Abstract
Parasite communities were examined in johnny darters (Etheostoma nigrum) collected from five localities in the St. Lawrence River in southwestern Quebec: two reference localities, one polluted locality upstream of the Island of Montreal and downstream of industrial and agricultural activity, and two polluted localities downstream of the Island of Montreal in the plume from the wastewater treatment facility. Twenty-four helminth species were found. Fish from the upstream polluted locality had the highest parasite species richness and total parasite numbers, and fish from the downstream polluted localities the lowest. Nonmetric multivariate analyses were conducted using square-root-transformed Bray–Curtis dissimilarity index. An analysis of similarity, dendrogram of centroids, and a permutational multivariate analysis of variance with contrasts all showed that fish from the reference localities had different parasite community composition than those from the polluted localities, and fish from the upstream polluted locality had different parasite communities than fish from the downstream polluted localities. Differences between reference and polluted localities were mainly due to higher abundances of the brain-encysting trematode, Ornithodiplostomum sp., at the reference localities. Differences between upstream and downstream polluted localities were mainly due to a higher diversity and abundance of trematodes in fish at the upstream locality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic systems are affected by a variety of anthropogenic activities that decrease water quality through the introduction of organic and inorganic pollutants. One general trend reported from polluted aquatic environments is a decrease in diversity of macroparasite communities in fish (Cone et al. 1993; MacKenzie et al. 1995; Kennedy 1997; Lafferty 1997; Overstreet 1997; MacKenzie 1999; Sures 2004; Marcogliese 2005; Blanar et al. 2009). This typically occurs through the loss of parasites with short-lived free-living stages that are assumed to be sensitive to pollutants (Pietrock and Marcogliese 2003) or through the loss of parasites with indirect life cycles that use intermediate hosts that are sensitive to pollution (MacKenzie et al. 1995; Marcogliese 2005; Pietrock et al. 2008). Digenetic trematodes in particular may be lost from communities under contaminant stress because of reductions in survival, longevity, or infectivity of their free-living stages or reductions in survival of their molluscan intermediate hosts (Marcogliese and Cone 1997; Marcogliese 2005). Diversity also may be lower in polluted environments due to higher rates of toxin-induced mortality of infected hosts (Lafferty 1997; Barber et al. 2000; Sures 2004, 2008). In contrast, fish hosts in stressed environments often have a proliferation of directly transmitted ectoparasites, such as ciliates and monogeneans, due to immunosupressive effects of toxicants on hosts (Khan and Thulin 1991; Poulin 1992; Morley et al. 2006).
Despite these commonly occurring patterns of decreased diversity and/or increased infections with particular parasites, the role of pollutants in creating these patterns is complex and not always predictable. Particular effects of pollutants on parasite communities may vary by type of pollution (MacKenzie et al. 1995; Lafferty 1997; Marcogliese 2005; Blanar et al. 2009). In natural environments, pollutants typically occur as combinations of chemicals. These complex mixtures can produce unpredictable or nonlinear affects on aquatic life (Pietrock and Marcogliese 2003; Dautremepuits et al. 2009). Their toxicity may be further affected by other abiotic factors such as pH and temperature (Poulin 1992; Lafferty 1997; Pietrock and Marcogliese 2003; Marcogliese 2005).
The St. Lawrence River in southwestern Quebec receives anthropogenic inputs from a number of sources, including municipal sewage, industrial activity, and agriculture. Various locations are exposed to differing amounts of these contaminants. Within this system, effects of pollution have been examined on immune response, stress response, and parasitism in spottail shiners (Notropis hudsonius) and yellow perch (Perca flavescens) (Marcogliese and Cone 2001; Marcogliese et al. 2005, 2006; Thilakaratne et al. 2007; Dautremepuits et al. 2009); however, no effects of pollution have been measured for a benthic fish in this river. Effects of pollutants on benthic fish may be of particular interest because of the close proximity of these fish to the sediments; similar studies have been conducted on flatfish in marine environments (e.g. Schmidt et al. 2003; Khan and Billiard 2007).
Johnny darters (Percidae: Etheostoma nigrum) are small, benthic fish commonly found in both clean and polluted areas of the St. Lawrence River and are host to 54 species of macroparasites in North America (Margolis and Arthur 1979; McDonald and Margolis 1995; Hoffman 1999). Unlike some darter species that undergo spawning migrations, johnny darters tend to remain in the same area over the entire year (Ingersoll et al. 1984). This characteristic makes them appropriate subjects on which to study the chronic effects of local long-term exposure to sublethal concentrations of pollutants on benthic fish (Marcogliese 2005). We examined parasite communities of johnny darters from reference and contaminated localities in the St. Lawrence River to determine if pollution mixtures from different sources had a similar or different impact on diversity and species composition.
Materials and methods
Study localities
Fish were collected from three polluted and two reference localities in the St. Lawrence River in southwestern Quebec in June 2008. The pollution status of all five localities has been previously characterized based on sediment measurements of metals and polychlorinated biphenyls (PCBs) (Loiselle et al. 1997; Marcogliese et al. 2005, 2006; Dautremepuits et al. 2009). Sediment measurements are appropriate because they are relatively stable over time (Dautremepuits et al. 2009). They are also of particular biological importance for our study system because johnny darters are benthic fish and therefore are in close contact with them.
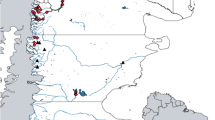
Two of the polluted localities, Îlet Vert (IVT; 45°42.230′ N; 73°27.143′ W) and Île Beauregard (IBE; 45°44.965′ N; 73°24.910′ W), are 4 and 10 km downstream of the City of Montreal sewage treatment plant outfall, respectively (Fig. 1). They both have high concentrations of fecal coliforms, which is typical of areas receiving sewage input (Marcogliese and Cone 2001; Marcogliese et al. 2006). Concentrations of contaminants such as metals, PCBs, organochlorines, and xenoestrogens are elevated downstream of the outfall (Pham et al. 1999; Sabik et al. 2003; Marcogliese et al. 2006; Magella Pelletier, Environment Canada, pers. comm.). Levels of chromium at IVT and levels of PCBs at IBE surpass the Canadian Environmental Quality Guidelines Probable Effects Level (PEL) for aquatic life (http://ceqg-rcqe.ccme.ca/) (Marcogliese et al. 2006; Magella Pelletier, Environment Canada, personal communication).
The third polluted locality, Beauharnois (BEA; 45°19.051′ N; 73°52.020′ W) is upstream of the sewage treatment plant, at the mouth of the St. Louis River. The water quality at this locality is affected by industrial and agricultural activity upstream and constitutes a different type of contaminant mixture than that downstream of the Montreal sewage treatment outfall. BEA has high concentrations of PCBs, organochlorines, and heavy metals including copper, arsenic, lead, chromium, nickel, and zinc. Mercury levels are particularly high and surpass the PEL (Marcogliese et al. 2005; Dautremepuits et al. 2009).
The two reference localities, Îles de la Paix (IPA; 45°20.022′ N; 73°51.362′ W) and Île Dorval (IDO; 45°26.016′ N; 73°44.234′ W), are located in Lake St. Louis upstream of the sewage outfall. No contaminants surpassing the PEL have been measured at either locality (Marcogliese et al. 2006).
IVT, IBE, and IPA are located in the water mass draining the Great Lakes, whereas IDO receives mainly Great Lakes water with some input from the Ottawa River (Marcogliese et al. 2006). BEA is situated in St. Louis River water, which has high conductivity (Thilakaratne et al. 2007). All five sampling localities have sufficiently high conductivity (and thus dissolved calcium concentrations) to support populations of invertebrate intermediate hosts such as gastropods (Marcogliese et al. 2006).
Study organisms
Fish were collected using a beach seine (22.6 × 1.15 m; 3-mm mesh). They were transported to the laboratory, where they were kept in 60-L gravel-lined tanks of dechlorinated tap water. Fish from each locality were kept in separate tanks at room temperature and fed with Nutrafin™ fish flakes ad libitum. They were maintained in the laboratory for 6 to 14 days for an accompanying behaviour study. After this study, fish were killed by an overdose of clove oil (50 mg/L) solution and individually packaged and frozen at -20°C for later necropsy. All fish used were presumed to be from the same age class, 1+, as determined by the length frequency distributions seen at each locality (Bagenal and Tesch 1978).
Examination for parasites
Frozen mass (mg) and standard length (mm) were measured for each fish prior to necropsy. Tissues and organs, including skin, fins, gills, eyes, brain, body cavity, liver, gastrointestinal tract, heart, spleen, gonads, and muscle, of each fish were examined for macroparasites, following protocols from Marcogliese (2002). During necropsy, all parasites were identified to genus (except nongyrodactylid monogeneans, acanthocephalans, and a few rare trematodes, which could only be identified to a higher taxonomic level) and counted. A subset of parasites representing all taxa found at each locality was preserved in 70% ethanol for species identification. Trematodes, cestodes, acanthocephalans, and some monogeneans were stained with acetocarmine and cleared with xylene before being mounted in either Permount or Canada balsam. Nematodes, copepods, and some monogeneans were cleared in glycerine alcohol and examined in temporary mounts. Parasite identifications were based on keys and descriptions in Beverley-Burton (1984), Kabata (1988), Caira (1989), Moravec (1994), Gibson (1996), Scholz (1997) and Hoffman (1999). Voucher specimens of adult parasites (Allocreadium boleosoma, Crepidostomum isostomum, Phyllodistomum etheostomae, Gyrodactylus etheostomae, Aethycteron nigrei, Bothriocephalus cuspidatus, and Camallanus lacustris) have been deposited in the Canadian Museum of Nature (Acquisition numbers are available from the senior author).
Data analysis
Standard length was compared among localities and between polluted and reference localities. One-way analyses of variance (ANOVAs) were used for comparisons among localities and between polluted and reference localities. Because there were significant differences in standard length between some localities and types of localities, it was initially included as a covariate in analyses using permutational multivariate ANOVAs (PERMANOVA), as described below.
Differences in total parasite numbers and species richness among localities were examined with an ANOVA using ranked data (Scheirer et al. 1976) followed by a Tukey–Kramer HSD test. Differences in standard length, total parasite numbers, and species richness were tested using JMP® 7.0.1 (© 2007 SAS Institute Inc.).
Nonparametric statistics based on the Bray–Curtis dissimilarity index were used to further characterize parasite communities with PRIMER and PERMANOVA + add-on (© 2009 Plymouth Routines In Multivariate Ecological Research, Plymouth, UK), following procedures outlined in Clarke and Gorley (2001), Clarke and Warwick (2001) and Anderson et al. (2008). The Bray–Curtis dissimilarity index is commonly used in describing community data because it takes into account both species identity and abundances in a single measure of dissimilarity between two samples, independent of all other samples. The Bray–Curtis index emphasizes abundant species, so parasite abundance data were square-root-transformed to decrease the weight of common species relative to rare species in the index. A nonmetric multidimensional scaling (MDS) plot was used to analyze ranked within-group and between-group Bray–Curtis dissimilarities. MDS poorly represents fish without infections, so one fish from IPA infected only with gyrodactylids was excluded from the plot. Relative similarities between localities based on average community composition, as calculated by centroids, were qualitatively assessed using a dendrogram. The magnitude of relative differences between communities at different localities was tested using a one-way analysis of similarity (ANOSIM) on ranked square root-transformed Bray–Curtis dissimilarities. ANOSIM is a nonparametric test analogous to a one-way ANOVA. The resulting test statistic “R,” a measure of the between-group to within-group dissimilarity, varies from 0 to 1, where R = 0 is the null hypothesis, and R = 1 when within-group dissimilarities of all samples are smaller than between-group dissimilarities. A Similarity Percentages (SIMPER) analysis was performed to identify the parasite species driving the differences between communities at different localities.
The dendrogram and pairwise ANOSIM suggested that the grouping of parasite communities of different localities was correlated with the pollution status of localities, so a PERMANOVA was used to test for community differences among localities and between types of localities. PERMANOVA is a nonparametric multivariate test analogous to a multivariate ANOVA. The “Pseudo-F,” the test statistic calculated in PERMANOVA, is analogous to the “F” statistic of parametric tests and is a measure of between-group to within-group variability. Because there are low numbers of replicate localities, differences between localities of different pollution status were tested for using contrasts. Two contrasts were tested: polluted versus reference localities (BEA, IBE, and IVT vs. IDO and IPA), and upstream versus downstream polluted localities (BEA vs. IBE and IVT), reflecting different sources of pollution.
All parasite community ecology terminology follows definitions of Bush et al. (1997). Prevalence is the percentage of hosts from a sample infected with a particular parasite. Mean abundance is the average number of parasites over a sample. Mean intensity is the average number of parasites in infected individuals in a sample. Infracommunity refers to all parasites within a single fish.
Results
All of the darters were infected with at least one species of helminth. Twenty-four species were found, including 15 digeneans (4 adults, 11 metacercariae), 2 monogeneans, 2 cestodes (1 adult, 1 plerocercoid), 3 nematodes (1 adult, 2 juveniles), 1 larval acanthocephalan, and 1 juvenile copepod. The prevalence and mean intensity of each species at each locality are presented in Table 1. Gyrodactylids were present at all localities and were included in the preliminary analysis. However, because they have very short generation times and proliferate rapidly under laboratory conditions (Scott and Anderson 1984), prevalence and intensities after the 6- to 14-day acclimation period were likely inflated. They were therefore excluded from the final analysis. Analysis with and without the gyrodactylids showed no major differences.
Standard length of fish from BEA (51.03 mm ± 6.92 SD) was significantly greater than that from IDO (46.88 ± 5.28 mm) (F 4,173 = 3.62, p = 0.007), but there was no significant difference in the standard lengths of fish from other localities (Fig. 2). When fish were pooled by treatment, those from the polluted localities (49.67 mm ± 5.46 SD) were significantly longer than those from the reference localities (47.48 mm ± 5.16 SD) (F 1,176 = 7.18, p = 0.0081).
Mean standard length (mm) ± standard error of johnny darters (E. nigrum) from five localities in June 2008 in the St. Lawrence River, Quebec, Canada: one upstream polluted locality (light grey)—BEA; two downstream polluted localities (dark grey)—IBE, IVT; and two reference localities (white)—IDO, IPA. Different letters indicate significant differences between localities
Total parasite numbers differed between localities; fish from BEA and IDO had significantly greater numbers of parasites than fish from IVT and IBE (F 4,173 = 31.73, p < 0.0001) (Fig. 3). Species richness also differed significantly between localities (F 4,173 = 38.48, p < 0.0001). It was greater in fish at BEA than at IPA and IDO, which in turn was greater than at IVT and IBE (Fig. 4). Differences in total parasite numbers and species richness were not tested between treatments because the greatest differences in both these measures were between the upstream and downstream polluted localities. Analysis of Bray–Curtis dissimilarities of infections in fish from all five localities revealed that those from the upstream polluted locality (BEA), those from the two reference localities (IDO, IPA), and those from the downstream polluted localities (IVT, IBE) formed three distinct clusters in the MDS plot (Fig. 5) and the dendrogram (Fig. 6).
Mean total parasite numbers ± standard error in johnny darters (E. nigrum) from five localities in June 2008 in the St. Lawrence River, Quebec, Canada: one upstream polluted locality (light grey)—BEA; two downstream polluted localities (dark grey)—IBE, IVT; and two reference localities (white)—IDO, IPA. Different letters indicate significant differences between localities
Mean species richness ± standard error in johnny darters (E. nigrum) from five localities in June 2008 in the St. Lawrence River, Quebec, Canada: one upstream polluted locality (light grey)—BEA; two downstream polluted localities (dark grey)—IBE, IVT; and two reference localities (white)—IDO, IPA. Different letters indicate significant differences between localities
Two-dimensional nonmetric MDS plot of ranked square root transformed Bray–Curtis dissimilarities between parasite communities in johnny darters (E. nigrum) from five localities in June 2008 in the St. Lawrence River, Quebec, Canada: one upstream polluted locality (light grey)—BEA; two downstream polluted localities (dark grey)—IBE, IVT; and two reference localities (white)—IDO, IPA. The stress level of the plot is 0.27
The difference between communities at the polluted and reference localities was primarily due to higher abundances of Ornithodiplostomum sp. at the reference localities (Table 2). The difference between the upstream and downstream polluted localities was mainly due to higher abundances of Neochasmus sp., Ichthyocotylurus sp., and Cryptogonimus sp. at the upstream polluted locality.
The global ANOSIM (Global R = 0.51, p = 0.001) and the pairwise ANOSIMs (Table 3) were significant. Based on the pairwise comparisons, BEA appeared to be the most distinct of the five localities. Distinctions between reference and downstream polluted localities were greater than within these localities. A PERMANOVA performed on the Bray–Curtis dissimilarities confirmed ANOSIM results of differences between localities, pollution status, and the upstream/downstream polluted localities (Table 4).
Discussion
This is the first study to examine parasite communities of johnny darters in the St. Lawrence River. Most parasite species encountered were larval stages (≥16), most of which could at best only be identified to the generic level. Most of these were trematodes, consistent with results of other studies on fish parasite communities in the St. Lawrence River (Marcogliese et al. 2006; Thilakaratne et al. 2007). Assuming each larval type identified represents a single species, two of the larval trematodes recovered, Ichthyocotylurus sp. and Tylodelphys sp. are new records for johnny darters, as is the intestinal nematode, C. lacustris, and the cestode T. nodulus. A further two trematodes, P. etheostomae and the metacercariae of Cryptogonimus sp., are new reports for this host in Canada (Margolis and Arthur 1979; McDonald and Margolis 1995; Gibson 1996; Hoffman 1999). Recent studies have shown that the larval trematode communities of fish in the St. Lawrence River are much more diverse than previously recognized (Moszczynska et al. 2009; Locke et al. 2010) and two of the “species” identified in this study (Diplostomum spp. and Apatemon spp.) are known to occur as species complexes in fish. Thus, the estimate of number of larval trematode species is likely conservative.
Parasite communities at polluted and reference localities showed some of the trends consistent with predicted parasite diversity in stressed environments. The two downstream polluted localities, IVT and IBE, had significantly different parasite communities from the reference localities, in both abundance and composition. Fish from the former two localities had the lowest mean total parasite numbers and mean species richness. They also had significantly lower abundances of metacercariae of the trematode Ornithodiplostomum sp. A large percentage of the differences between these localities and the reference localities was due to higher abundances of Ornithodiplstomum sp. at the reference localities. This is consistent with studies showing decreased longevity and infectivity of trematode cercariae (Morley et al. 2003; Pietrock and Marcogliese 2003) and in particular decreased infectivity of cercariae of Ornithodiplostomum following exposure to metal pollution (Pietrock and Goater 2005). Alternatively, it may reflect lower densities of the obligatory gastropod intermediate host at IVT and IBE (Marcogliese et al. 2006). However, Ornithodiplostomum ptychocheilus is common in spottail shiners at IBE and BEA, and its abundance does not appear to reflect snail densities (Marcogliese et al. 2006; Thilakaratne et al. 2007). Otherwise, parasite communities of the pelagic spottail shiner from these localities show a similar general pattern. Marcogliese et al. (2006) found that fish downstream of the sewage outfall had lower total parasite abundances than fish from reference localities in Lake St. Louis. Thilakaratne et al. (2007) reported a lower diversity of helminths at IVT than at the reference localities in Lake St. Louis. These patterns of lower parasite abundance and diversity in polluted environments have also been reported in field studies from other systems (reviewed by Marcogliese 2005). For example, parasite communities of grey mullet (Liza aurata and Liza ramada) in a polluted estuary were missing helminth parasites with indirect life cycles that were present in a nearby reference estuary (Dzikowski et al. 2003). Exposure to urban effluent was reflected in lower species richness of macroparasite communities of flounder (Platichthys flesus) in the German Bight in the North Sea (Schmidt et al. 2003). Winter flounder (Pleuronectes americanus) in the vicinity of a pulp and paper mill had significantly lower abundances of intestinal helminths than those at a nearby reference locality (Khan and Billiard 2007). A fish community in a river in southern Slovakia contaminated with cyanide and heavy metals had half the diversity of gastrointestinal helminth parasites as a similar community in a nearby reference river due to a loss of species that use sensitive invertebrate species as obligatory intermediate hosts (Oros and Hanzelová 2009).
However, the relationship between pollution and parasite community diversity is seldom simple to interpret and may be confounded by the type of pollution or by other abiotic or ecological factors (Poulin 1992; MacKenzie et al. 1995; Kennedy 1997; Lafferty 1997; Marcogliese 2005). The upstream polluted locality, BEA, differs from the pattern of parasite community impoverishment seen in the two downstream polluted localities. Composition of parasite communities at BEA differed from both of the two downstream polluted localities and the two reference localities. BEA had the highest species richness and among the highest overall parasite abundances of both polluted and reference localities. In particular, differences between BEA and the other localities were due to higher abundances of three trematodes, Neochasmus sp., Ichthyocotylurus sp., and Cryptogonimus sp. at BEA. Similar to the other polluted localities, BEA had lower abundances of Ornithodiplostomum sp. than the reference localities. The different parasite community composition between the upstream and downstream polluted localities may reflect differences in the types and sources of pollution. IVT and IBE are impacted primarily by effluent from a major sewage treatment facility that empties into the St. Lawrence River directly upstream of them. In contrast, BEA is downstream of industrial and agricultural activity along the St. Louis River. Differences in pollution include chemicals and metals, but also nutrient inputs. Both upstream and downstream polluted localities experience eutrophication; however, the types of nutrient input differ due to their different sources. Agricultural runoff typically includes chemical fertilizers, whereas sewage is composed of organic material and bacteria (Khan and Ansari 2005). There are also differences in the free-living biotic communities between the upstream and downstream polluted localities, which may reflect the type of eutrophication experienced. Diets of organisms downstream of the municipal effluents are more detritus-based than those upstream, possibly reflecting simpler food webs resulting in relatively low parasite infracommunity diversity (deBruyn et al. 2003; Marcogliese et al. 2006). Conversely, BEA has high productivity and fish diversity (Marcogliese, personal observation), both of which may lead to an increase in parasite abundance and diversity. Thus, our data suggest that the type of contaminant mixture may impact parasite communities of fish differently, resulting in measures of diversity and abundance that do not always follow previously widely accepted patterns (see MacKenzie et al. 1995; Lafferty 1997; Marcogliese 2005; Blanar et al. 2009).
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: guide to software and statistical methods. PRIMER-E Ltd, Aukland
Bagenal TB, Tesch FW (1978) Age and growth. In: Bagenal T (ed) Methods for assessment of fish production in fresh waters. Blackwell Scientific Publications, Oxford
Barber I, Hoare D, Krause J (2000) Effects of parasites on fish behaviour: a review and evolutionary perspective. Rev Fish Biol Fish 10:131–165
Beverley-Burton M (1984) Monogenea and Turbellaria. In: Margolis L, Kabata Z (eds) Guide to the parasites of fishes of Canada, Part I. Can Spec Publ Fish Aquat Sci. No 74. pp 1–209
Blanar CA, Munkittrick KR, Houlahan J, MacLatchy DL, Marcogliese DJ (2009) Pollution and parasitism in aquatic animals: a meta-analysis of effect size. Aquat Toxicol 93:18–28
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Caira JN (1989) A revision of the North American papillose Allocreadiidae (Digena) with independent cladistic analyses of larval and adult forms. Bull Univ Nebr State Mus 11:58
Clarke KR, Gorley RN (2001) PRIMER v6: user manual/tutorial. PRIMER-E Ltd, Plymouth
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E Ltd, Plymouth
Cone DK, Marcogliese DJ, Watt WD (1993) Metazoan parasite communities of yellow eels (Anguilla rostrata) in acidic and limed rivers of Nova Scotia. Can J Zool 71:177–184
Dautremepuits C, Marcogliese DJ, Gendron AD, Fournier M (2009) Gill and head kidney antioxidant processes and innate immune system responses of yellow perch (Perca flavescens) exposed to different contaminants in the St. Lawrence River, Canada. Sci Total Environ 407:1055–1064
deBruyn AMH, Marcogliese DJ, Rasmussen JB (2003) The role of sewage in a large river food web. Can J Fish Aquat Sci 60:1332–1344
Dzikowski R, Diamant A, Paperna I (2003) Trematode metacercariae of fishes as sentinels for a changing limnological environment. Dis Aquat Org 55:145–150
Gibson DI (1996) Trematoda. In: Margolis L, Kabata Z (eds) Guide to the parasites of fishes of Canada, Part IV. Can Spec Publ Fish Aquat Sci. No 124. pp 1–373
Hoffman GL (1999) Parasites of North American freshwater fishes. University of California Press, Berkley
Ingersoll CG, Hlohowskyj I, Mundahl ND (1984) Movements and densities of the darters Etheostoma flabellare, Etheostoma spectabile and Etheostoma nigrum during spring spawning. J Fresh Ecol 2:345–352
Kabata Z (1988) Copepoda and branchiura. In: Margolis L, Kabata Z (eds) Guide to the parasites of fishes of Canada, Part II—Crustacea. Can Spec Publ Fish Aquat Sci. No 101. pp 3–127
Kennedy CR (1997) Freshwater fish parasites and environmental quality: an overview and caution. Parassitologia 39:249–254
Khan FA, Ansari AA (2005) Eutrophication: an ecological vision. Bot Rev 71:449–482
Khan RA, Billiard SM (2007) Parasites of winter flounder (Pleuronectes americanus) as an additional bioindicator of stress-related exposure to untreated pulp and paper mill effluent: a 5-year field study. Arch Environ Contam Toxicol 52:243–250
Khan RA, Thulin J (1991) Influence of pollution on parasites of aquatic animals. Adv Parasitol 30:201–238
Lafferty KD (1997) Environmental parasitology: what can parasites tell us about human impacts on the environment? Parasitol Today 13:251–255
Locke SA, McLaughlin JD, Dayanandan S, Marcogliese DJ (2010) Diversity and specificity in Diplostomum spp. metacercariae in freshwater fishes revealed by cytochrome c oxidase I and internal transcribed spacer sequences. Int J Parasitol 40:333–343. doi:10.1016/j.ijpara.2009.08.012
Loiselle C, Fortin GR, Lorraine S, Pelletier M (1997) Dynamics and contamination of the St. Lawrence River sediment. St. Lawrence Centre, Environment Canada, Montreal
MacKenzie K (1999) Parasites as pollution indicators in marine ecosystems: a proposed early warning system. Marine Poll Bull 38:955–959
MacKenzie K, Williams HH, Williams B, Mcvicar AH, Siddall R (1995) Parasites as indicators of water-quality and the potential use of helminth transmission in marine pollution studies. Adv Parasitol 35:85–144
Marcogliese DJ (2002) Protocols for measuring biodiversity: parasites of fishes in fresh water. Ecological Monitoring and Assessment Network, Environment Canada. http://www.eman-rese.ca/eman/ecotools/protocols/freshwater/parasites
Marcogliese DJ (2005) Parasites of the superorganism: are they indicators of ecosystem health? Int J Parasitol 35:705–716
Marcogliese DJ, Cone DK (1997) Parasite communities as indicators of ecosystem stress. Parassitologia 39:227–232
Marcogliese DJ, Cone DK (2001) Myxozoan communities parasitizing Notropis hudsonius (Cyprinidae) at selected localities on the St. Lawrence River, Quebec: possible effects of urban effluents. J Parasitol 87:951–956
Marcogliese DJ, Brambilla LG, Gagne F, Gendron AD (2005) Joint effects of parasitism and pollution on oxidative stress biomarkers in yellow perch Perca flavescens. Dis Aquat Org 63:77–84
Marcogliese DJ, Gendron AD, Plante C, Fournier M, Cyr D (2006) Parasites of spottail shiners (Notropis hudsonius) in the St. Lawrence River: effects of municipal effluents and habitat. Can J Zool 84:1461–1481
Margolis L, Arthur JR (1979) Synopsis of the parasites of fishes of Canada. Bull Fish Res Board Can 179:1–269
McDonald TE, Margolis L (1995) Synopsis of the parasites of fishes of Canada: supplement (1978–1993). Can Spec Publ Fish Aquat Sci 122:1–265
Moravec F (1994) Parasitic nematodes of freshwater fishes of Europe. Kluwer Academic Publishers, Dordrecht
Morley NJ, Irwin SWB, Lewis JW (2003) Pollution toxicity to the transmission of larval digeneans through their molluscan hosts. Parasitology 126:S5–S26
Morley NJ, Lewis JW, Hoole D (2006) Pollutant-induced effects on immunological and physiological interactions in aquatic host–trematode systems: implications for parasite transmission. J Helminthol 80:137–149
Moszczynska A, Locke SA, McLaughlin JD, Marcogliese DJ, Crease TJ (2009) Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Mol Ecol Resour 9:S75–S82
Oros M, Hanzelová V (2009) Re-establishment of the fish parasite fauna in the Tisa River system (Slovakia) after a catastrophic pollution event. Parasitol Res 104:1497–1506
Overstreet RM (1997) Parasitological data as monitors of environmental health. Parassitologia 39:169–175
Pham T, Proulx S, Brochu C, Moore S (1999) Composition of PCBs and PAHs in the Montreal Urban Community wastewater and in the surface water of the St. Lawrence River (Canada). Water Air Soil Pollut 111:251–270
Pietrock M, Goater CP (2005) Infectivity of Ornithodiplostomum ptychocheilus and Posthodiplostomum minimum (Trematoda: Diplostomidae) cercariae following exposure to cadmium. J Parasitol 91:854–856
Pietrock M, Marcogliese DJ (2003) Free-living endohelminth stages: at the mercy of environmental conditions. Trends Parasitol 19:293–299
Pietrock M, Meinelt T, Marcogliese DJ (2008) Effects of cadmium exposure on embryogenesis of Stagnicola elodes (Mollusca, Gastropoda): potential consequences for parasite transmission. Arch Environ Contam Toxicol 55:43–48
Poulin R (1992) Toxic pollution and parasitism in freshwater fish. Parasitol Today 8:58–61
Sabik H, Gagné F, Blaise C, Marcogliese DJ, Jeannot R (2003) Occurrence of alkylphenol polyethoxylates in the St. Lawrence River and their bioconcentration by mussels (Elliptio complanata). Chemosphere 51:349–356
Scheirer CJ, Ray WS, Hare N (1976) The analysis of ranked data derived from completely randomized factorial designs. Biometrics 32:429–434
Schmidt V, Zander S, Koerting W, Steinhagen D (2003) Parasites of the flounder Platichthys flesus (L.) from the German Bight, North Sea, and their potential use in ecosystem monitoring. Helgol Mar Res 57:236–251
Scholz T (1997) A revision of the species of Bothriocephalus Rudolphi, 1808 (Cestoda: Pseudophyllidea) parasitic in American freshwater fishes. Syst Parasitol 36:85–107
Scott ME, Anderson RM (1984) The population dynamics of Gyrodactylus bullatarudis (Monogenea) within laboratory populations of the fish host Poecilia reticulata. Parasitology 89:159–194
Sures B (2004) Environmental parasitology: relevancy of parasites in monitoring environmental pollution. Trends Parasitol 20:170–177
Sures B (2008) Host-parasite interactions in polluted environments. J Fish Biol 73:2133–2142
Thilakaratne IDSIP, Mclaughlin JD, Marcogliese DJ (2007) Effects of pollution and parasites on biomarkers of fish health in spottail shiners, N. hudsonius (Clinton). J Fish Biol 71:519–538
Acknowledgements
We acknowledge assistance in the field in the collection of specimens by Michel Arseneau, Coralie Beaudry, Germain Brault, Melanie Gelinas, Ariane Laurence, Claude Lessard, and Sophie Trépanier. We thank Lia Clark and Asra Toobaie for help in caring for fish in the laboratory, Sean Locke for help in identifying parasite specimens, and François Boudreault for preparing the map. Thank you also to an anonymous reviewer for providing helpful feedback on the manuscript. Funding was provided in part through a Natural Sciences and Engineering Research Council of Canada Discovery Grant A6979 to J.D. McLaughlin and the St. Lawrence Action Plan (Environment Canada) to D.J. Marcogliese.
Ethical standards
Animal use was in accordance with Canadian law and the Concordia University Certification of Ethical Acceptability for Research or Teaching Involving the Use of Animals in place at the time of the study, certification AREC-2006-MCLA to J.D. McLaughlin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krause, R.J., McLaughlin, J.D. & Marcogliese, D.J. Parasite fauna of Etheostoma nigrum (Percidae: Etheostomatinae) in localities of varying pollution stress in the St. Lawrence River, Quebec, Canada. Parasitol Res 107, 285–294 (2010). https://doi.org/10.1007/s00436-010-1862-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1862-6