even stronger, with facilitation in the majority of years (>50%) for relative aggressiveness of D. rufipennis above 0.14 and a maximum of 85% of years for an index aggressiveness value of 0.6.
Discussion
Likelihood of bark beetle invasions in North American spruce forests
There are many factors that may affect establishment of insects in new habitats, including climate suitability, introduction thresholds, and availability and suitability of host trees. The results from our colonization and breeding experiments indicate that difference in spruce species is not an insurmountable barrier to the establishment of I. typographus in North America. The high production of offspring in all of the species suggests that I. typographus is capable of breeding in dead trees of all of the six North American spruce species tested in our experiments. Offspring production is believed to vary with qualities of trees, forest stands, local climate and the complex population dynamics of bark beetles (Anderbrant et al. 1985; Anderbrant 1990; Økland and Bjørnstad 2003). Our experiments do not allow analysis of how these factors influence the differences in offspring production between the spruce species. However, a standardization of the test conditions on a few trees allow us to conclude that breeding of I. typographus appears to be possible on all of the six major spruce species that are widespread in North America (Fig. 1). Also it seems likely that live trees can be colonized, as the maternal gallery lengths during the initial attacks were about the same for all spruce species in our experiment. The success of colonization and production of offspring in live trees certainly depends on several factors, such as tree vigour (Franceschi et al. 2005) and population densities of beetles (Weslien et al. 1989). Successful tree-killing of Sitka spruce by I. typographus have been observed in different European plantations (Browne and Laurie 1968, Ulf Johansson pers. comm.). Since D. rufipennis is widespread and found on most spruce species in North America (Holsten et al. 2000), it does not seem unrealistic that this significant spruce-killing bark beetle in North America can utilize Norway spruce as well. However, we lack experimental results to verify this, and there is no statistics about interception frequency of D. rufipennis at Scandinavian ports of entry.
Lower levels of colonization success and productivity of the beetles on North American tree species may influence the likelihood of becoming established. For example, trees may vary in chemical precursors necessary for production of aggregation pheromone of bark beetles, and hence affect mate location and successful host colonization (Wood 1982a; Erbilgin and Raffa 2000). In our experiments, many of the North American spruce species produced somewhat lighter offspring of I. typographus compared to the native host Norway spruce. It is known that smaller beetles may result in fewer and smaller offspring in the next generation (Anderbrant et al. 1985), which in turn may contribute to a maternal effect in the population dynamics (Turchin 2003). Lower productivity of the beetles may indirectly influence the strength of Allee effects and add to other potential sources of Allee effects, such as mating failure or too low population size for sufficient aggregation to colonize trees (Stephen and Grégoire 2001), or lack of benefits from aggregation behaviour (Wallin and Raffa 2004). A significant Allee effect may be important for the invasive population dynamics and the probability of establishment in the new environment with different host trees (Taylor and Hastings 2005; Liebhold and Tobin 2008). It is difficult to evaluate how a slightly lower colonization success and productivity of the beetles will influence the likelihood of becoming established without implementing this information into the complex population dynamics of bark beetles in a spatio-temporal setting.
Even though Allee effects may reduce the establishment success of alien bark beetles that arrive on a new continent, the probabilistic nature of the systems indicates that we cannot ignore the risk of exotic aggressive bark beetles to become established. The more often a species arrives at a location, the more likely it is to invade. For example, Brockerhoff et al. (2006) showed that frequently intercepted bark beetle species are about four times more likely to successfully invade an area than species that are intercepted rarely. We recently developed a generic model for the first step of the invasion process for trade-imported pests, and applied it to potentially harmful bark beetles (Skarpaas and Økland 2009). The model results suggested that current timber import practices are likely to lead to introductions of new bark beetle species, and may in some cases lead to beetle immigration in densities corresponding to outbreak levels at timber storage sites at the port of entry. Furthermore, the results suggested that arrivals may often go undetected by pheromone traps (Skarpaas and Økland 2009), which may lead to a delay in detection (Lee 2002; Liebhold and Tobin 2008). In some cases, populations may require multiple introductions to improve the gene pool for population adaptation and growth (Ellstrand and Schierenbeck 2000; Kolbe et al. 2004; Suarez and Tsutsui 2008), or the local immigrant population may remain small for a long time due to emigration (Shigesada and Kawasaki 1997; Kean and Barlow 2000).
Direct interaction effects between non-indigenous and native tree-killing bark beetles on outbreak dynamics
While most bark beetles depend on dead trees, the tree-killing species can attack healthy trees during periods of epidemic outbreaks. In general, several feedback mechanisms may be operating at different thresholds of interaction prior to bark beetle epidemics, such as host availability and suitability, changes in beetle population density, weather and escape from natural enemies (Raffa et al. 2008). The population density is particularly important for initiation of successful mass colonization of living trees, which in turn can lead to tree mortality at the landscape-level (Weslien et al. 1989; Økland and Bjørnstad 2003; Baier et al. 2007).
Non-indigenous invasive bark beetles may play an important role at the landscape level due to their direct or indirect interactions with native bark beetles. For example, if an invasive bark beetle is capable of colonizing and breeding in native tree species (as suggested in our experiment with I. typographus and six North-American spruce species), situations may arise where the invasive and native beetle species are forced to partition similar resources on the same tree. Given that both species are successful in this joint habitat, their competition for the same resources may reduce the reproductive success of both species. Alternatively, their collaborative tree-killing effect may have a positive impact on attack density, i.e. a relatively lower number of beetles per species may be required to kill a host tree, which may enable each species to attack and kill more trees. Our simulations of interaction effects indicate that positive interactions may be frequent if the most significant spruce-killing bark beetle in North America, D. rufipennis, becomes established in the habitat of I. typographus in Scandinavia. The results show frequent facilitations even if D. rufipennis as a non-indigenous species in a new environment should prove to be far less productive and aggressive than I. typographus. This suggests that an introduction of D. rufipennis in Scandinavia could lead to more frequent eruptions than when I. typographus acts alone.
Effects of bark beetle outbreaks on economy and ecosystem function
Bark beetle outbreaks cause direct economical loss by killing large volumes of trees and indirect losses by negatively impacting forest industries and activities relying on non-timber aspects of forest resources. For example, between 1979 and 1983, D. ponderosae killed 80 million pine trees (ca. 30 million m3) in the Northwestern USA (McGregor 1985). During the same period D. frontalis killed pines equivalent to 17.4 million m3 in the southern USA (Hoffard 1985). Outbreaks of D. rufipennis have caused extensive spruce mortality from Alaska to Arizona in western North America, occurring in every forest with substantial spruce stands (Holsten et al. 2000). More recently, in western Canada, the current outbreak of D. ponderosae has killed 620 million m3 of wood (worth more than 40 billion Canadian $) in the period 2001–2009. It is predicted that it will kill 80% of the mature forest of lodgepole pine (Pinus contorta Douglas ex Loudon 1838) by 2013 (www.nrcan-rncan.gc.ca). Other examples of major tree-killing bark beetles in North America are the Douglas fir beetle (D. pseudotsugae Hopkins), and the Northern spruce engraver (Ips perturbatus (Eichhoff)) (Wood 1972; Bright 1976; Wood 1982b; Wood and Van Sickle 1992; Holsten and Werner 1997). Likewise, I. typographus has caused catastrophic timber loss in Central and Northern Europe (Annila 1969; Bakke 1989; Grégoire and Evans 2004). Since the late 1940s, excluding the recent outbreaks (Slovakia and Sweden), it has killed around 50 million m3 of Norway spruce in Europe (Christiansen and Bakke 1988). For introduced bark beetles, the potential cost of different treatment and control measures may be as high as those estimated for emerald ash borer (Kovacs et al. 2010), Asian longhorn beetle and citrus longhorn beetle (Haack et al. 2010).
Catastrophic events may be difficult to predict from information obtained at a single scale (Peters et al. 2004; Raffa et al. 2005). A better understanding of a bark beetle–forest system may require more knowledge about how processes at different biological levels and spatio-temporal scales interact (McMahon and Diez 2007; Raffa et al. 2008). The following synthesis should be viewed as possible scenarios when major tree-killing bark beetles become sympatric due to invasion, and if our model observations of facilitations between the species (Fig. 2) become dominant in large-scale ecosystems.
By killing trees over extensive areas during periodic outbreaks, invasive bark beetles may impact forest structure and species composition, understorey vegetation, hydrological and nutrient regimes, and animal biodiversity (see Table 3 for a survey of major effects). The literature on ecosystem effects of bark beetle outbreaks is limited. However, we may expect many similarities with the effects described for other insect herbivores in forests. According to a review of effects caused by alien insect herbivores in the mixed deciduous and hemlock forests, disturbances have altered the dynamics of canopy gaps, coarse woody debris, biogeochemical cycling, and ecological interactions among organisms in terrestrial and aquatic systems, with consequent effects on forest composition, structure, and function (Gandhi and Herms 2010). Further, it has been suggested that the carbon sink/source relationship is upset by bark beetle outbreaks, as widespread tree mortality reduces forest carbon uptake (sink) and increases emissions from decaying dead trees (source) (Kurz et al. 2008). For example, estimates based on the current outbreaks of D. ponderosae in British Columbia (Canada) showed that the cumulative impact of the beetle outbreak from 2000 to 2020 would be 270 megatonnes of carbon release, including the combined impact of the beetle, forest fires and harvesting (Kurz et al. 2008). This is particularly alarming considering the role of boreal forests in northern Canada as a carbon pool, taking up and storing atmospheric carbon.
The outcome of ecosystem effects is assumed to differ between regions. The level of changes in community structure will depend on several factors, such as tree species composition, presence of competing tree species, abundance of suitable host trees for aggressive beetle species, climatic variables (such as drought and temperature), and species composition of plants and animals prior to the outbreak (Lance and Howell 2000; Lance et al. 2006; Werner et al. 2006). Community changes due to indirect effects are particularly hard to predict without specific information about the ecology and the responses and inter-specific interactions of the local species.
Large areas could be affected if a major bark beetle was introduced. As an example, some of the major bark beetles on spruce could become sympatric, such as I. typographus, D. rufipennis or I. pertubatus, which could lead to amplified outbreaks and ecosystem-wide effects (Table 3) in their distribution ranges (Fig. 1). I. typographus is widely distributed in the areas where Norway spruce and Siberian spruce (P. obovata Ledeb. 1833) are dominant in Europe and Asia, respectively (Fig. 1). These spruce species are closely related and hybridize in some regions (Farjón 1990). I. typographus can also utilize oriental spruce (P. orientalis (L.) Link) around the Black Sea and other species in the diverse genus of Picea (Farjón 1990; Yamaoka et al. 1997; Ran et al. 2006; Akkuzu and Guner 2008), including planted Sitka spruce (Browne and Laurie 1968). In addition, a variety or subspecies I. typographus f. japonicus Niisima 1909 attacks Yezo spruce (Picea jezoensis (Siebold et Zuccarini) Carrière 1855) in Japan and the Far East of Russia (Wood and Bright 1992; Yamaoka et al. 1997). D. rufipennis is widespread throughout the range of Picea spp. in North America, attacking white (P. glauca (Moench) Voss 1907) and black spruce (P. mariana (Miller) Britton Sterns, & Poggenburg 1888) in the north, Engelmann and Sitka spruce in the west, and red spruce in the east (Fig. 1). The beetle is widespread particularly in western parts of North America, ranging from Alaska to Mexico, and along the Rocky Mountains (Wood 1982b). In recent years, there has been an increase in tree killing by I. pertubatus in interior and south-central Alaska, especially in white and Lutz spruce forests.
New interactions could lead to large-scale ecosystem effects if major spruce-killing bark beetles become sympatric due to invasion. At the population level, the model suggests facilitation that could lead to increased outbreak frequency depending on the relative aggressiveness of the species and the overlap between species. All of the species I. typographus, D. rufipennis and I. pertubatus, exhibit aggressive behaviour under certain conditions though our understanding of how their behaviours might change in the introduced range is limited due to absence of empirical data. We have species like Dendroctonus valens, which is a secondary pest in its historical range, but has become a tree-killing species in the introduced range (Yan et al. 2005). In our study, we only focused on tree-killing species, not secondary bark beetles because apparently their behaviour is quite different from tree-killing bark beetles. However, frequent facilitations were found even when D. rufipennis was assumed to have a relatively low aggressiveness as an introduced species (Fig. 2). The life cycles of the three species would allow a significant temporal and spatial overlap, which could give frequent facilitations, more frequent and severe outbreaks and ecosystem effects as described in Table 3. All three species have a univoltine life cycle with main dispersal flight from mid-May to early June in some portion of their native range, including northern Europe for I. typographus (Lange et al. 2006), and lowland northern areas of North America for D. rufipennis and I. pertubatus (Beckwith 1972; Wood 1982b). In some regions, life cycle asynchrony between invasive and native species may possibly lead to little or no temporal overlap, resulting in an advantage for the earlier colonizer. If the invasive species is not outcompeted, it may possibly reduce productivity and frequency of outbreaks of the native species, which may reduce the ecosystem impacts that are described in Table 3.
Absence of native competitors could also enhance significant ecosystem effects of bark beetle introductions. For example, Scots pine in Northern Europe does not harbour any major tree-killing bark beetle species, as opposed to important pine species in North and Central America, where huge areas are ravaged by tree-killing bark beetles. Although several species of defoliators and shoot-feeding species have affected Scots pine forests in some regions of Northern Europe, these outbreaks are on a much smaller scale than the extensive bark beetle outbreaks in North American pines. Scots pine is the most abundant and widely distributed tree species in Fennoscandia, where its standing volume constitutes 41.6% of the total forest volume. One possible candidate to invade the Scots pine forests in this region is mountain pine beetle, which inhabits various pine species in Canada and the northern USA. Planted Scots pines in North America are apparently highly suitable hosts for this species (M. Furniss pers. comm.). Comparing the climate in localities of D. ponderosae in North America with Scandinavia (with the software CLIMEX) indicates that the Scandinavian climate should be suitable for this bark beetle. If D. ponderosae should become established and a successful tree killer in its new environment, it could cause extensive ecosystem effects, greater than any experienced previously in the postglacial period of Scots pine forests in Scandinavia. To the extent that interactions with Diprion pini, Bupalus piniaria and Tomicus piniperda would occur, the weakening of pines by these defoliators and shoot-feeding species could promote eruptions by D. ponderosae. It remains to be analysed which regions of Northern Europe would offer the most suitable climate for a life cycle synchrony leading to successful mass attacks (Bentz et al. 1991; Logan and Powell 2001; Lange et al. 2006), and if there are other factors in the levels of biological hierarchy and spatiotemporal scales that would promote or limit outbreaks of D. ponderosae beetles in the Scots pine ecosystems.
Management implications
Introductions of major spruce-killing bark beetle species on other continents are likely to happen with the present import practices. A delay in detection of beetles can be expected since immigration often may go undetected even when pheromone traps are used. Once alien pest species have become established in their new habitats, they may be extremely difficult to eradicate, and the costs of damage and control programmes may be high (Pimentel 2002; Haack et al. 2010; Kovacs et al. 2010; Økland et al. 2010). Even though there are examples of successful eradications of invasive species (Simberloff 2009), we are not optimistic about eradicating highly mobile and productive alien bark beetles after they have become established in forests. Based on our simulation models, frequent inter-specific facilitations between native and invasive beetles is likely and may potentially result in more frequent and severe outbreaks with serious impacts both on the economy and ecosystem function. Pre-emptive measures such as ISPM-15 (Haack and Petrice 2009) can reduce arrivals of alien species by wood packaging material, and various measures have been suggested to reduce establishment success of true bark beetles that are transported with timber (Skarpaas and Økland 2009). Considering that introductions often are irreversible, there are good reasons to argue for application of the precautionary principle and a wide perspective in the cost-benefit calculations of risk assessments (Simberloff 2005). Thus, investments in import routines that reduce entries and lower the likelihood of introduction appear to be the most cost-efficient approach to this problem.
References
Akkuzu E, Guner S (2008) Defoliation levels of oriental spruce by Ips typographus (L.) in relation to elevation and exposure. J Environ Biol 29:223–226
Allen JL, Wesser S, Markon CJ, Winterberger KC (2006) Stand and landscape level effects of a major outbreak of spruce beetles on forest vegetation in the Copper River Basin, Alaska. For Ecol Manag 227:257–266. doi:10.1016/j.foreco.2006.02.040
Anderbrant O (1990) Gallery construction and oviposition of the bark beetle Ips typographus (Coleoptera: Scolytidae) at different breeding densities. Ecol Entomol 15:1–8
Anderbrant O, Schlyter F, Birgersson G (1985) Intraspecific competition affecting parents and offspring in the bark beetle Ips typographus. Oikos 45:89–98
Annila E (1969) Influence of temperature opon the development and voltinism of Ips typographus L. (Coleoptera, Scolytidae). Ann Zool Fennici 6:161–208
Baier P, Pennerstorfer J, Schopf A (2007) PHENIPS–A comprehensive phenology model of Ips typographus (L.) (Col., Scolytinae) as a tool for hazard rating of bark beetle infestation. For Ecol Manag 249:171–186. doi:10.1016/j.foreco.2007.05.020
Bakke A (1989) The recent Ips typographus outbreak in Norway–experiences from a control program. Hol Ecol 12:515–519
Bakke A, Kvamme T (1993) Beetles attracted to Norway spruce under attack by Ips typographus. Commun Skogforsk 45:1–24
Beckwith RC (1972) Scolytid flight in white spruce stands in Alaska. Can Entomol 104:1977–1983
Bentz BJ, Logan JA, Amman GD (1991) Temperature-dependent development of the mountain pine beetle (Coleoptera: Scolytidae) and simulation of its phenology. Can Entomol 123:1083–1094
Bethlahmy N (1974) More streamflow after a bark beetle epidemic. J Hydrol 23:185–189
Bethlahmy N (1975) A Colorado episode: beetle epidemic, ghost forests, more stream flow. Northwest Sci 49:95–105
Bright DE (1976) The insects and arachnids of Canada, Part 2. Canadian Department of Agricultural Publication, Canada, 1576, p 241
Brockerhoff EG, Bain J, Kimberley M, Knizek M (2006) Interception frequency of exotic bark and ambrosia beetles (Coleoptera : Scolytinae) and relationship with establishment in New Zealand and worldwide. Can J For Res 36:289–298. doi:10.1139/x05-250
Browne FG, Laurie MV (1968) Pests and diseases of forest plantation trees: an annotated list of the principal species occurring in the British Commonwealth. Clarendon Press, Oxford, xi, p 1330
Christiansen E, Bakke A (1988) The spruce bark beetle of Eurasia. In: Berryman AA (ed) Dynamics of forest insect populations. Plenum Press, New York, pp 479–503
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci U S A 97:7043–7050
Erbilgin N, Raffa KF (2000) Effects of host tree species on attractiveness of tunneling pine engravers, Ips pini, to conspecifics and insect predators. J Chem Ecol 26:823–840
Farjón A (1990) Pinaceae: drawings and descriptions of the genera Abies, Cedrus, Pseudolarix, Keteleeria, Nothotsuga, Tsuga, Cathaya, Pseudotsuga, Larix and Picea. Koeltz, Königstein, p 330
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–375
Gandhi KJK, Herms DA (2010) Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions 12:389–405. doi:10.1007/s10530-009-9627-9
Global Forest Resources Assessment (2010) Main Report. FAO Forestry paper 163. Food and Agriculture Organization of the United Nations, Rome, Italy, p 340
Grégoire J-C, Evans HF (2004) Damage and control of Bawbilt organisms–an overview. In: Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht, pp 19–37
Haack RA (2001) Intercepted Scolytidae (Coleoptera) at U.S. ports of entry: 1985–2000. Integr Pest Manag Rev 6:253–282
Haack RA (2006) Exotic bark- and wood- boring Coleoptera in the United States: recent establishments and interceptions. Can J For Res 36:269–288
Haack RA, Petrice TR (2009) Bark- and wood-borer colonization of logs and lumber after heat treatment to ispm 15 specifications: the role of residual bark. J Econ Entomol 102:1075–1084
Haack RA, Herard F, Sun JH, Turgeon JJ (2010) Managing invasive populations of Asian Longhorned Beetle and Citrus Longhorned Beetle: a worldwide perspective. Ann Rev Entomol 55:521–546. doi:10.1146/annurev-ento-112408-085427
Hansson L (1994) Vertebrate distributions relative to clear-cut edges in a boreal forest landscape. Landsc Ecol 9:105–115
Hoffard WH (1985) Southern pine beetle - a would-be manager of southern forests. In: Loomis RC, Tucker S, Hofacker TH (eds) Insect and disease conditions in the United States. USDA For Serv Gen Tech Rep WO-46, pp 32–37
Holsten EH, Werner RA (1997) Engraver beetles in Alaska forests. USDA For Serv. Leaflet, Missoula, p 6
Holsten EH, Werner RA, Develice RL (1995) Effects of a spruce beetle (Coleoptera: Scolytidae) outbreak and fire on Lutz spruce in Alaska. Environ Entomol 24:1539–1547
Holsten EH, Thier RW, Munson AS, Gibson KE (2000) The Spruce beetle. forest insect & disease leaflet 127. U. S. Department of Agriculture Forest Service, USA
Kean JM, Barlow ND (2000) Can host-parasitoid metapopulations explain successful biological control? Ecology 81:2188–2197
Kenis M, Auger-Rozenberg MA, Roques A, Timms L, Pere C, Cock M, Settele J, Augustin S, Lopez-Vaamonde C (2009) Ecological effects of invasive alien insects. Biol Invasions 11:21–45. doi:10.1007/s10530-008-9318-y
Kolbe JJ, Glor RE, Schettino LRG, Lara AC, Larson A, Losos JB (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177–181. doi:10.1038/nature02807
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in US communities, 2009–2019. Ecol Econ 69:569–578. doi:10.1016/j.ecolecon.2009.09.004
Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature 452:987–990
Lance EW, Howell S (2000) Survey of songbirds during a spruce beetle (Dendroctonus rufipennis) outbreak on the Kenai Peninsula, Alaska. Northwest Nat 81:1–10
Lance EW, Howell SM, Lance BK, Howlin S, Suring LH, Goldstein MI (2006) Spruce beetles and timber harvest in Alaska: implications for northern red-backed voles. For Ecol Manag 222:476–479. doi:10.1016/j.foreco.2005.11.023
Lange H, Økland B, Krokene P (2006) Thresholds in the life cycle of the spruce bark beetle under climate change. Interjournal for Complex Syst 1648:1–10
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Li JS, Chang GB, Song YS, Wang YW, Chang BS (2001) Control project on red turpentine beetle (Dendroctonus valens). For Pest Dis 4:41–44
Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Ann Rev Entomol 53:387–408. doi:10.1146/annurev.ento.52.110405.091401
Lindhe A, Lindelöw A (2004) Cut high stumps of spruce, birch, aspen and oak as breeding substrates for saproxylic beetles. For Ecol Manag 203:1–20. doi:10.1016/j.foreco.2004.07.047
Lindhe A, Lindelöw A, Asenblad N (2005) Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter. Biodivers Conserv 14:3033–3053. doi:10.1007/s10531-004-0314-y
Logan JA, Powell JA (2001) Ghost forests, global warming, and the mountain pine beetle. Am Entomol 47:160–173
Martikainen P, Siitonen J, Kaila L, Punttila P, Rauh J (1999) Bark beetles (Coleoptera, Scolytidae) and associated beetle species in mature managed and old-growth boreal forests in southern Finland. For Ecol Manag 116:233–245
McFarlane W, Logan JA, Wilcox L, Kern WR, Gordon BS (2009) Using an innovative aerial survey method, to monitor mountain pine beetle outbreaks in whitebark pine of greater Yellowstone ecosystem. IUFRO-meeting (Units 7.03.05/7.03.07). Forest Insects and Environmental Change, Jackson Hole
McGregor MD (1985) Mountain pine beetle–the conflict between people and the beetle. In: Loomis RC, Tucker S and Hofacker TH (eds) Insect and disease conditions in the United States. USDA For Serv Gen Tech Rep WO-4616-23
McMahon SM, Diez JM (2007) Scales of association: hierarchical linear models and the measurement of ecological systems. Ecol Lett 10:437–452. doi:10.1111/j.1461-0248.2007.01036.x
Morehouse K, Johns T, Kaye J, Kaye A (2008) Carbon and nitrogen cycling immediately following bark beetle outbreaks in southwestern ponderosa pine forests. For Ecol Manag 255:2698–2708. doi:10.1016/j.foreco.2008.01.050
Økland B (1996) Unlogged forests: important sites for preserving the diversity of mycetophilids (Diptera: Sciaroidea). Biol Conserv 76:297–310
Økland B (2002) Canopy cover favours sporocarp-visiting beetles in spruce forests. Nor J Entomol 49:29–39
Økland B, Bjørnstad ON (2003) Synchrony and geographical variation of the spruce bark beetle (Ips typographus) during a non-epidemic period. Popul Ecol 45:213–219
Økland B, Bjørnstad ON (2006) A resource depletion model of forest insect outbreaks. Ecology 87:283–290
Økland B, Bakke A, Hågvar S, Kvamme T (1996) What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway. Biodivers Conserv 5:75–100
Økland B, Skarpaas O, Kausrud K (2009) Threshold facilitations of interacting species. Popul Ecol 51:513–523. doi:10.1007/s10144-009-0141-9
Økland B, Skarpaas O, Schroeder M, Magnusson C, Lindelöw Å, Thunes K (2010) Is eradication of the pinewood nematode (Bursaphelenchus xylophilus) likely? An evaluation of current contingency plans. Risk Anal 30:1424–1439. doi: 10.1111/j.1539-6924.2010.01431.x
Peters DPC, Pielke RA, Bestelmeyer BT, Allen CD, Munson-McGee S, Havstad KM (2004) Cross-scale interactions, nonlinearities, and forecasting catastrophic events. Proc Natl Acad Sci U S A 101:15130–15135. doi:10.1073/pnas.0403822101
Piel F, Gilbert M, De Canniere C, Gregoire JC (2008) Coniferous round wood imports from Russia and Baltic countries to Belgium. A pathway analysis for assessing risks of exotic pest insect introductions. Divers Distributions 14:318–328. doi:10.1111/j.1472-4642.2007.00390.x
Pimentel D (2002) Biological Invasions: economic and environmental cost of alien plant, animal and microbe species. CRC Press, Boca Raton
Potts DF (1984) Hydrologic impacts of a large scale mountain pine beetle (Dendroctonus ponderosae Hopkins) epidemic. Water Res Bull Paper 83122:373–377
R Development Core Team (2009) R: a language and environment for statistical computing. The R foundation for statistical computing, Vienna, Austria www.r-project.org
Raffa K, Aukema B, Erbilgin N, Klepzig K, Wallin K (2005) Interactions among conifer terpenoids and bark beetles across multiple levels of scale: an attempt to understand links between population patterns and physiological processes. Rec Adv Phytochem 39:79–118
Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58:501–517. doi:10.1641/b580607
Ran JH, Wei XX, Wang XQ (2006) Molecular phylogeny and biogeography of Picea (Pinaceae): Implications for phylogeographical studies using cytoplasmic haplotypes. Mol Phylogenet Evol 41:405–419. doi:10.1016/j.ympev.2006.05.039
Shigesada N, Kawasaki K (1997) Biological invasions: theory and practice. Oxford University Press, Oxford, p 205
Siitonen J (2001) Forest management, coarse woody debris and saproxylic organisms: fennoscandian boreal forests as an example. Ecol Bull 49:11–41
Simberloff D (2005) The politics of assessing risk for biological invasions: the USA as a case study. Trend Ecol Evol 20:216–222. doi:10.1016/j.tree.2005.02.008
Simberloff D (2009) We can eliminate invasions or live with them. Successful management projects. Biol Invasions 11:149–157
Skarpaas O, Økland B (2009) Timber import and the risk of forest pest introductions. J Appl Ecol 46:55–63. doi:10.1111/j.1365-2664.2008.01561.x
Stenseth NC (1977) Food selection of the field vole Microtus agrestis. Oikos 29:511–524
Stephen FM, Grégoire JC (2001) Introduction and establishment of exotic bark beetles. Risks of exotic forest pests and their impact on trade. International Online Workshop April 16–29. http://www.apsnet.org/online/proceedings/exoticpest/Papers/stephen.htm
Suarez AV, Tsutsui ND (2008) The evolutionary consequences of biological invasions. Mol Ecol 17:351–360
Taylor CM, Hastings A (2005) Allee effects in biological invasions. Ecol Lett 8:895–908. doi:10.1111/j.1461-0248.2005.00787.x
Tollefsrud MM, Sonstebo JH, Brochmann C, Johnsen O, Skroppa T, Vendramin GG (2009) Combined analysis of nuclear and mitochondrial markers provide new insight into the genetic structure of North European Picea abies. Heredity 102:549–562. doi:10.1038/hdy.2009.16
Turchin P (2003) Complex population dynamics: a theoretical/empirical synthesis. Princeton University Press, New Jersey, p 451
Uunila L, Guy B, Pike R (2006) Hydrologic effects of mountain pine beetle in the interior pine forests of British Columbia: key questions and current knowledge. Watershed Manag Bull 9:2–6
Veblen TT, Hadley KS, Reid MS, Rebertus AJ (1991) The response of sub-alpine forests to spruce beetle outbreak in Colorado. Ecology 72:213–231
Wallin KF, Raffa KF (2004) Feedback between individual host selection behavior and population dynamics in an eruptive herbivore. Ecol Monogr 74:101–116
Wermelinger B (2004) Ecology and management of the spruce bark beetle Ips typographus—a review of recent research. For Ecol Manag 202:67–82
Werner RA, Holsten EH, Matsuoka SM, Burnside RE (2006) Spruce beetles and forest ecosystems in south-central Alaska: A review of 30 years of research. For Ecol Manag 227:195–206. doi:10.1016/j.foreco.2006.02.050
Weslien J, Annila E, Bakke A, Bejer B, Eidmann HH, Narvestad K, Nikula A, Ravn HP (1989) Estimating risks for spruce bark beetle (Ips typographus (L.)) damage using pheromone-baited traps and trees. Scand J For Res 4:87–98
Williamson MH (1996) Biological Invasions. Chapman & Hall, London
Wood DL (1972) Selection and colonization of ponderosa pine by bark beetles. In: van Emden HF (ed) Insect/plant relationships. Blackwell Scientific, London, pp 101–117
Wood DL (1982a) The role of pheromones, kairomones, and allomones in the host selection and colonization behaviour of bark beetles. Ann Rev Entomol 27:411–446
Wood SL (1982b) The bark and Ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat mem 6:1–1359
Wood SL, Bright DE (1992) A catalog of Scolytidae and Platypodidae (Coleoptera), part 2: taxonomic index. Great Basin Nat Mem 13:1–1553
Wood CS, Van Sickle GA (1992) Forest insect, disease conditions British Colombia, Yukon–1992. Information Report BC-X-340. Forestry Canada Pacific Forestry Centre, Victoria
Yamaoka Y, Wingfield MJ, Takahashi I, Solheim H (1997) Ophiostomatoid fungi associated with the spruce bark beetle Ips typographus f. japonicus in Japan. Mycol Res 101:1215–1227
Yan Z, Sun J, Don O, Zhang Z (2005) The red turpentine beetle, Dendroctonus valens LeConte (Scolytidae): an exotic invasive pest of pine in China. Biodivers Conserv 14:1735–1760
Acknowledgments
Gro Wollebæk, Ulf Johansson, Morgan Erixon and Anna Björklund are thanked for technical assistance in the field experiment. Thanks to Mari Mette Tollefsen and Eva Solbjørg Flo Heggem for their contributions in developing distribution map of spruce species, to Ken Raffa for useful references about ecosystem function effects, to Stein Tomter for compiling statistics about forest cover and to Wendy Fjeldstad for text comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Økland, B., Erbilgin, N., Skarpaas, O. et al. Inter-species interactions and ecosystem effects of non-indigenous invasive and native tree-killing bark beetles. Biol Invasions 13, 1151–1164 (2011). https://doi.org/10.1007/s10530-011-9957-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-9957-2