Abstract
A literature survey identified 403 primary research publications that investigated the ecological effects of invasive alien insects and/or the mechanisms underlying these effects. The majority of these studies were published in the last 8 years and nearly two-thirds were carried out in North America. These publications concerned 72 invasive insect species, of which two ant species, Solenopsis invicta and Linepithema humile, accounted for 18% and 14% of the studies, respectively. Most publications investigated effects on native biodiversity at population or community level. Genetic effects and, to a lesser extent, effects on ecosystem services and processes were rarely explored. We review the effects caused by different insect invaders according to: their ecosystem roles, i.e. herbivores, predators, parasites, parasitoids and pollinators; the level of biological organisation at which they occur; and the direct and indirect mechanisms underlying these effects. The best documented effects occur in invasive ants, Eurasian forest herbivores invasive in North America, and honeybees. Impacts may occur through simple trophic interactions such as herbivory, predation or parasitism. Alien species may also affect native species and communities through more complex mechanisms such as competition for resources, disease transmission, apparent competition, or pollination disruption, among others. Finally, some invasive insects, particularly forest herbivores and ants, are known to affect ecosystem processes through cascading effects. We identify biases and gaps in our knowledge of ecological effects of invasive insects and suggest further opportunities for research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The threat posed by invasive alien species on biodiversity is widely recognized (Williamson 1996; Wittenberg and Cock 2001; Pimentel 2002). Although insects form a large part of the alien fauna worldwide, invasive alien insects appear to have received disproportionately less attention regarding their effects on the environment compared to plants, vertebrates, or aquatic organisms (Parker et al. 1999; Levine et al. 2003; Long 2003). Alien insects can affect native biodiversity through direct interactions, e.g. a herbivore feeding on a native plant (Jenkins 2003), a predator or a parasitoid attacking a native prey or host (Boettner et al. 2000; Snyder and Evans 2006), an alien species hybridizing with a native species (Jensen et al. 2005), etc. They can also affect native species and ecosystems indirectly, through cascading effects, or through various mechanisms, such as carrying diseases, competing for food or space or sharing natural enemies with native species (NRC 2002).
Ecological impact by invasive species can occur at different levels of biological organisation: genetic effects; effects on individuals, populations or communities of species; and effects on ecosystem processes (Parker et al. 1999). It can also occur at different spatial scales, from microhabitat to landscape (Williamson 1996). Parker et al. (1999) surveyed for published reports of quantitative data on impacts by various categories of invasive organisms. The majority focused on population effects and most studies were carried out in a correlative manner—e.g. comparing sites before and after invasion, or sites inside and outside the invasion range, but only a few of these studies used designed experiments to assess the mechanisms or pathways through which these impacts occur. For terrestrial invertebrates, they identified two dozen publications, more than half of them illustrating population-level effects.
To date, reviews on the ecological effect of invasive alien insects have been published on particular taxa, such as ants (Holway et al. 2002), bees (Goulson 2003; Moritz et al. 2005) and mosquitoes (Juliano and Lounibos 2005), for specific regions, such as the Galapagos Islands (Causton et al. 2006), or for particular impact mechanisms, such as the ecological impact of generalist predators (Snyder and Evans 2006). This paper provides the first comprehensive literature review of the ecological effects of invasive insects. We first make a general analysis of the literature presently available on the topic and review the impacts caused by different invaders according to their ecosystem roles—herbivores, predators, parasites, parasitoids and pollinators. Within these groups we analyse ecological effects at different levels of biological organisation—genetic, population/community and ecosystem—and through different ecological mechanisms, e.g. herbivory, predation, parasitism, resource competition, and various indirect mechanisms. We also try to identify gaps in knowledge of ecological hazards by invasive insects and to stimulate new approaches to fill these gaps. The study was carried out as part of the EU project ALARM (Settele et al. 2005).
Published studies on the ecological impact of invasive insects
Relevant primary research publications on the ecological effects of invasive alien insects were first identified by electronic searches in CAB Abstracts covering the period 1900–2007. Since the terminology used in the context of invasive species has changed during this period, the widest possible variety of terms (e.g. invasive or alien or non-indigenous or exotic; impact or effect, displacement) were entered in the search engine, in various combinations. Then the references in these sources were examined for additional relevant publications. Only papers published until 2007 were included in the general analysis, but some relevant papers in press are cited in the text. In a couple of cases, general publications were included in the analysis when they described essential unpublished research. Studies describing the effect on single individuals without information on the effect at population level (e.g. an alien parasitoid emerged from a native species, or an alien herbivore found attacking an alien plant) were not taken into account, unless the mortality described in the paper affected a measurable and significant proportion of the regional or world population of a native species (e.g. Stiling and Moon 2001; Fowler 2004). Studies showing negative results (i.e. no ecological effect) were included. In contrast, papers on risk assessments or on alien biological control agents showing a positive effect on the environment, either through the control of a pest of ecological importance or through pesticide reduction, were excluded. Papers describing a negative impact of phytosanitary methods implemented to control invasive insects were also excluded, as well as those mentioning interference with pests in purely agricultural systems. Finally, we did not consider in the analyses the effect of alien species on other alien species, unless this effect had an indirect consequence on native biodiversity.
Papers were classified following the biological organisation level at which the investigated effects occur (genetic, population/community, and ecosystem) and based on how the effect was quantified: (1) studies based on field observations, usually comparative studies between invaded and non invaded sites, or comparing data before and after invasion; (2) field studies with a significant experimental component, e.g. exclusion experiments, exposure of sentinel animals or plants, etc.; (3) laboratory experiments or mathematical models used to investigate impact mechanisms. Studies of the third type were included in the database only when they were based on field data showing or suggesting that impact by the invasive insect already occurs. The identity of the invasive species, the year of publication and the continent/region in which the study was carried out were also included in the database.
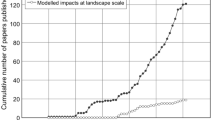
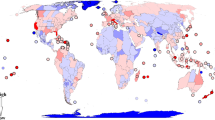
A total of 403 primary research papers were identified that investigate the ecological effect of invasive insects and/or the mechanisms underlying these effects (See electronic Appendix A available on the Biological Invasions web site). Although these represent only a fraction of the publications available, we believe that they are a representative sample of the published literature on the topic. Nearly 60% of the papers were published in the last 8 years, even though many of the alien insects were introduced several decades earlier (Fig. 1). Few papers on invasive alien insects published before the 1990s were included in our analyses because, in most cases, earlier papers described damage on individuals, economic injuries or anecdotal observations on ecological effects, but did not provide reliable and measurable data on the effects on native populations, communities and ecosystem processes. This suggests that the ecological impact of insects is a relatively new area of research, or that the ecological effects of invasive alien insects were of little concern until recently, and we can expect that much more information on impacts and impact mechanisms will become available in the next few years. About 62% of the studies were carried out in North America, followed by Oceanic Islands (13%) and Australia/New Zealand (8%) (Fig. 2). Only a few studies were conducted in the other continents Vilá et al. (2006) found a similar pattern for plants. They showed that 59% and 21% of studies assessing the ecological effects of invasive plants were carried out in North America and Oceania, respectively. This discrepancy in the number of studies on the effect of invasive species between regions and continents probably reflects the higher incidence of invasive species usually observed in Oceanic islands, Oceania, and North America compared to other regions (Simberloff 1986; Niemelä and Mattson 1996; Pimentel 2002). However, literature searches being usually made in English and references in other languages being generally under-represented in peer-reviewed literature, a bias towards English-speaking countries cannot be ruled out.
Only nine of these 403 publications describe investigations on genetic effects. Publications on the effect of invasive insects on ecosystem processes are more numerous (25 publications), but concern nearly exclusively ants (e.g. Solenopsis invicta Buren) and, especially, forest herbivores in North America (e.g. Adelges tsugae Annand and Lymantria dispar (L.)). Most studies analyse effects at the population or community level. Effects were most commonly assessed or studied through field comparisons of populations in invaded and non-invaded areas (224 publications), but field studies involving an experimental component were more frequent than previously expected (106 publications). Laboratory experiments (104 publications) concern mainly intraguild predation tests with the invasive predators. Parker et al. (1999) also observed that population level effects are by far the most commonly documented ecological effects for invasive terrestrial invertebrates, as for all other taxonomic groups.
Impact studies were found for 72 invasive insect species and evidence for ecological effects in the field was found for 54 of them (Table 1). Two ant species, S. invicta and Linepithema humile (Mayr), account for 18% and 14% of the studies, respectively. Other extensively studied species include the honey bee Apis mellifera L.(7%), three Eurasian forest pests introduced into North America, L. dispar (6%), Adelges piceae (Ratzeburg) (5%) and A. tsugae (5%), and a biological control agent, the Asian ladybird Harmonia axyridis (Pallas) (6%). All together, invasive ants were the target of 41% of the studies, other predators 19%, parasitoids and parasites 6%, herbivores 24% and pollinators 10% (Fig. 3).
Genetic effects
Hybridization between invasive and native species may be of major concern because of the disturbances it can induce in native genetic resources (Huxel 1999; Mallet 2005). Hybridization has been well documented in vertebrates and plants and, in several cases, has been shown to have a strong negative impact on native species (Rhymer and Simberloff 1996; Vilà et al. 2000, 2006; Long 2003). In contrast, genetic impacts related to invasions of insects and other terrestrial invertebrates remain largely unexplored. Indeed, most studies focused on the genetic structure of insect invaders (Tsutsui and Case 2001; Lee 2002), especially with the aim of tracing their origin (Scheffer and Grissell 2003; Grapputo et al. 2005; Havill et al. 2006). No case of horizontal gene transfer is reported except in some laboratory tests (Labrador et al. 1999) and good examples of interspecific hybridization are scarce, and mainly concern laboratory experiments, e.g. with bumblebees (Mitsuhata and Ono 1996). More examples concern hybridization between native and introduced bees and bumblebee subspecies. The shipment of vast numbers of non-native honeybees and bumblebees throughout the world has already resulted in noticeable genetic effects. The massive introduction in north-western Europe of two subspecies of A. mellifera originating from southern Europe, A. m. ligustica S. and A. m. carnica Pollmann, has caused large-scale gene flow and introgression between these subspecies and the native black honeybee, A. m. mellifera (Jensen et al. 2005), whose native populations are now threatened in north-west Europe (Goulson 2003) and in the Canary islands (De La Rùa et al. 2002). A similar problem exists with bumblebee subspecies, Bombus terrestris dalmatinus Dalle Torre and B. t. sassaricus Tournier, originating from the Middle East and Sardinia respectively, which have been introduced in vast numbers worldwide as managed pollinators of glasshouse crops. Indeed, there is a real risk that commercial and native subspecies will hybridize (Ings et al. 2005a, b). Even if growers are advised to prevent the escape of sexuals (queens and males) that could interbreed with native bumblebees, this measure might not be enough since workers can successfully produce males (by arrhenotokous parthenogenesis) by invading congener colonies (Lopez-Vaamonde et al. 2004). However, suitable molecular markers still need to be identified to genetically characterize B. terrestris subspecies and to identify evidence for recent hybridization.
Other cases of hybridization between native and invasive species or subspecies are rather speculative, since they have not been confirmed by proper genetic studies. A frequently cited example is the Australian lycaenid butterfly Zizina labradus (Godart), which has apparently displaced the endemic Z. oxleyi (Felder) in several regions in New Zealand (Barlow and Goldson 2002). Hybridization is the most likely mechanism responsible for the displacement because the two species—often regarded as sub-species—interbreed freely at sites where they still occur sympatrically (Gibbs 1980, 1987). However, other impact mechanisms such as competition for resources or indirect competition through shared natural enemies cannot be ruled out. Interestingly, hybridization was also suspected to be the cause of the observed displacement of the native Torymus beneficus Yasumatsu and Kamijo by the introduced Torymus sinensis Kamijo, hymenopteran parasitoids of the chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu, in Japan (Yara 2006). However, recent molecular studies revealed that hybridization between the two species in the field was marginal and, thus, was probably not playing a significant role in the displacement of the native species (Yara et al. 2007).
Due to the limited number of studies, the question of whether genetic risks, especially hybridization, due to invasive populations are weak or just underestimated in insects remains open. Further research is badly needed, in particular the molecular quantification of gene flow between introduced and native species.
Ecological effects due to herbivores
Direct effect on native plant populations
Alien invertebrate herbivores can be particularly harmful to native plant populations, sometimes driving them to local extinction. However, most publications reporting ecological effects of alien herbivores do not properly quantify these effects. The best documented effects of invasive invertebrate herbivores are undoubtedly those caused by forest insects. North America has been particularly affected by invasive forest insects from Eurasia. For example the balsam woolly adelgid, A. piceae, and the hemlock woolly adelgid, A. tsugae, are threatening unique forest ecosystems in eastern North America by killing Fraser fir (Abies fraseri (Pursh) Poir) and Eastern and Carolina hemlock (Tsuga canadensis (L.) Carr. and T. caroliniana Engelm.) on a large scale, so that they are gradually replaced by other tree species (e.g. Smith and Nicholas 2000; Jenkins 2003; Small et al. 2005; Weckel et al. 2006). In particular, A. tsugae poses a major threat to the viability of Carolina hemlock, a rare endemic tree species in the Appalachian Mountains. Since its accidental introduction from Europe to North America in the nineteenth century, the gypsy moth, L. dispar, has become the main pest of broadleaved trees in Eastern North America. Repeated defoliation may lead to severe tree mortality, particularly in oak (Quercus spp.) stands (Kegg 1971; Allen and Bowersox 1989; Liebhold et al. 1995; Fajvan and Wood 1996). Other examples of Eurasian insects causing serious concern for North American tree species include the spruce aphid, Elatobium abietinum (Walker), threatening Engelmann spruce, Picea engelmannii Parry ex. Engelm. (Lynch 2004), and the newly introduced emerald ash borer, Agrilus planipennis Fairmaire, which, in a few years, has already killed 15 million ash trees (Fraxinus spp.) (Poland and McCullough 2006).
Endemic flora on islands are particularly vulnerable to herbivore invasions. In St Helena, the scale insect Orthezia insignis Browne was in the process of pushing the endemic gumwood, Commidendrum robustum (Roxb.), to extinction when a successful biological control programme was implemented (Fowler 2004). Similarly, in the Galapagos, another scale, Iceria purchasi Maskell, has severely affected populations of endangered plants (Roque-Albelo 2003). Here again the introduction of its natural enemy, Rhodolia cardinalis Mulsant, clearly mitigated its effect. Sometimes, biological control agents introduced against exotic weeds may have an adverse effect on native plants. The weevil Rhinocyllus conicus (Frölich), introduced in North America to control the exotic weed nodding thistle, Carduus nutans L., now feeds on many native thistles including the endangered Pitcher’s thistle (Cirsium pitcheri (Torr. ex Eaton) Torr. & A. Gray), significantly reducing the seed production of native thistles (e.g. Louda et al. 1997, 2005; Russel and Louda 2005). Native thistles, in particular Tracy’s thistle, Cirsium undulatum var. tracyi (Rydb.) Welsh, are also threatened by another European weevil, Larinus planus (F.) accidentally introduced in North America but deliberately distributed within the continent as a biological control agent (Louda and O’Brien 2002). The cactus moth, Cactoblastis cactorum (Berg), was introduced to the Caribbean to successfully control prickly pear cacti, Opuntia spp., in 1956. In 1989, it was found in Florida, where it is now threatening the survival of already endangered indigenous Opuntia species (Stiling and Moon 2001; Stiling et al. 2004). It is also spreading along the coast towards Mexico, an important centre of diversity and endemism for Opuntia spp., where some species are also of significant economic importance (Perez-Sandi 2001).
Indirect effects on native plant communities
By killing and reducing host plant populations, invasive herbivores also indirectly affect populations of other native plant species and native plant communities. Defoliation by L. dispar can cause a major shift in tree species in North America, either directly through tree mortality (Allen and Bowersox 1989; Fajvan and Wood 1996) or via seed failures and mortality of oak seedlings (Gottschalk 1990). The dramatic mortality observed in Fraser fir and Eastern hemlock due to Adelges spp. has totally altered forest plant communities in these forest ecosystems (Jenkins 2003; Eschtruth et al. 2006; Weckel et al. 2006). Busing and Pauley (1994) showed that the loss of Fraser fir due to A. piceae has increased wind exposure and, consequently, mortality of remaining canopy trees, in particular red spruce, Picea rubens Sarg.
Indirect effects on native fauna
Invasive herbivores may affect populations and communities of native herbivores by competing for the same resource, although mechanisms underlying competition are not always fully understood (Reitz and Trumble 2002). An Asian adelgid, Pineus boerneri Annand, has been shown to be competitively superior and to displace a native congener, P. coloradensis (Gilette) in red pine (Pinus resinosa Aiton) plantations in Eastern USA, probably by reducing host plant quality and forcing P. coloradensis to less suitable sites (McClure 1984, 1989). The European weevil R. conicus, feeding on flowerheads of native thistles in North America, significantly decreases the density of native tephritid flies, which also feed on the flowerheads, at high weevil density (Louda et al. 1997). The scale insect I. purchasi, by killing endangered plants in the Galapagos, has also caused local extinctions of host-specific Lepidoptera (Roque-Albelo 2003). Fabre et al. (2004) suspect resource competition between native and exotic seed chalcids of the genus Megastigmus spp. in Europe, and displacement of the native species. The African stem borer Busseola fusca (Fuller) seems to be displaced from grain sorghum fields by the Asian stem borer Chilo partellus (Swinhoe) (Kfir 1997), perhaps because the native species is deterred by the invasive species, or because of differences in their phenology. Oak defoliation by gypsy moth, L. dispar, may negatively affect populations of the northern tiger swallowtail, Papilio canadensis Rothschild & Jordan. Adult female swallowtails are incapable of distinguishing between damaged and undamaged leaves and laboratory experiments showed that defoliation by gypsy moths depressed swallowtail growth rate and survival (Redman and Scriber 2000).
An invasive herbivore can also displace other indigenous species via behavioural interference. A striking example is the rampant invasion of biotype B of the sweet potato whitefly, Bemisia tabaci (Gennadius). This biotype is one of the world’s most damaging agricultural pests and has displaced two indigenous biotypes of this species because invading males interfere with mating by native males and invading females produce more female offspring (Liu et al. 2007).
Biological control may provide an opportunity to confirm competitive displacement a posteriori. For example, the displacement of native Lepidoptera by the exotic noctuid moth Penicillaria jocosatrix Guenée in Guam was confirmed by a successful biological control program against P. jocosatrix, which allowed the native species to recover (Schreiner and Nafus 1993).
Invasive herbivores do not only affect closely-related species. The disturbance of Fraser fir forests by the balsam woolly aphid has had a detrimental effect on local birds, 10 out of 11 species declining, and six species by more than 50% (Rabenold et al. 1998). Similarly, the decline of eastern hemlock due to A. tsugae in North America strongly affects bird species composition (Tingley et al. 2002) and also has an effect on salamander populations (Brooks 2001), and on deer survival through modifications in forest microclimates (Lishawa et al. 2007). The indirect consequences of L. dispar outbreaks on native birds, have been extensively studied (e.g. Bell and Whitmore 2000; Gale et al. 2001). However, in contrast to Adelges spp., defoliations by L. dispar induced only temporary changes to bird populations and communities, probably because the general impact on the dominant tree species is less dramatic for L. dispar than for the two adelgids. Interestingly, Thurber et al. (1994) observed that nests in sites defoliated by L. dispar suffered a higher predation rate than did those in non-defoliated sites.
Finally, invasive species may also affect native predators through intoxication. The glassy-winged sharpshooter, Homalodisca coagulate (Say), has recently invaded islands of French Polynesia, where it represents a poisonous prey for native spiders (Suttle and Hoddle 2006). Laboratory experiments showed that H. coagulata can be lethal for two native spider species, and preliminary field surveys suggest that the invasive species may already have adversely affected an endemic spider population on at least one island.
Indirect impact as vectors of plant and insect diseases
Invasive herbivores may affect native plants by transmitting or facilitating diseases. The European elm bark beetle, Scolytus multistriatus (Marsham) is the vector of the infamous Dutch elm disease, Ophiostoma ulmi (Buisman) Nannf., and O. novi-ulmi (Brasier) in North America (Brasier 2000). The European beech scale, Cryptococcus fagisuga Lindinger is associated with the fungus, Neonectria faginata (Lohman et al.) Castl. & Rossman, to cause beech bark disease, which devastates American beech (Fagus grandifolia Ehrh.) in North America (Houston 1994; Morin et al. 2007). The insects themselves are relatively minor pests, but the related diseases have a tremendous impact on North American forest species and ecosystems.
Non native insects may also affect native insects by transmitting diseases. An interesting case is the extinction of the Madeiran large white, Pieris brassicae wollastoni Butler. This remarkable endemic disappeared a few years after the introduction in Madeira of the congeneric pest species, Pieris rapae (L.), which is now one of the most abundant butterflies in the island (Wakeham-Dawson et al. 2002; Aguiar-Franquinho and Karsholt 2006). Gardiner (2003) suggests that the introduction of P. rapae brought a different strain of the granulosis virus for which P. brassicae wollastoni had no resistance, which in turn lead to the sharp decline and ultimate extinction of this island endemic.
Indirect effects through apparent competition
Apparent competition occurs when the presence of one species indirectly decreases the fitness of another through the increased presence of a shared enemy (Holt 1977). Very few studies have investigated such interactions in invertebrates, and fewer still in the context of invasive insects. The earliest of these studies investigated the correlation between invasion of the variegated leafhopper, Erythroneura variabilis Beamer, and decreases in populations of a congeneric native grape leafhopper, E. elegantula Osborn, in California vineyards (Settle and Wilson 1990). Field experiments and collections revealed that although neither species was superior in direct competition, declines in E. elegantula populations were correlated with increased levels of parasitism by a native mymarid wasp, Anagrus epos Girault, in the presence of E. variabilis.
The invasion of L. dispar in North America has provided numerous possibilities for apparent competition with native species. Efforts to biologically control the gypsy moth have led to the introduction of over 60 species of parasitoids from Europe and Asia. Many of the natural enemies found attacking gypsy moth in North America are generalist parasitoids of Lepidoptera. These include the polyphagous tachinid fly Compsilura concinnata (Meigen), which has been implicated in the decline of several endangered saturniid moths (Boettner et al. 2000) (see section on parasitoids below). Redman and Scriber (2000) also investigated the effect of gypsy moth on native northern tiger swallowtails, P. canadensis, through a suite of indirect interactions. Although they did not examine the competitive interactions between the two species in the absence of shared enemies, they did find that parasitism rates of the native caterpillar more than doubled in the presence of the gypsy moth (Redman and Scriber 2000). Gypsy moth outbreaks also favour a generalist predator, the white-footed mouse, resulting in an increase in tick populations and in the incidence of Lyme disease (Jones et al. 1998).
Some studies failed to observe apparent competition between invasive and native insects. Schönrogge and Crawley (2000) used quantitative linkage webs to investigate the effect of alien cynipid gall wasps on native gall wasps in the UK through shared native parasitoids and inquilines. They concluded that the recruitment of parasitoids and inquilines by the invading species was unlikely to have a strong effect on the native species because the native parasitoids did not exhibit strong responses to the invasive wasps. In other cases, shared natural enemies between an invasive and a native species may favour the latter. In North America, Hoogendoorn and Heimpel (2002) compared parasitism rates in an invasive ladybeetle, H. axyridis, and a native ladybeetle, Coleomegilla maculata (DeGeer), by a native braconid wasp, Dinocampus coccinellae (Schrank). They used parasitism rates from field collections and a calculated measure of host susceptibility from lab trials to parameterize a model of parasitoid-mediated interactions between the two species. Hoogendoorn and Heimpel (2002) concluded that invasion by H. axyridis may actually be beneficial to C. maculata because limited susceptibility of the invader to D. coccinellae can allow it to act as an ‘egg sink’, reducing the abundance of the parasitoid in the community.
Effects on ecosystem processes
Good studies on the effect of invasive insects on ecosystem processes are rare and most examples concern the effect of herbivores on forest ecosystems through tree defoliation or mortality. Effects on North American oak forests by L. dispar defoliation have been extensively investigated (see review in Lovett et al. 2002, 2006). Defoliation decreases transpiration, tree growth and seed production and increases tree mortality, light penetration to the forest floor and water drainage. It alters tree species composition and consequently, faunistic composition. It may also alter carbon allocation and nitrogen cycling, which may have consequences such as acidification of stream waters.
Adelges tsugae provides another example of a forest insect for which the impact on ecosystem processes has been well studied. A major effect of Eastern hemlock mortality caused by the adelgid is a dramatic increase in inorganic N availability and nitrification rates, resulting in nitrate leaching in regions experiencing adelgid infestations (Jenkins et al. 1999; Kizlinski et al. 2002). Yorks et al. (2003) made similar observations when girdling trees to simulate an adelgid attack. Stadler et al. (2005) showed that infestations by adelgids increased the presence of bacteria, yeast and filamentous fungi in the canopy, and strongly altered the chemistry, quantity and spatial pattern of throughfall. Tree mortality due to the adelgid may also modify forest floor microclimate (Cobb et al. 2006; Lishawa et al. 2007) and hydrologic processes (Ford and Vose 2007). Other invasive forest insects such as A. piceae, C. fagisuga (and its associated fungus N. coccinea var. faginata), E. abietinum and A. planipennis are probably responsible for serious changes in forest ecosystems because they kill important tree species on a large scale, but the precise impacts, as well as the processes underlying these impacts, are largely unknown.
Ecological effects due to predators and detritivores
Effects on native animal populations
Many studies show that invasive predatory insects displace native species, but most fail to identify the mechanism behind displacement (Reitz and Trumble 2002). This can be caused by extensive direct predation, exploitative competition for food or space, relative immunity from shared natural enemies or a disruptive mating system (see review in Snyder and Evans 2006). Many invasive predators are especially detrimental to related native predators. For example, H. axyridis, and the European seven-spotted ladybird, Coccinella septempunctata L., are both strongly suspected to displace other aphidophagous ladybirds in various agricultural environments in North America (e.g. Elliott et al. 1996; Brown and Miller 1998; Colunga-Garcia and Gage 1998; Michaud 2002; Evans 2004). But it is not clear whether the main mechanism of displacement is predation on native coccinellids or local depletion of aphids. Several laboratory experiments showed that larvae of H. axyridis and, to a lesser extent, C. septempunctata, are aggressive intraguild predators and will successfully prey on immature stages of most indigenous ladybirds (e.g. Burgio et al. 2002; Snyder et al. 2004; Yasuda et al. 2004; Ware and Majerus 2008). In contrast, there are few studies that investigate the more complex mechanism of displacement of native ladybirds by food depletion. Those who did include the factor of food availability in laboratory competition tests (e.g. Obrycki et al. 1998) failed to reach firm conclusions regarding the mechanisms involved in the displacement. Both invasive ladybirds have a particularly broad diet, allowing them to persist at sites where aphid density is low and purely aphidophagous species have left (Michaud 2002; Evans 2004). Invasive ladybirds are not only detrimental to other ladybirds. A recent study (Mizell 2007) showed that the invasion of H. axyridis also dramatically reduced other groups of aphid predators and parasitoids in pecan and crape myrtle.
Among predatory insects, alien ants show the highest and best documented records of ecological damage on the native fauna. The most dramatic impacts of invasive ants occur when native ants are displaced through resource competition or direct predation. But other invertebrates or even vertebrates may also be displaced through the same mechanisms. Only some examples are given here. More can be found in Holway et al. (2002). The red imported fire ant, S. invicta, is probably the invasive insect which has received the most attention for its impact on native biodiversity. Originating from South America, it has invaded southern North America, where it threatens several arthropods, molluscs, reptiles, birds, amphibians, and mammals (e.g. Porter and Savignano 1990; Vinson 1997; Allen et al. 1997, 2000, 2001; Forys et al. 2001; Morrison 2002). It also attacks beneficial insects such as parasitoids and predators (Eubanks et al. 2002; Ness 2003). The argentine ant, L. humile, has invaded most continents and is known to displace native ants, other arthropods, birds, lizards and mammals through a variety of mechanisms such as predation, by competition for nesting sites or by tending arthropods and plants (e.g. Human and Gordon 1996, 1997; Laakkonen et al. 2001; Gómez and Oliveras 2003; Carpintero et al. 2005; Suarez et al. 2005; Lach 2007, 2008). The crazy ant, Anoplolepis gracilipes (Jerdon), greatly reduces populations of red land crab on Christmas Island (O’Dowd et al. 2003), ants and other invertebrate species in Tokelau (Lester and Tavite 2004; Sarty et al. 2007), many invertebrates in the Seychelles (Hill et al. 2003; Gerlach 2004) and, in conjunction with the big-headed ant, Pheidole megacephala (F.), excludes native spiders in the genus Tetragnatha from native and disturbed forests in Hawaii (Gillespie and Reimer 1993). Pheidole megacephala is also introduced in northern Australia, where it displaces native ants and other invertebrates (Hoffmann et al. 1999; Hoffmann and Parr 2008) in Florida, where it may pose a threat to native fauna, including sea turtle and sea bird nestlings (Wetterer and O’Hara 2002), and in Mexico, where it has a negative effect on termite populations (Dejean et al. 2007). The little fire ant, Wasmannia auropunctata (Roger), and the tropical fire ant, Solenopsis geminata (F.), reduce the diversity and abundance of invertebrates, birds and reptiles in the Galapagos (Causton et al. 2006). Wasmannia auropunctata is also present in Central Africa, where it is known to displace native ants (Walker 2006) and in New Caledonia, where it has a negative effect on populations of native arthropods and lizards (Jourdan 1997; Jourdan et al. 2001). Finally, the crazy ant Paratrechina fulva (Mayr), introduced in Colombia for the control of leaf-cutting ants and poisonous snakes, is now threatening local biodiversity, in particular soil insects, snakes and lizards (de Zenner-Polania and Wilches 1992). In some cases, however, climatic requirements may limit the impact of invasive ants and other species. For example, after 150 or more years of residence in Madeira, P. megacephala and L. humile occupy only a small part of the island and appear to have little impact. Most of the island may be too cool for P. megacephala and too moist for L. humile, which are excluded by a dominant, better adapted native ant, Lasius grandis Forel (Wetterer et al. 2006).
Although most of these impacts on the native fauna were investigated by comparing infested and uninfested areas, quite a few used experimental designs, for example by using poisonous baits, hot water or sticky barriers to exclude the invasive species (Allen et al. 2001; King and Tschinkel 2006; Lach 2007), food baits to assess the importance of resource competition (Sarty et al. 2007) or artificial nests to assess predation on birds (Suarez et al. 2005).
Social wasps can also be particularly damaging for both native wasp species and other indigenous species. European wasps, Vespula germanica (F.) and V. vulgaris (L.), have invaded New Zealand beech forests where they prey on vulnerable native invertebrates and strongly compete with rare birds and invertebrates for food (Beggs 2001). Vespula germanica is also invasive in Australia, where it is suspected of outcompeting the native paper wasp Polistes humilis (F.) because of its broader diet (Kasper et al. 2004). Another paper wasp, Polistes versicolor (Olivier) is considered as highly invasive in the Galapagos. It is estimated to prey on 17–154 g insects per ha per day, mainly Lepidoptera, therefore competing for food with finches and other arthropod predators (Parent 2000, in Causton et al. 2006). A congeneric species, the European Polistes dominulus (Christ) has invaded North America where it may be competing with the native Polistes fuscatus (F.). Although displacement has not yet been proven, several studies showed that the invasive species is competitively superior to the native species (Gamboa et al. 2002; Armstrong and Stamp 2003; Curtis et al. 2005).
Invasive mosquitoes have the potential to affect populations of native mosquitoes by various mechanisms. However, the only well illustrated cases of displacement are between two invasive species, particularly Aedes albopictus (Skuse) displacing A. aegypti (L.) in various regions (see Juliano and Lounibos 2005, for review). For example Juliano (1998) investigated the competitive interactions between A. albopictus, recent invader in Florida, and A. aegypti, the previously resident invader, which had been shown to be displaced by A. albopictus in some environments and not others (Juliano et al. 2004). Juliano (1998) used field and lab experiments to test both the effects of shared parasites, Ascogregarina sp., and interspecific competition on mosquito communities. His results show that A. albopictus was a superior competitor in the absence of the parasite, and that its rate of parasite infection in the field was actually higher than that found in A. aegypti. The variation in the outcome of the interaction between the two mosquitoes was attributed to differences in habitat suitability. Juliano (1998) concluded that, when it occurred, direct competition was the mechanism driving the replacement of A. aegypti by A. albopictus.
Displacements of native mosquito species have been less extensively studied. The invasive Culex quinquefasciatus Say may have displaced the native Culex tarsalis Coquillett in California through competition for resource and by degrading larval breeding sites (Smith et al. 1995). A laboratory experiment showed that C. quinquefasciatus displaces C. tarsalis in laboratory cages within a single generation (Smith et al. 1995). Carrieri et al. (2003) carried out laboratory experiments to investigate potential competitive interactions between A. albopictus and the native mosquito Culex pipiens L. in Italy. They found evidence that A. albopictus is superior in resource competition with C. pipiens but, to date, the displacement of C. pipiens has not been demonstrated in the field. Similarly, although laboratory experiments consistently showed that the invasive A. albopictus was competitively superior to the native North American Ochlerotata triseriatus (Say), there is no evidence for decline of O. triseriatus in the field (Lounibos et al. 2001). In some cases, extensive research programmes on abundant invasive predators fail to show a significant effect on the native fauna, as for the European carabid beetle Pterostichus melanarius Illiger, invasive but apparently harmless for native carabid populations in North America (Niemelä and Spence 1991; Niemelä et al. 1997).
Several calliphorid blow flies have been introduced from the Old World to the Americas. While some are strictly saprophagous species feeding mainly on carrion, at least two species, Chrysomya albiceps (Wiedemann) and C. rufifacies (Maquart), are facultative predators on other maggots. Both species are displacing native flies, in particular the American species Cochliomyia macellaria (F.), in both field and laboratory experiments (Wells and Greenberg 1992; Wells and Kurahashi 1997; Del Bianco Faria et al. 1999). Interestingly, laboratory experiments showed that Old World species having co-evolved with the predatory species are more resistant to predation than C. macellaria (Wells and Kurahashi 1997; Del Bianco Faria et al. 1999).
Effects as vectors of animal diseases
Several invasive mosquitoes are vectors of various animal and human diseases (Juliano and Lounibos 2005). Some of them have severe consequences for biodiversity. For example, invasive Culex spp. are responsible for the transmission of avian malaria that devastates endemic bird populations in Hawaii, particularly at low elevations (Van Riper 1991; Atkinson et al. 1995; Woodworth et al. 2005). In New Zealand, Tompkins and Gleeson (2006) found a correlation between the distribution of the invasive C. quinquefasciatus and the occurrence of avian malaria.
Indirect effects on plant communities and ecosystem processes
Predators having a significant effect on native species may also indirectly affect plant communities and ecosystem processes through cascading effects. Well described cascading effects by invasive predators are rare, except for some invasive ants. For example, the yellow crazy ant, A. gracilipes, has caused a rapid, catastrophic shift in the rain forest ecosystem of Christmas Island by greatly reducing populations of the red land crab, which is the main endemic consumer of the forest floor (O’Dowd et al. 2003). The displacement of crab populations results in slower litter breakdown, followed by a release of seedling recruitment and an increase in tree and shrub species richness. Furthermore, new associations between the alien ant and scale insects have led to tree dieback and changes in tree community composition. Similar observations were made in other parts of the world where A. gracilipes has been introduced, such as in the Seychelles, where it developed an association with scale insects resulting in tree mortality (Hill et al. 2003).
The invasion of the argentine ant, L. humile, in many parts of the world has disturbed seed dispersal through the displacement of myrmecochorous ants (Christian 2001; Carney et al. 2003; Gómez and Oliveras 2003; Gómez et al. 2003; Witt et al. 2004). It is also known to reduce fruit-set and seed set of some native plants (Blancafort and Gómez 2005). Lach (2007, 2008) observed that L. humile displaces floral arthropods, including pollinators, on various plants of the South African fynbos, but this decline had no detectable effect on seed sets. In addition, the argentine ant is strongly suspected to affect soil chemistry, turnover and erosion. Nest building and foraging activities of the red imported fire ant, S. invicta, affect physical and chemical soil properties and strongly enhances plant growth though the increase of NH4 + (Lafleur et al. 2005). In general, the importance of ecosystem process effects in invasive ants is largely unknown and deserves further studies (Folgarait 1998; Holway et al. 2002).
The Chinese mantis, Tenodera sinensis (Sauss.), in North America provides another interesting case of a trophic cascade effect triggered by a generalist predator. The mantis preys on both herbivores and spiders, which feed on the same herbivores. The result is a net herbivore reduction increasing plant biomass (Moran et al. 1996; Moran and Hurd 1998). In other cases, effects on ecosystem processes are strongly suspected but not fully ascertained. For example, in New Zealand, European wasps are thought to alter nutrient cycling in New Zealand beech forests by removing honeydew, which reduces the flow of carbon to microorganisms in the phyllosphere and the soil (Beggs 2001).
Ecological effects due to parasitoids and parasites
About 2,000 arthropod species have been released in new regions for biological control purposes, the majority of them being parasitoids (van Lenteren et al. 2006). A small number of these parasitoids have been subsequently reared from non target species. In several cases an effect on native non-target hosts and native parasitoids has been either documented or suspected (see examples in Lynch and Thomas 2000; van Lenteren et al. 2006; Parry 2008).
The earliest example is probably that of the tachinid, Bessa remota (Aldrich), released in Fiji in the 1920s, which is suspected to have caused the extinction of the questionably native target species, the coconut moth Levuana iridescens Bethune-Baker, but also of a non-target native moth, Heteropan dolens Druce (Tothill et al. 1930; Kuris 2003). However, assessing the effect of alien parasitoids on non-target hosts/preys and native parasitoids long after their introduction is a complicated task because, in most cases, the necessary quantitative data on native species populations before the introduction, or in non-invaded areas, are not available. A good example is the introduction of the tachinid fly Trigonospila brevifacies (Hardy) from Australia to New Zealand to control the tortricid moth Epiphyas postvittana (Walker). An extensive study on the parasitoid food web of Tortricidae in New Zealand showed that the tachinid has become the dominant parasitoid of many Tortricidae in broadleaf/podocarp forests in central North Island (Munro and Henderson 2002). Although the introduced parasitoid is suspected to affect both native tortricid populations and their parasitoids, the authors conclude that, “as no pre-release data on the composition of the parasitoid guild or the relative abundance of lepidopteran species were gathered before the release of T. brevifacies, it is difficult to determine the exact effect the tachinid has had on the native fauna in this system. Empirical studies of a simplified controlled host–parasitoid community would be required to determine if native parasitoid displacement were actually occurring”.
Another tachinid, C. concinnata, has been implicated in the decline of several species of native Lepidoptera since shortly after its release in North America in 1906 for control of the gypsy moth. Boettner et al. (2000) state that C. concinnata has significantly contributed to the decline of several native saturniid moths. They base their conclusion, firstly, on the fact that field exposures of saturniid caterpillars resulted in very high parasitism by C. concinnata and, secondly, by presenting arguments that alternative hypotheses for the decline were very unlikely. A similar study by Kellogg et al. (2003) demonstrated high rates of parasitism by C. concinnata on native luna moths, Actias luna (L.), in Virginia, but concluded that long-term studies would be needed to determine the actual effects of the parasitoid on luna moth populations.
Life tables are sometimes used to assess the effect of alien natural enemies on native species. For example, Johnson et al. (2005) applied life table studies to show that the decline of the native Hawaiian koa bug, Coleotichus blackburniae White, was due to accidentally introduced egg predators rather than parasitoids introduced for biological control. Alternatively, models may be used to assess the role of invasive species in the decline of native fauna. Keeler et al. (2006) used a stochastic simulation model to assess the respective role of an alien parasitoid, an alien plant and the loss of native host plants in the decline of the native butterfly Pieris napi oleracea Harris in North America. The model showed that the role of the parasitoid was probably negligible compared to the two other factors. Similarly, Barlow et al. (2004) and Barron (2007) modelled the impact of two introduced parasitoids in New Zealand on non-target hosts, using the intrinsic rate of host increase, the average abundance of the host in the presence of parasitism and the estimated mortality caused by the parasitoid. Barlow et al. (2004) predicted that the introduction of the alien braconid Microctonus aethiopoides Loan would decrease populations of some native weevil species by 8–30%. The models developed by Barron (2007) showed that the introduced pteromalid parasitoid, Pteromalus puparum (L.), is probably not responsible for the decline of populations of the endemic red admiral butterfly Bassaris gonerilla (F.).
Accidentally introduced parasitoids may also cause ecological damage. For example, the ichneumonid Echthromorpha intricatoria (F.) is suspected to be partly responsible for the decline of B. gonerilla in New Zealand (Barron et al. 2004). Kenis et al. (2007) suspect that many parasitoids that are thought of as occurring on several continents may have been more or less recently introduced accidentally with their host or host plant. Several of these may cause undetected hazards on new hosts.
It has often been suggested that introduced parasitoids may also displace native parasitoids by competition (Bennett 1993), but reliable examples are rare. The Nearctic aphid parasitoid Lysiphlebus testaceipes (Cresson), introduced in the Mediterranean region to control Aphis spiraecola Patch, has become a dominant parasitoid of other aphid species, including Toxoptera aurantii (Boyer de Fonscolombe), in which it may have displaced two congeneric parasitoid species, L. fabarum (Marshall) and L. confuses Tremblay & Eady (Tremblay 1984). Similarly, Schellhorn et al. (2002) provide evidence that the exotic braconid parasitoid Aphidius ervi (Haliday), introduced into North America to control the pea aphid Acyrthosiphon pisum Harris, has caused the decline of the native Praon pequadorum Viereck. Another example is the probable displacement of Encarsia margaritiventris (Mercet) as dominant parasitoid of the viburnum whitefly, Aleurotuba jelineki (Frauen.), in Italy, following the introduction of the exotic parasitoid Cales noaki Howard (Viggiani 1994). A recent study by Parry (2008) suggests that Compsilura concinnata may be involved in the apparent disappearance from areas of New England of Lespesia frenchii (Williston), a native polyphagous tachinid competing for the same lepidopteran hosts. Other potential cases are listed and discussed in Lynch and Thomas (2000) and van Lenteren et al. (2006).
The small hive beetle, Aethina tumida Murray, a nest parasite/scavenger native to Sub-Saharan Africa has invaded North America and Australia, where it parasitizes domesticated honey bees. It also attacks and develops on bumble bees, and there is growing concern that may affect populations of native pollinators (Hoffmann et al. 2008).
Some exotic ectoparasites are considered as ecological pests because of the damage on vertebrates. In the Galapagos, a parasitic fly, Philornis downsi Dodge & Aitken, significantly decreases fledging success of finches by infesting and killing juvenile birds (Fessl et al. 2006). A chewing louse, Damalina (Cervicola), is suspected to cause hair-loss syndrome in black-tail deer in North America, although firm evidence is still lacking (Bildfell et al. 2004).
Ecological effects due to invasive pollinators
Invasive pollinators, in addition to causing hazards through hybridization (see section above), may also compete with native pollinators for floral resources and nesting sites. Other undesirable effects include co-introduction of natural enemies, inadequate pollination of native flora or undesirable pollination of exotic flora (Goulson 2003; Goulson et al. 2008). The honeybee, A. mellifera, has been widely introduced in many regions for pollination and honey production (Moritz et al. 2005). Although its introduction is often considered positive, various detrimental effects have been investigated and reported. In particular, it has been often reported to cause a decline in native bee and bird species, particularly on islands (e.g. Roubik 1978; Kato et al. 1999; Hansen et al. 2002; Dupont et al. 2003). Another important invasive pollinator, the European bumblebee, Bombus terrestris (L.), displaces native megachilid bees in Tasmania, where it is also suspected to have a negative effect on plant pollination (Hingston and McQuillan 1999). In addition, it has been shown that B. terrestris has superior reproductive rate than native Japanese bumblebees and there is serious risk of outcompetition since there is overlap in forage use and time of foraging (Matsumura et al. 2004; Inari et al. 2005).
Non native pollinators can be vectors of pathogens which can threat native pollinators. An interesting case is the spread of bumblebee-specific pathogens (Critihidia bombi Lipa and Triggiani and Nosema bombi Fantham & Porter) and tracheal mite (Locustacarus buchneri Stammer) due to trafficking of commercial bumblebee colonies. Pathogen spillover from commercial bumblebee colonies can potentially have a devastating effect on native bumblebee populations (Colla et al. 2006). Indeed, Colla et al. (2006) have shown that commercial colonies have greater parasite load than wild colonies and that pathogen loads in wild bumblebee populations near commercial greenhouses are significantly increased.
Introduced bees are also known to reduce fitness of some native plant species (Roubik 1996; Gross and Mackay 1998). On the other hand, they may enhance pollination and, consequently, invasiveness of exotic weeds, as shown by Barthell et al. (2001) for Centaurea solstitialis L. in North America and Stout et al. (2002) for Lupinus arboreus Sims in Tasmania. Another interesting example is provided by fig wasps. Many fig species are dependent on highly specific fig wasps (Agaonidae) for pollination, and without them the fig tree will bear no seeds. Three exotic fig trees, Ficus microcarpa L., F. benghalensis L., and F. altissima Blume grown in Florida gardens for over a century only started spreading and became invasive in the 1980s, when their fig wasp pollinators arrived (Nadel et al. 1992).
It must be noted that no single experiment has clearly demonstrated long-term reductions in populations of native organisms following the introduction of exotic pollinators (Goulson 2003; Moritz et al. 2005). As stated by Goulson (2003) this probably reflects more the difficulty of carrying out convincing competition studies rather than a true absence of competitive effects. Whereas most studies on the ecological effect of introduced pollinators rely on correlational data or other indirect measures, two recent investigations use experimental approaches. Kenta et al. (2007) tested, in a greenhouse experiment, the potential disturbance caused by the introduction of the European bumblebee on native plant–pollinator interactions. They concluded that the alien bumblebee can disturb pollination on a plant even when only representing a small fraction of the total pollinator community. Thomson (2004) provides the first experimental demonstration of the negative effects of non native honey bees on native bumblebees. She experimentally introduced honey bees and found that proximity to hives significantly reduced the foraging rates and reproductive success of Bombus occidentalis Greene colonies. In addition, Thomson (2006) found significant niche overlap between foraging preferences of native bumble bees and introduced honey bees, which is maximum at the end of the season when floral resources are more limited. This indicates that both native bumblebees and introduced honey bees largely rely on the same restricted suite of plant species.
Conclusions and future research
This review has shown that invasive alien insects can affect native species and ecosystems through a variety of mechanisms. A surprisingly high number of primary research publications (403) were found that describe or investigate the ecological effect of invasive alien insects. Nevertheless, these studies concern only 72 species, and a clear impact in field conditions has been ascertained for only 54 of them. This represents a very low proportion of the alien insects in the world. For example, 311 alien insect species are established in Switzerland (Kenis 2006), more than 2,000 alien arthropods are found in the Continental USA and more than 2,500 in Hawaii (Pimentel 2002). It is not clear whether the low proportion of alien insects known to have an effect on biodiversity reflects a lack of effect or a lack of investigations. The vast majority of studies found during our survey (>80%) reported a significant effect, suggesting that more investigations would reveal more impacts. However, it may also be partly due to a publication bias towards studies showing significant results.
Other important biases are observed towards species and ecosystems that are also considered important for the economy or public health, as illustrated by the high number of studies investigating the impact of alien ants, honey bees, plant pests or mosquitoes. Many studies showing effects on native insect biodiversity focused on groups that are considered as more “attractive” for the public and the researchers, e.g. butterflies or ladybirds, although there is no scientific reason to believe that, for example, aphids or flies are less affected by invasive species.
The vast majority of studies on the effect of alien herbivorous insects have focused on forest pests and their damage on trees and forest ecosystems, probably because their effect is more visible and concerns keystone species of forest ecosystems. However, many other invasive herbivores would deserve more attention for their potential effect on indigenous plants. For example, in eastern North America, dozens of studies have investigated the impact of alien forest pests such as L. dispar, A. piceae and A. tsugae, whereas none has focused on the ecological effects of the lily leaf beetle, Lilioceris lilii (Scopoli), and the viburnum leaf beetle, Pyrrhalta viburni (Paykull), two species that may seriously threaten the survival of wild lilies (Lilium spp.) and Viburnum spp. in the same region (Ernst et al. 2007; Weston et al. 2007).
Similarly, many investigations have focused on the effect of insects released for biological control because of the follow-up studies carried out in biological control programmes and because of the particular interest of conservation ecologists for the non-target effect of alien biological control agents (Louda et al. 2005; van Lenteren et al. 2006). The Asian ladybird, H. axyridis, a biological control agent that invaded North America and Europe is presently the target of extensive studies on its potential impact on native ladybirds (Burgio et al. 2002; Michaud 2002; Snyder et al. 2004; Yasuda et al. 2004; Ware and Majerus 2008) whereas the impact of many alien predators accidentally introduced in the same continents remains totally unexplored.
Furthermore, all examples of ecological impact cited here concern terrestrial ecosystems, with the exception of mosquitoes, but freshwater ecosystems are probably not immune. Insects as effective biological control agents of alien water weeds clearly can have a profound effect on freshwater plant populations and hence ecosystem functioning (Mbati and Neuenschwander 2005). Thus, there are clear and important gaps in our knowledge of the effect of alien insects on native biodiversity and ecosystems, particularly in species and habitats that are of lower importance for the economy and the general public. Nevertheless, these gaps also represent exciting opportunities for further research and the remarkable increase in the number of studies on the ecological impact of invasive insects shows that these opportunities are presently being taken.
Examples of effects on species populations and communities are far more numerous than those on ecosystem processes, an observation also made by Parker et al. (1999) for several groups of invasive species. In contrast, Levine et al. (2003) found that roughly equal numbers of studies on invasive plants examined effects on species and communities and effects on ecosystem processes. In general, effects of invasive species on native species populations are more easily observed, i.e. through comparative studies between invaded and non-invaded areas, or between conditions before and after invasion. However, many of these observations are rather anecdotal or quantified at very local scales only, and the mechanisms by which the impacts arise are often not clearly understood. Furthermore, making assessments of invasion impact on the basis of temporal or spatial correlations may be misleading (Thomson 2006). To fully assess and understand the ecological effects of an invasive species and the mechanisms behind variations in populations observed, or not, in the field, experimental approaches are needed, preferably under field conditions. This is particularly true for effects occurring at the same trophic level, i.e., the displacement of an herbivore by another herbivore or a predator by another predator. In cases such as these, the mechanisms underlying the impact are often indirect and complex. For example, until now the numerous field observations and laboratory experiments carried out to assess the effect H. axyridis on native ladybirds have failed to understand whether displacement is due to direct intraguild predation or through resource competition. The question may only be answered by field experiments involving the manipulation of prey and ladybird densities and analysis of gut contents.
Direct effects of invasive insects on lower trophic levels through herbivory, predation and parasitism are easier to assess, at least at a local scale. Evaluating the effect on native species at a regional scale is often more complicated because it has to take into account the geographic, ecological and temporal variability throughout the distribution range. The effect of an invasive insect is known to vary with time, space and system, and, understandably, studies tend to focus on sites and systems where the impact is most likely. However, it would be of utmost importance to conduct parallel studies in systems where impacts are suspected to be lower. This would improve assessments of the regional importance of the invasive species, as well as increasing our understanding of the mechanisms underlying impacts. Finally it could also allow us to understand how native ecosystems and communities resist the impact of invaders, which could be the key for restoring invasion-resistant ecosystems and for developing control strategies (Levine et al. 2003).
Investigations on ecosystem effects, albeit less numerous than population and community effects, are often of good quality because they require longer studies based on a priori hypotheses on impact processes. Nevertheless, as pointed out by Levine et al. (2003) for plant invasions, the consequences of alterations in ecosystem processes for species populations and community structure are poorly explored. For example, invasive forest herbivores such as L. dispar and A. tsugae are known to alter nitrogen cycling in the invaded forests, but how these changes affect plant and animal communities remains unclear.
References
Aguiar-Franquinho AM, Karsholt O (2006) Systematic catalogue of the entomofauna of the Madeira Archipelago and Selvagens islands. Bol Mus Municipal do Funchal 9:5–139
Allen D, Bowersox TW (1989) Regeneration in oak stands following gypsy moth defoliations. In: Rink G, Budelsky CA (eds) Proceedings of the 7th central hardwood conference, General Technical Report, NC-132. United States Department of Agriculture Forest Service, North Central Experiment Station, pp 67–73
Allen CR, Demarais S, Lutz RS (1997) Effects of red imported fire ants on recruitment of white-tailed deer fawns. J Wildl Manage 61:911–916
Allen CR, Willey RD, Myers PE, Horton PM, Buffa J (2000) Impact of red imported fire ant infestation on northern bobwhite quail abundance trends in southeastern United States. J Agric Urban Entomol 17:43–51
Allen CR, Lutz RS, Lockley T, Phillips SA Jr, Demarais S (2001) The non-indigenous ant Solenopsis invicta, reduces loggerhead shrike and native insect abundance. J Agric Urban Entomol 18:249–259
Armstrong TR, Stamp NE (2003) Colony productivity and foundress behaviour of a native wasp versus an invasive social wasp. Ecol Entomol 28:635–644
Atkinson CT, Woods KL, Dusek RJ, Sileo LS, Iko WM (1995) Wildlife disease and conservation in Hawaii: pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected Iiwi (Vestaria coccinea). Parasitology 111:S59–S69
Barlow ND, Goldson SL (2002) Alien invertebrates in New Zealand. In: Pimentel D (ed) Biological invasions: economic and environmental costs of alien plant, animal and microbe species. CRC Press, New York, pp 195–216
Barlow ND, Barrat BIP, Ferguson CM, Barron MC (2004) Using models to estimate parasitoid impacts on non-target host abundance. Environ Entomol 33:941–948
Barron MC (2007) Retrospective modelling indicates minimum impact of non-target parasitism by Pteromalus puparum on red admiral butterfly (Bassaris gonerilla) abundance. Biol Control 41:53–63
Barron MC, Wratten SD, Barlow ND (2004) Phenology and parasitism of the red admiral butterfly Bassaris gonerilla (Lepidioptera: Nymphalidae). New Zeal J Ecol 28:105–111
Barthell JF, Randall JM, Thorp RW, Wenner AM (2001) Promotion of seed set in yellow star-thistle by honey bees: evidence of an invasive mutualism. Ecol Appl 11:1870–1883
Beggs J (2001) The ecological consequences of social wasps (Vespula spp.) invading an ecosystem that has an abundant carbohydrate resource. Biol Conserv 99:17–28
Bell JL, Whitmore RC (2000) Bird nesting ecology in a forest defoliated by gypsy moths. Wilson Bull 112:524–531
Bennett FD (1993) Do introduced parasitoids displace native ones? Fla Entomol 76:54–63
Bildfell RJ, Mertins JW, Mortensen JA, Cottam DF (2004) Hair-loss syndrome in black-tailed deer of the Pacific Northwest. J Wildl Dis 40:670–681
Blancafort X, Gómez C (2005) Consequences of the Argentine ant, Linepithema humile (Mayr), invasion on pollination of Euphorbia characias (L.) (Euphorbiaceae). Acta Oecol 28:49–55
Boettner GH, Elkinton JS, Boettner CJ (2000) Effects of a biological control introduction on three non-target native species of saturniid moth. Conserv Biol 14:1798–1806
Brasier CM (2000) Intercontinental spread and continuing evolution of the Dutch elm research pathogens. Kluwer Academic Publishers, Boston, pp 61–72
Brooks RT (2001) Effects of the removal of overstory hemlock from hemlock-dominated forests on eastern redback salamanders. For Ecol Manage 149:197–204
Brown MW, Miller SS (1998) Coccinellidae (Coleoptera) in apple orchards of eastern West Virginia and the impact of invasion by Harmonia axyridis. Entomol News 109:143–151
Burgio G, Santi F, Maini S (2002) On intra-guild predation and cannibalism in Harmonia axyridis (Pallas) and Adalia bipunctata L. (Coleoptera: Coccinellidae). Biol Control 24:110–116
Busing RT, Pauley EF (1994) Mortality trends in a southern Appalachian red spruce population. For Ecol Manage 64:41–45
Carney SE, Byerley MB, Holway DA (2003) Invasive Argentine ants (Linepithema humile) do not replace native ants as seed dispersers of Dendromecon rigida (Papaveraceae) in California, USA. Oecologia 135:576–582
Carpintero S, Reyes-López J, Arias de Reyna L (2005) Impact of Argentine ants (Linepithema humile) on an arboreal ant community in Doñana National Park, Spain. Biol Conserv 14:151–163
Carrieri M, Bacchi M, Bellini R, Maini S (2003) On the competition occurring between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environ Entomol 32:1313–1321
Causton CE, Peck SB, Sinclair BJ, Roque-Albel L, Hodgson CJ, Landry B (2006) Alien insects: threats and implications for conservation of Galápagos Islands. Ann Entomol Soc Am 99:121–143
Christian CE (2001) Consequences of a biological invasion reveals the importance of mutualism for plant communities. Nature 413:635–639
Cobb RC, Orwig DA, Currie S (2006) Decomposition of green foliage in eastern hemlock forests of southern New England impacted by hemlock woolly adelgid infestations. Can J For Res 36:1331–1341
Colla SR, Otterstatter MC, Gegear RJ, Thomson JD (2006) Plight of the bumble bee: pathogen spillover from commercial to wild populations. Biol Conserv 129:461–467
Colunga-Garcia M, Gage S (1998) Arrival, establishment, and habitat use of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) in a Michigan landscape. Environ Entomol 27:1574–1580
Curtis TR, Aponte Y, Stamp NE (2005) Nest absorbency, toughness, and protein concentration of a native vs. an invasive wasp. J Anim Ecol 31:1089–1100
De La Rùa P, Serrano J, Galian J (2002) Biodiversity of Apis mellifera populations from Tenerife (Canary Islands) and hybridisation with East European races. Biol Conserv 11:59–67
de Zenner-Polania I, Wilches OM (1992) Impacto ecologico de la hormiga loca, Paratrechina fulva (Mayr), en el municipio de Cimitarra (Santander). Rev Colomb Entomol 18:14–22
Dejean A, Kenne M, Moreau CS (2007) Predatory abilities favour the success of the invasive ant Pheidole megacephala in an introduced area. J Appl Entomol 131:625–629
Del Bianco Faria L, Orsi L, Trinca LA, Godoy WAC (1999) Larval predation by Chrysomya albiceps on Cochliomyia macellaria, Chrysomya megacephala and Chrysomya putoria. Entomol Exp Appl 90:149–155
Dupont YL, Hanse DM, Valido A, Olesen JM (2003) Impact of introduced honeybees on native pollination interactions of the endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands. Biol Conserv 118:301–311
Elliott N, Kieckhefer R, Kauffman W (1996) Effects of an invading coccinellid on native coccinellids in an agricultural landscape. Oecologia 105:537–544
Ernst C, Cappuccino N, Arnason JT (2007) Potential novel hosts for the lily leaf beetle Lilioceris lilii Scopoli (Coleoptera: Chrysomelidae) in eastern North America. Ecol Entomol 32:45–52
Eschtruth AK, Cleavitt NL, Battles JJ, Evans RA, Fahey TJ (2006) Vegetation dynamics in declining eastern hemlock stands: 9 years of forest response to hemlock woolly adelgid infestation. Can J For Res 36:1435–1450
Eubanks MD, Blackwell SA, Parrish CJ, Delamar ZD, Hull-Sanders H (2002) Intraguild predation of beneficial arthropods by red imported fire ants in cotton. Environ Entomol 31:1168–1174
Evans EW (2004) Habitat displacement of North American ladybirds by an introduced species. Ecology 85:637–647
Fabre JP, Auger-Rozenberg MA, Chalon A, Boivin S, Roques A (2004) Competition between exotic and native insects for seed resources in trees of a Mediterranean forest ecosystem. Biol Invasions 6:11–22
Fajvan M, Wood JM (1996) Stand structure and development after gypsy moth defoliation in the Appalachian Plateau. For Ecol Manage 89:79–88
Fessl B, Sinclair BJ, Kleindorfer S (2006) The life-cycle of Philornis downsi (Diptera: Muscidae) parasitizing Darwin’s finches and its impacts on nestling survival. Parasitology 133:739–747
Folgarait PJ (1998) Ant biodiversity and its relationship to ecosystem functioning: a review. Biol Conserv 7:1221–1244
Ford CR, Vose JM (2007) Tsuga canadensis (L.) Carr. mortality will impact hydrologic processes in Southern Appalachian forest ecosystems. Ecol Appl 17:1156–1167
Forys EA, Quistorff A, Allen CR, Wojcik DP (2001) The likely cause of extinction of the tree snail Orthalicus reses reses (Say). J Molluscan Stud 67:369–376
Fowler SV (2004) Biological control of an exotic scale. Orthezia insignis Browne (Homoptera: Ortheziidae), saves the endemic gumwood tree Commidendrum robustum (Roxb.) DC. (Asteraceae) on the Island of St. Helena. Biol Control 29:367–374
Gale GA, DeCecco JA, Marshall MR, McClain WR, Cooper RJ (2001) Effects of gypsy moth defoliation on forest birds: an assessment using breeding bird census data. J Field Ornithol 72:291–304
Gamboa GJ, Greig EI, Thom MC (2002) The comparative biology of two sympatric paper wasps, the native Polistes fuscatus and the invasive Polistes dominulus (Hymenoptera, Vespidae). Insects Soc 29:45–49
Gardiner BOC (2003) The possible cause of extinction of Pieris brassicae wollastoni Butler (Lepidoptera: Pieridae). Entomol Gaz 54:267–268
Gerlach J (2004) Impact of the invasive crazy ant Anoplolepis gracilipes on Bird Island, Seychelles. J Insect Conserv 8:15–25
Gibbs GW (1980) New Zealand butterflies: identification and natural history. Collins, Auckland
Gibbs GW (1987) Butterfly blues. For Bird 18:18–20
Gillespie RG, Reimer N (1993) The effect of alien predatory ants (Hymenoptera: Formicidae) on Hawaiian endemic spiders (Araneae: Tetragnathidae). Pac Sci 47:21–33
Gómez C, Oliveras J (2003) Can the Argentine ant (Linepithema humile Mayr) replace native ants in myrmecochory? Acta Oecol 24:47–53
Gómez C, Pons P, Bas JM (2003) Effects of the Argentine ant Linepithema humile on seed dispersal and seedling emergence of Rhamnus alaternus. Ecography 26:532–538
Gottschalk KW (1990) Gypsy moth effects on mast production. In: Charles E (ed) Proceedings of the workshop, Southern Appalachian mast management, University of Tennessee, Knoxville, pp 42–50
Goulson D (2003) Effects of introduced bees on native ecosystems. Annu Rev Ecol Syst 34:1–26
Goulson D, Lye GC, Darvill B (2008) Decline and conservation of bumblebees. Annu Rev Entomol 53:191–208
Grapputo A, Boman S, Lindstrom L, Lyytinen A, Mappes J (2005) The voyage of an invasive species across continents: genetic diversity of North American and European Colorado potato beetle populations. Mol Ecol 14:4207–4219
Gross CL, Mackay D (1998) Honeybees reduce fitness in the pioneer shrub Melastoma affine (Melastomataceae). Biol Conserv 86:169–178
Hansen DM, Olesen JM, Jones CG (2002) Trees birds and bees in Mauritius: exploitable competition between introduced honeybees and endemic nectarivorous birds. J Biogeogr 29:721–734
Havill NP, Montgomery ME, Yu G, Shiyake S, Caccone A (2006) Mitochondrial DNA from hemlock woolly adelgid (Hemiptera: Adelgidae) suggests cryptic speciation and pinpoints the source of the introduction to eastern North America. Ann Entomol Soc Am 99:195–203
Hill M, Holm K, Vel T, Shah NJ, Matyot P (2003) Impact of the introduced yellow crazy ant Anoplolepis gracilipes on Bird Island, Seychelles. Biol Conserv 12:1969–1984
Hingston AB, McQuillan PB (1999) Displacement of Tasmanian native megachilid bees by the recently introduced bumblebee Bombus terrestris (Linnaeus, 1758) (Hymenoptera: Apidae). Aust J Zool 47:59–65
Hoffmann BD, Parr CL (2008) An invasion revisited: the African big-headed ant (Pheidole megacephala) in northern Australia. Biol Invasions. doi:10.1007/s10530-007-9194-x
Hoffmann BD, Andersen AN, Hill GJE (1999) Impact of an introduced ant on native rain forest invertebrates: Pheidole megacephala in monsoonal Australia. Oecologia 120:595–604
Hoffmann D, Pettis JS, Neumann P (2008) Potential host shift of the small hive beetle (Aethina tumida) to bumblebee colonies (Bombus impatiens). Insect Soc. doi:10.1007/s00040-008-0982-9
Holt RD (1977) Predation, apparent competition and the structure of prey communities. Theor Popul Biol 12:197–229
Holway DA, Lach L, Suarez A, Tsutsui N, Case TJ (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–233
Hoogendoorn M, Heimpel GE (2002) Indirect interactions between an introduced and a native ladybird beetle species mediated by a shared parasitoid. Biol Control 25:224–230
Houston DR (1994) Major new tree disease epidemics: beech bark disease. Annu Rev Phytopathol 32:75–87
Human KG, Gordon DM (1996) Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia 105:405–441
Human KG, Gordon DM (1997) Effects of Argentine ants on invertebrate biodiversity in northern California. Conserv Biol 11:1242–1248
Huxel GR (1999) Rapid displacement of native species by invasive species: effects of hybridization. Biol Conserv 89:143–152
Inari N, Nagamitsu T, Kenta T, Goka K, Hiura T (2005) Spatial and temporal pattern of introduced Bombus terrestris abundance in Hokkaido, Japan, and its potential impact on native bumblebees. Popul Ecol 47:77–82
Ings TC, Raine NE, Chittka L (2005a) Mating preference of commercially imported bumblebees (Bombus terrestris) in Britain (Hymenoptera: Apidae). Entomol Gen 28:233–238
Ings TC, Schikora J, Chittka L (2005b) Bumblebees, humble pollinators or assiduous invaders? A population comparison of foraging performance in Bombus terrestris. Oecologia 144:508–516
Jenkins MA (2003) Impact of the balsam woolly adelgid (Adelges piceae Ratz.) on an Abies fraseri (Pursh) Poir. dominated stand near the summit of Mount LeConte, Tennessee. Castanea 68:109–118
Jenkins JC, Aber JD, Canham CD (1999) Hemlock woolly adelgid impacts on community structure and N cycling rates in eastern hemlock forests. Can J For Res 29:630–645
Jensen AB, Palmer KA, Boomsma JJ, Pedersen BV (2005) Varying degrees of Apis mellifera ligustica introgression in protected populations of the black honeybee, Apis mellifera mellifera, in northwest Europe. Mol Ecol 14:93–106
Johnson MT, Follett PA, Taylor AD, Jones VP (2005) Impacts of biological control and invasive species on a non-target native Hawaiian insect. Oecologia 142:529–540
Jones CG, Ostfeld RS, Richar MP, Scauber EM, Wolff JO (1998) Chain reaction linking acorns to gypsy moth outbreaks and lyme disease risk. Science 279:1023–1026
Jourdan H (1997) Threats on Pacific Islands: the spread of the tramp ant Wasmannia auropunctata (Hymenoptera: Formicidae). Pac Conserv Biol 3:61–64
Jourdan H, Sadlier RA, Bauer AM (2001) Little fire ant invasion (Wasmannia auropunctata) as a threat to New Caledonian lizards: evidences from a Sclerophyll forest (Hymenoptera: Formicidae). Sociobiology 38:283–301
Juliano SA (1998) Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology 79:255–268
Juliano SA, Lounibos LP (2005) Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett 8:558–574
Juliano SA, Olson JR, Murrell EG, Hatle JD (2004) Plasticity and canalization of insect reproduction: testing alternative models of life history transitions. Ecology 85:2986–2996
Kasper ML, Reeson AF, Cooper SJB, Perry KD, Austin AD (2004) Assessment of prey overlap between a native (Polistes humilis) and an introduced (Vespula germanica) social wasp using morphology and phylogenetic analyses of 16S rDNA. Mol Ecol 13:2037–2048
Kato M, Shibata A, Yasui T, Nagamasu H (1999) Impact of introduced honeybees, Apis mellifera, upon native bee communities in the Bonin (Ogasawara) Islands. Res Popul Ecol 41:217–228
Keeler MS, Chew FS, Goodale BC, Reed JM (2006) Modelling the impacts of two exotic invasive species on a native butterfly: top-down vs. bottom-up effects. J Anim Ecol 75:777–788
Kegg JD (1971) The impact of gypsy moth: repeated defoliation of Oak in New Jersey. J For 69:852–854
Kellogg SK, Fink LS, Brower LP (2003) Parasitism of native luna moths, Actias luna (L.) (Lepidoptera: Saturniidae) by the introduced Compsilura concinnata (Meigen) (Diptera: Tachinidae) in Central Virginia, and their hyperparasitism by trigonalid wasps. Environ Entomol 32:1019–1027
Kenis M (2006) Insects. In: Wittenberg R (ed) Invasive alien species in Switzerland. An inventory of alien species and their threat to biodiversity and economy in Switzerland. Environmental Studies, vol 29, no 6. Federal Office for the Environment
Kenis M, Rabitsch W, Auger-Rozenberg MA, Roques A (2007) How can alien species inventories and interception data help us prevent insect invasions? Bull Entomol Res 97:489–502
Kenta T, Inari N, Nagamitsu T, Goka K, Hiura T (2007) Commercialized European bumblebee can cause pollination disturbance: an experiment on seven native plant species in Japan. Biol Conserv 134:298–309
Kfir R (1997) Competitive displacement of Busseola fusca (Lepidoptera: Noctuidae) by Chilo partellus (Lepidoptera: Pyralidae). Ann Entomol Soc Am 90:619–624
King JR, Tschinkel WR (2006) Experimental evidence that the introduced fire ant, Solenopsis invicta, does not competitively suppress co-occurring ants in a disturbed habitat. J Anim Ecol 75:1370–1378
Kizlinski ML, Orwig DA, Cobb RC, Foster DR (2002) Direct and indirect ecosystem consequences of an invasive pest on forests dominated by eastern hemlock. J Biogeogr 29:1489–1503
Kuris AM (2003) Did biological control cause extinction of the coconut moth, Levuana iridescens, in Fidji? Biol Invasions 5:131–141
Laakkonen J, Fisher RN, Case TJ (2001) Effect of land cover, habitat fragmentation and ant colonies on the distribution and abundance of shrews in southern California. J Anim Ecol 70:776–788
Labrador M, Farré M, Utzet F, FontdeVilà A (1999) Interspecific hybridization increases transposition rates of Osvaldo. Mol Biol Evol 16:931–937
Lach L (2007) A mutualism with native membracid facilitates pollinator displacement by Argentine ants. Ecology 88:1994–2004
Lach L (2008) Argentine ants displace floral arthropods in a biodiversity hotspot. Divers Distrib 14:281–290
Lafleur B, Hooper-Bù LM, Mumma EP, Geaghan JP (2005) Soil fertility and plant growth in soils from pine forests and plantations: effect of invasive red imported fire ants Solenopsis invicta (Buren). Pedobiologia 49:415–423
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Lester PJ, Tavite A (2004) Long-legged ants, Anoplolepis gracilipes (Hymenoptera: Formicidae), have invaded Tokelau, changing composition and dynamics of ant and invertebrate communities. Pac Sci 58:391–401
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impact of exotic plant invasions. Proc R Soc Lond B Biol Sci 270:775–781
Liebhold AM, Macdonald WL, Bergdahl D, Mastro VC (1995) Invasion by exotic forest pests: a threat to forest ecosystems. For Sci Monogr 30:1–49
Lishawa SC, Bergdahl DR, Costa SD (2007) Winter conditions in eastern hemlock and mixed-hardwood deer wintering areas of Vermont. Can J For Res 37:697–703
Liu SS, De Barro PJ, Xu J, Luan JB, Zang LS, Ruan YM, Wan FH (2007) Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 318:1769–1772
Long JL (2003) Introduced mammals of the world. Their history distribution and influence. CABI, Wallingford
Lopez-Vaamonde CJ, Koning W, Brown RM, Jordan WC, Bourke AFG (2004) Social parasitism by male-producing reproductive workers in a eusocial insect. Nature 430:557–560
Louda SM, O’Brien CW (2002) Unexpected ecological effects of distributing the exotic weevil Larinus planus (F.) for the biological control of Canada thistle. Conserv Biol 16:717–727
Louda SM, Kendall D, Connor J, Simberloff D (1997) Ecological effects of an insect introduced for the biological control of weeds. Science 277:1088–1090
Louda SM, Rand TA, Arnett AE, McClay AS, Shea K, McEachern AK (2005) Evaluation of ecological risk to populations of a threatened plant from an invasive biocontrol insect. Ecol Appl 15:234–249
Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA (2001) Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biol Invasions 3:151–166
Lovett GM, Christenson LM, Groffman PM, Jones CG, Hart JE, Mitchell MJ (2002) Insect defoliating and nitrogen cycling in forests. Bioscience 52:335–341
Lovett GM, Canham CD, Arthur MA, Weathers KC, Fitzhugh RD (2006) Forest ecosystem responses to exotic pests and pathogens in eastern North America. Bioscience 56:395–405
Lynch AM (2004) Fate and characteristics of Picea damaged by Elatobium abietinum (Walker) (Homoptera: Aphididae) in the White Mountains of Arizona. West N Am Nat 64:7–17
Lynch LD, Thomas MB (2000) Nontarget effects in the biocontrol of insects with insects, nematodes and microbial agents: the evidence. Biocontrol News Inf 21:117N–130N
Mallet J (2005) Hybridization as an invasion of the genome. Trends Ecol Evol 20:229–237
Matsumura C, Nakajima M, Yokoyama J, Washitani I (2004) High reproductive ability of an alien bumblebee invader, Bombus terrestris, L., in the Hidaka region of southern Hokkaido, Japan. Jpn J Conserv Ecol 9:93–102
Mbati G, Neuenschwander P (2005) Biological control of three floating water weeds, Eichhornia crassipes, Pistia stratiotes, and Salvinia molesta in the Republic of Congo. Biocontrol 50:635–345
McClure MS (1984) Influence of cohabitation and resinosis on site selection and survival of Pineus boerneri Annand and P. coloradensis (Gillette) (Homoptera: Adelgidae) on red pine. Environ Entomol 13:657–663
McClure MS (1989) Biology, population trends, and damage of Pineus boerneri and P. coloradensis (Homoptera: Adelgidae) on red pine. Environ Entomol 18:1066–1073
Michaud JP (2002) Invasion of the Florida citrus ecosystem by Harmonia axyridis (Coleoptera: Coccinellidae) and asymmetric competition with a native species, Cycloneda sanguinea. Environ Entomol 31:827–835
Mitsuhata M, Ono M (1996) Hybridization between Japanese and European bumblebees (Bombus spp.). In: 7th International pollination symposium, Lethbridge, Canada
Mizell RFIII (2007) Impact of Harmonia axyridis (Colleoptera: Coccinellidae) on native arthropod predators in pecan and crabe myrtle. Fla Entomol 90:524–536
Moran MD, Hurd LE (1998) A trophic cascade in a diverse arthropod community caused by a generalist arthropod predator. Oecologia 113:126–132
Moran MD, Rooney TR, Hurd LE (1996) Top-down cascade from a bitrophic predator in an old-field community. Ecology 77:2219–2227
Morin RS, Liebhold AM, Tobin PC, Gottschalk KW, Luzader E (2007) Spread of beech bark disease in the eastern United States and its relationship to regional forest composition. Can J For Res 37:726–736
Moritz RFA, Härtel S, Neumann P (2005) Global invasions of the western honeybee (Apis mellifera) and the consequences for biodiversity. Ecoscience 12:289–301
Morrison LW (2002) Long-term impacts of an arthropod-community invasion by the imported fire ant, Solenopsis invicta. Ecology 83:2337–2345
Munro VMW, Henderson IM (2002) Non target effect of entomophagous biocontrol: shared parasitism between native Lepidopteran parasitoids and the biocontrol agent Trigonospila brevifascies (Diptera: Tachnidae) in forest habitats. Environ Entomol 31:388–396
Nadel H, Frank JH, Knight RJ Jr (1992) Symposium: insect behavioral ecology—’91: escapees and accomplices: the naturalization of exotic Ficus and their associated faunas in Florida. Fla Entomol 75:29–38
Ness JH (2003) Contrasting exotic Solenopsis invicta and native Forelius pruinosus ants as mutualists with Catalpa bignonioides, a native plant. Ecol Entomol 28:247–251
Niemelä P, Mattson WJ (1996) Invasion of North America forest by European phytophagous insects. Bioscience 46:741–753
Niemelä J, Spence JR (1991) Distribution and abundance of an exotic ground-beetle (Carabidae): a test of community impact. Oikos 62:351–359
Niemelä J, Spence JR, Cárcamo H (1997) Establishment and interactions of carabid populations: an experiment with native and introduced species. Ecography 20:643–652
NRC (2002) Predicting invasions of nonindigenous plants and plant pests. National Academy Press, Washington
O’Dowd DJ, Green PT, Lake PS (2003) Invasion “meltdown” on an oceanic island. Ecol Lett 6:812–817
Obrycki J, Giles K, Ormord A (1998) Interactions between an introduced and indigenous coccinellid species at different prey densities. Oecologia 117:279–285
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Parry D (2008) Beyond Pandora’s box: quantitatively evaluating non-target effects of parasitoids in classical biological control. Biol Invasions. doi:10.1007/s10530-008-9319-x
Perez-Sandi CM (2001) Addressing the threat of Cactoblastis cactorum (Lepidoptera: Pyralidae), to Opuntia in Mexico. Fla Entomol 84:499–502
Pimentel D (ed) (2002) Biological invasions. Economic and environmental costs of alien plants, animal and microbe species. CRC Press, Boca Raton
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. J For 104:118–124
Porter SD, Savignano DA (1990) Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 71:2095–2106
Rabenold KN, Fauth PT, Goodner BW, Sadowski JA, Parker PG (1998) Response of avian communities to disturbance by an exotic insect in spruce-fir forests of the southern Appalachians. Conserv Biol 12:177–189
Redman AM, Scriber JM (2000) Competition between the gypsy moth, Lymantria dispar, and the Northern tiger swallowtail, Papilio canadensis: interactions mediated by host plant chemistry, pathogens and parasitoids. Oecologia 125:218–228
Reitz SR, Trumble JT (2002) Competitive displacement among insects and arachnids. Annu Rev Entomol 47:435–465
Rhymer JM, Simberloff D (1996) Extinction by hybridization and introgression. Annu Rev Ecol Syst 27:83–109
Roque-Albelo L (2003) Population decline of Galápagos endemic Lepidoptera on Volcano Alcedo (Isabela Island, Galápagos Islands, Ecuador): an effect of the introduction of the cottony cushion scale? Bull Inst R Sci Nat Belg Entomol 73:1–4
Roubik DW (1978) Competitive interactions between neotropical pollinators and Africanized honey bees. Science 201:1030–1032
Roubik DW (1996) African honey bees as exotic pollinators in French Guiana. In: Matheson A, Buchmann SL, O’Toole C, Westrich P, Williams ID (eds) The conservation of bees. Academic, London, pp 173–182
Russel FL, Louda SM (2005) Indirect interaction between two native thistles mediated by an invasive exotic floral herbivore. Oecologia 146:373–384
Sarty M, Abbott KL, Lester PJ (2007) Community level impacts of an ant invader and food mediated coexistence. Insect Soc 54:166–173
Scheffer SJ, Grissell EE (2003) Tracing the geographical origin of Megastigmus transvaalensis (Hymenoptera: Torymidae): an African wasp feeding on a South American plant in North America. Mol Ecol 12:415–421
Schellhorn NA, Kuhman TR, Olson AC, Ives AR (2002) Competition between native and introduced parasitoids of aphids: non-target effects and biological control. Ecology 83:2745–2757
Schönrogge K, Crawley MJ (2000) Quantitative webs as a means of assessing the impact of alien insects. J Anim Ecol 69:841–868
Schreiner IH, Nafus DM (1993) Population increases of native moths following biological control of an introduced pest moth. Micronesia (Suppl) 4:49–56
Settele J, Hammen V, Hulme PE, Karlson U, Klotz S, Kotarac M, Kunin WE, Marion G, O’Connor M, Petanidou T, Peterseon K, Potts S, Pritchard H, Pyšek P, Rounsevell M, Spangenberg J, Steffan-Dewenter I, Sykes MT, Vighi M, Zobel M, Kühn I (2005) ALARM: assessing large scale environmental risks for biodiversity with tested methods. GAIA—Ecol Perspect Sci Humanit Econ 14:69–72
Settle WH, Wilson LT (1990) Invasion by the variegated leafhopper and biotic interactions: parasitism, competition and apparent competition. Ecology 71:1461–1470
Simberloff D (1986) Introduced insects: a biogeographic and systematic perspective. In: Mooney HA, Drake JA (eds) Ecology of biological invasions of North America and Hawaii. Springer Verlag, New York, pp 3–26
Small MJ, Small CJ, Dreyer GD (2005) Changes in a hemlock-dominated forest following woolly adelgid infestation in southern New England. J Torrey Bot Soc 132:458–470
Smith GF, Nicholas NS (2000) Size- and age-class distributions of Fraser fir following balsam woolly adelgid infestation. Can J For Res 30:948–957
Smith PT, Reisen WK, Cowles DA (1995) Interspecific competition between Culex tarsalis and Culex quinquefasciatus. J Vector Ecol 20:139–146
Snyder WE, Evans EW (2006) Ecological effects of invasive arthropod generalist predators. Annu Rev Ecol Evol Syst 37:95–122
Snyder WE, Clevenger GM, Eigenbrode SD (2004) Intraguild predation and successful invasion by introduced ladybird beetles. Oecologia 140:559–565
Stadler B, Müller T, Orwig D, Cobb R (2005) Hemlock woolly adelgid in New England forests: canopy impacts transforming ecosystem processes and landscapes. Ecosystems 8:233–247
Stiling PD, Moon DC (2001) Protecting rare Florida cacti from attack by the exotic cactus moth, Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla Entomol 84:506–509
Stiling PD, Moon D, Gordon D (2004) Endangered cactus restoration: mitigating the non-target effects of a biological control agent (Cactoblastis cactorum) in Florida. Restor Ecol 12:605–610
Stout JC, Kells AR, Goulson D (2002) Pollination of the invasive exotic shrub Lupinus arboreus (Fabaceae) by introduced bees in Tasmania. Biol Conserv 106:425–434
Suarez AV, Yeh P, Case TJ (2005) Impacts of Argentine ants on avian nesting success. Insect Soc 52:378–382
Suttle KB, Hoddle MS (2006) Engineering enemy-free space: an invasive pest that kills its predators. Biol Invasions 8:639–649
Thomson DM (2004) Competitive effects of the invasive European honey bee on the reproductive success of a native bumble bee. Ecology 85:458–470
Thomson DM (2006) Detecting the effects of introduced species: a case study of competition between Apis and Bombus. Oikos 114:407–418
Thurber DK, McClain WR, Whitmore RC (1994) Indirect effects of gypsy moth defoliation on nest predation. J Wildl Manage 58:493–500
Tingley MW, Orwig DA, Field R, Motzkin G (2002) Avian response to removal of a forest dominant: consequences of hemlock woolly adelgid infestations. J Biogeogr 29:1505–1516
Tompkins DM, Gleeson DM (2006) Relationship between avian malaria distribution and an exotic invasive mosquito in New Zealand. J R Soc NZ 36:51–62
Tothill JD, Taylor THC, Paine RW (1930) The coconut moth in Fiji—a history of its control by means of its parasites. Imperial Bureaux of Entomology, London
Tremblay E (1984) The parasitoid complex (Hym.: Ichneumonoidea) of Toxoptera aurantii (Hom.: Aphidoidea) in the Mediterranean area. Entomophaga 29:2003–2010
Tsutsui ND, Case TJ (2001) Population genetics and colony structure of the argentine ant (Linepithema humile) in its native and introduced range. Evolution 55:976–985
van Lenteren JC, Bale JS, Bigler F, Hokkanen HMT, Loomans AJM (2006) Assessing risks of releasing exotic biological control agents of arthropod pests. Annu Rev Entomol 51:609–634
Van Riper CIII (1991) The impact of introduced vectors and avian malaria on insular passeriform bird populations in Hawaii. Bull Soc Vector Ecol 16:59–83
Viggiani G (1994) Recent cases of interspecific competition between parasitoids of the family Aphelinidae (Hymenoptera: Chalcidoidea). Norw J Agric Sci Suppl 16:353–359
Vilá M, Bacher S, Hulme P, Kenis M, Kobelt M, Nentwig W, Sol D, Solarz W (2006) Impactos ecológicos de las invasions de plantas y vertebrados terrestres en Europa. Ecosistemas 2:1–12
Vilà M, Weber E, D’Antonio C (2000) Conservation implications of invasion by plant hybridization. Biol Invasions 2:207–217
Vinson SB (1997) Invasion of the red imported fire ant (Hymenoptera: Formicidae): spread, biology, and impact. Am Entomol 43:23–39
Wakeham-Dawson A, Franquinho Aguiar AM, Martin G (2002) The distribution of endemic butterflies (Lepidoptera) on the island of Madeira, Portugal since 1850, with comments on their current conservation status. Entomol Gaz 53:153–180
Walker KL (2006) Impact of the little fire ant, Wasmannia auropunctata, on native forest ants in Gabon. Biotropica 38:666–673
Ware RL, Majerus MEN (2008) Intraguild predation of immature stages of British and Japanese coccinellids by the invasive ladybird Harmonia axyridis. Biocontrol 53:169–188
Weckel M, Tirpak JM, Nagy C, Christie R (2006) Structural and compositional change in an old-growth eastern hemlock Tsuga canadensis forest, 1965–2004. For Ecol Manage 231:114–118
Wells JD, Greenberg B (1992) Interaction between Chrysomya rufifacies and Cochliomyia macellaria (Diptera: calliphoridae): the possible consequences of an invasion. Bull Entomol Res 82:133–137
Wells JD, Kurahashi H (1997) Chrysomya megacephala (Fabr.) is more resistant to attack by Ch. rufifacies (Macquart) in a laboratory arena than is Cochliomyia macellaria (Fabr.) (Diptera: Calliphoridae). Pan-Pac Entomol 73:16–20
Weston PA, Desurmont G, Hoebeke ER (2007) Viburnum leaf beetle (Coleoptera: Chrysomelidae): biology, invasion history in North America, and management options. Am Entomol 53:96–101
Wetterer JK, O’Hara BC (2002) Ants (Hymenoptera: Formicidae) of the dry Tortugas, the outermost Florida keys. Fla Entomol 85:303–307
Wetterer JK, Espadaler X, Wetterer AL, Aquin-Pombo D, Franquinho-Aguilar AM (2006) Long-term impact of exotic ants on the native ants of Madeira. Ecol Entomol 31:358–368
Williamson M (1996) Biological invasions. Chapman and Hall, London
Witt ABR, Geertsema H, Giliomee JH (2004) The impact of an invasive ant, Linepithema humile (Mayr), on the dispersal of the elaiosome-bearing seeds of six plant species. Afr Entomol 12:223–230
Wittenberg R, Cock MJW (2001) Invasive alien species: a toolkit of best prevention and management practices. CAB International, Wallingford
Woodworth BL, Atkinson CT, LaPointe DA et al (2005) Host population persistence in the face of introduced vector-borne diseases: Hawaii amakihi and avian malaria. Proc Natl Acad Sci USA 102:1532–1536
Yara K (2006) Identification of Torymus sinensis and T. beneficus (Hymenoptera: Torymidae), introduced and indigenous parasitoids of the chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae), using the ribosomal ITS2 region. Biol Control 36:15–21
Yara K, Sasawaki T, Kunimi Y (2007) Displacement of Torymus beneficus (Hymenoptera: Torymidae) by T. sinensis, an indigenous and introduced parasitoid of the chestnut gall wasp, Dryocosmus kuriphilus (Hymenoptera: Cynipidae), in Japanese chestnut fields: possible involvement in hybridization. Biol Control 42:148–154
Yasuda H, Evans EW, Kajita Y, Urakawa K, Takizawa T (2004) Asymmetric larval interactions between introduced and indigenous ladybirds in North America. Oecologia 141:722–731
Yorks TE, Leopold DJ, Raynal DJ (2003) Effects of Tsuga canadensis mortality on soil water chemistry and understory vegetation: possible consequences of an invasive insect herbivore. Can J For Res 33:1525–1537
Acknowledgments
We thank Ecki Brockerhoff, Wolfgang Rabitsch and an anonymous reviewer for their useful comments on the manuscript, and Jon Sweeney and Dave Langor for having invited us to participate in this special issue. We also thank all the colleagues who provided assistance in literature search and acknowledge support from the European Commission Framework Programme 6 via the Integrated Project ALARM (Assessing LArge scale environmental Risks for biodiversity with tested Methods; GOCE-CT-2003-506675; www.alarmproject.net; Settele et al. (2005)).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Kenis, M., Auger-Rozenberg, MA., Roques, A. et al. Ecological effects of invasive alien insects. Biol Invasions 11, 21–45 (2009). https://doi.org/10.1007/s10530-008-9318-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-008-9318-y