Abstract
Salmonids are fish from the Northern Hemisphere which have been introduced and acclimated to many regions in the Southern Hemisphere for commercial (aquaculture) and recreational (sport fishing) purposes. In some cases a species like brown trout Salmo trutta rapidly spread across the host ecosystem and became invasive, threatening local fauna, and even outcompeting other exotic fish. We have analyzed life-history traits in combination with genetic variation of Atlantic salmon and brown trout adapted to the lake systems of the Argentinean Patagonia (South America). We have identified two main characteristics that conferred invasive capacity to those exotic species: undomesticated status and lifelong growth. Stocks originated from wild populations adapted better than long-term domestic lineages, and their geographic origin seems to be less important for adaptation to exotic environments. We propose that considering these characteristics in future planning of commercial aquaculture projects by selecting non-invasive lineages will minimise the impact of accidental escapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are to a great extent a consequence of human activity. Organisms are often inadvertently transported by humans (Carlton and Geller 1993; Vitousek et al. 1997; Smith et al. 1999; Fofonoff et al. 2003; Perez et al. 2007), and new ways for spontaneous dissemination of many species like roads and channels (Ruiz et al. 2006; Gollasch 2006) are constructed every year. In addition, deliberate releases of exotic species have been carried out with purposes of acclimation to new habitats for further exploitation. Salmonids fall within the last category. Native to the Northern Hemisphere, millions of eggs of many Salmonidae species have been transplanted to different regions of the Southern Hemisphere (Davaine and Beall 1982, 1997; Pascual et al. 2002; McDowall 2003; Baigún and Ferriz 2003; Becker et al. 2007; Aigo et al. 2008; Correa and Gross 2008; Inoue et al. 2009). In the new habitats their fate has been quite diverse, depending on various factors that are not yet well understood. In some cases they have displaced local native species and have become a pest, like brown trout in New Zealand (Townsend 2003), where the cost of their adaptation has been the loss of some native Galaxiidae species (McDowall 1990, 2006; Townsend 1996; McIntosh 2000). In other cases they have adapted to coexist with local organisms and can be considered naturalized, like various salmon and trout species in South America (Ciancio et al. 2005; Astorga et al. 2008). Finally, in some cases they have been unable to show successful adaptation –or may have been outcompeted by other exotic salmonids- and are extinct, like in some systems of the Kerguelen Islands (Ayllon et al. 2004a).
One of the key questions for understanding what makes a salmonid species invasive is: what are the traits which confer invasive capacity? Is it genetics, that is, animals from some genetic lineages possess an inherent tendency to explore and colonize other habitats? Or is it associated with one or various life-history traits, like growth (Kinnison et al. 1998; Macchi et al. 2007; Pascual et al. 2007), migratory behaviour (Valiente et al. 2010) and/or others? Although the role of genetic variability per se has not been confirmed in conferring invasiveness in Salmonids (Valiente et al. 2007), the answer is likely a combination of different environmental and genetic factors.
Even when a species exhibits colonizer capacity and can theoretically become a pest, not all their lineages are equally invasive. Domestication may interfere with invasive capacity. An example is brown trout. Domestic stocks are able to adapt to new environments and advantageously compete with wild ones of the same species (Lura and Saegrov 1991; Naylor et al. 2005; Braithwaite and Salvanes 2005), substituting wild populations in some environments, such as in areas of low water flow (e.g. Moran et al. 1991; Ford and Myers 2008). However, in other cases domestic trout failed to colonize new habitats (e.g. Moran et al. 1991; Ayllon et al. 2006). It is crucial to determine what are the main factors involved in invasiveness in order to set up control mechanisms of invasive species.
New stock transfers or common garden experiments have inherent risks because total containment of experimental animals is difficult to ensure, particularly in aquatic environments. However, one possibility is to study the outcome of old transplants, when more than one species and population have been transferred to the same exotic habitat. Such situations could be considered a sort of non-deliberate common garden experiment. In this study we have identified a region where Atlantic salmon Salmo salar and brown trout S. trutta of different origins and domestication status have been released: the Argentine province of Neuquen (Patagonia) in South America. We have analyzed growth and genetic diversity of the extant naturalized populations and compared them with founder stocks (or stocks inhabiting their regions of origin). The results allowed us to identify some key factors that determined adaptation and –by extension- invasiveness in these two species.
Materials and methods
Study sites
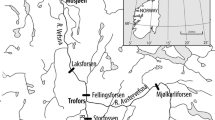
In Patagonia we have studied two river systems where the two salmonid species considered have been introduced: Río Blanco (Curruhué system) and Río Traful, within the province of Neuquén (Argentina) (Fig. 1). The watersheds of the two systems are derived from Andean glacial lakes. There is no evidence that Salmonids established sea migrating stocks in the region (Riva Rossi 2008), and native and introduced species coexist (Table 1).
Salmonids introductions to North Patagonia have been described in detail by Macchi et al. (2008). Atlantic salmon was introduced first in the Neuquén region (Table 2). Landlocked salmon from lakes Sebago and Grand (Maine, USA) were probably the first consignment and arrived successfully in Patagonia in 1904 (Tulian 1908; MacCrimmon and Gots 1979). They were likely wild or recently domesticated, as Atlantic salmon domestication first occurred around those years (Jensen 2002). That first introduction was followed by successive releases of eggs and alevins imported from domestic European stocks (Germany, British Isles). Brown trout was introduced later (Table 2), principally imported from Chile (Macchi et al. 2008) where it was naturalized, and to a minor extent from other European countries (Baigún and Quirós 1985). The origin of brown trout introduced to Chile is not totally well known. Of Atlantic origin as opposed to Mediterranean (Colihueque et al. 2003), they had been imported from different European countries to establish populations in Chilean rivers (Faundez et al. 1997), principally from Germany and some introductions from other European countries. It can be supposed that at least some of them were imported from Spain, given the historical commercial and cultural relationships between this country and South America. The German stocks were likely domestic and those native from South European regions were probably wild or recently domesticated, as little domestication activity of native brown trout exists in this area (Perez-Rubin 2006).
Measures of abundance
For the Patagonian systems there are no published systematic inventories of fish abundance, therefore the proportion of sport catches for each Salmo species (their relative mass in the total number of sport catches) was roughly considered as a proxy of their relative abundance.
Samples analyzed
A summary of the samples analysed and the type of analysis carried out is shown in Table 3. Representatives of Maine Atlantic salmon founders (Sebago Lake and Grand Lake) were analyzed. Detailed genetic data can be found in Valiente et al. (2007). For European founders, because Atlantic salmon is now extinct in Germany, the comparison with present German stocks is not possible. However, there are wild Atlantic salmon populations and domestic stocks in the British Isles; genetic data of hatchery stocks from these islands were taken from Säisä et al. (2005).
Brown trout samples from the Elbe and Rhine rivers were chosen as representative of founder German brown trout. Detailed data are published in Valiente et al. (2010). We have chosen a mixture of wild Spanish brown trout samples (n = 1284) as representative of South European imports. Genetic data from most of these samples have been published in Izquierdo et al. (2006).
Naturalized Atlantic salmon inhabiting the Blanco and Traful rivers systems were analyzed for both genetic variability (n = 100) and morphological traits (n = 308) (Table 3). Naturalized brown trout inhabiting the same systems were also genetically (n = 50) and morphologically (n = 226) analyzed (Table 3). Samples were obtained in 1994 and 1995.
Life-history traits analysis
Fork length and weight were obtained (directly measured) from salmonids caught by electrofishing in field surveys (March and July 1994 and 1995). To determine the cohort or brood year of the analyzed individuals, age determination of scales was performed according to Busacker et al. (1990).
Genetic analysis
The DNA source tissue was scales (donated by anglers). Theses scales (2–5 per specimen) were dried and preserved in paper envelopes for DNA extraction.
DNA was extracted based on Chelex methodology (Estoup et al. 1996). Five non-coding hypervariable microsatellite loci were analyzed. Microsatellite typing at loci SSOSL85, SSOSL311 and SSOSL417 (Slettan et al. 1995), BF002 (Sušnik et al. 1997), and SS4 (Martinez et al. 1999), was based on standard PCR-fragment size determination methodology by capillary electrophoresis in an ABI Prism 3100 DNA Sequencer and the GENSCAN V. 3.7 software (Burge and Karlin 1997) at the DNA Sequencing Unit of the University of Oviedo. Annealing temperatures in PCR cycles were the following: locus SSOSL85 and SSOSL311, 55°C (Slettan et al. 1995); SSOSL417, 52°C (Slettan et al. 1995), BF002, 50°C (Sušnik et al. 1997) and SS4, 58°C (Martinez et al. 1999); Reaction mixtures and cycle conditions followed standard protocols (for example Ayllon et al. 2004b). One protein-coding locus (LDH-C1*) was also analyzed. LDH-C1* genotypes were determined by restriction digestion of the gene DNA sequence (McMeel et al. 2001).
Statistical analyses
Life-history traits
Statistical analyses were performed with the program SSPS 8.0 to assess differences between mean length and weight of age 1 + juvenile salmonids (Atlantic salmon and brown trout). Homocedasticity (equality of variances) was checked employing Levene’s test. Means were compared employing t-tests for independent samples. The curve that best fitted the evolution of each morphological parameter (length, weight) through time was estimated employing the same program, which gives significance to each model curve. Comparison of linear growth slopes (when a linear model was the best-fit) of Atlantic salmon and brown trout inhabiting the same systems was made based on t-tests.
Genetic variation
Variation at the loci considered was quantified for two different purposes. Genetic variability at all loci was assessed in donor and derivative stocks for evaluating its possible role in adaptation. The parameters of genetic variability considered were the number of alleles per locus and heterozygosity observed and expected. They were estimated with the GENETIX (2000) computer package, which was also employed for calculating allele frequencies.
Genetic variation can also serve for determining the origin stock of naturalized Salmonids, when more than one donor type has been stocked in or has colonized a system. There are some stock-specific alleles, for example the locus SSOSL311 is continent-specific for Salmo salar (Säisä et al. 2005) and can be employed for differentiating naturalized salmon of American and European lineages. The microsatellite locus BF002 and the coding locus LDH-C1* are useful markers for differentiating domestic and wild stocks of brown trout in Southern Europe (Izquierdo et al. 2006). Allele variation at those loci was employed for identification of the proportion of each donor stock in naturalized individuals. When assignment was not direct (exclusive alleles), the program GENECLASS was employed for self- assignment of naturalized individuals to each donor stock based on all the loci analysed. Self-assignment tests (Bayesian method) were done using the program GENECLASS 1.0.02 (Cornuet et al. 1999). This is a likelihood-based method in which individuals are assigned to the population in which the likelihood of their genotype is highest. The following settings were used: 10,000 simulated individuals, threshold = 0.01 and Bayesian estimation of frequencies.
Results
Both Atlantic salmon and brown trout acclimated and constituted naturalized populations in the studied systems. Temperature was relatively low due to the altitude of the considered Patagonian systems (above 800 m). Accompanying fauna included many invertebrate and other native fish species.
Interspecific differences were found for morphological traits in juvenile stages (Fig. 2). Extant 1 + Atlantic salmon were shorter and lighter than brown trout in the River Traful (P < 0.001 for both traits), but in the River Blanco the initial growth was the opposite: Atlantic salmon were longer and heavier than brown trout (t = 4.214, 63 df, and 2.543, 118 df with P = 0.000 and P = 0.012 for length and weight respectively, after testing equality of variances with Levene’s test). However, when extending the analysis to the entire lifespan of salmon and trout the results were different. The coefficients of the logarithmic curve that best fitted growth were higher for brown trout than for Atlantic salmon (Fig. 3) indicating higher long-term growth of the former species. Adjusting size data to linear growth, the values b0 (intercepts) of the regression line were significantly higher for brown trout than for Atlantic salmon: 4.3 for brown trout versus 0.8 for Atlantic salmon (t = 6.8571, 7 df, P < 0.005).
Adaptation of Atlantic salmon and brown trout to the new environments was not identical for all the lineages of each species introduced in the Blanco and Traful rivers. Genetic markers (Table 4a) evidenced that in these Patagonian systems the extant naturalized Atlantic salmon was of North American origin, because they were fixed for one SSOSL311 allele exclusive of North America (allele 114) that was not present in European salmon (Table 4; Säisä et al. 2005). It seems that the European domestic salmon introduced in Patagonia failed.
With respect to brown trout, absolute markers of donor stocks were not found (Table 4). For BF002 allele variation, in the Patagonian systems most naturalized individuals exhibited the less frequent allele of domestic German populations (allele 122), which was in turn the most frequent in South European wild populations. Variation at the LDH-C1* locus revealed a frequency of 0.23 for the *100 allele in Patagonian trout (Table 4). LDH-C1*100 is the most frequent allele in South European populations and very infrequent in domestic German ones (less than 0.05), therefore the trout adapted to Patagonia is likely a mixture of donor stocks. The program GENECLASS assigned 16 and 22% of individuals to German and South European stocks respectively in the studied systems when all the loci analyzed were included. The rest of the individuals were not significantly assigned to any putative donor stock. South European alleles would be overrepresented, considering that releases of south European individuals were minor in comparison with the much more abundant domestic German imports (Table 5).
With respect to the levels of genetic variation at microsatellite loci found for these naturalized Salmonids, Atlantic salmon and brown trout exhibited similar values for the three parameters considered (Fig. 4). The differences between species in allelic richness, mean Ho and He were not significant (P > 0.1 for all cases).
Although the measures of species abundance were not precise in Patagonia because they were based on angling catches, increasing abundance of brown trout in parallel with Atlantic salmon declines seems to be clear. In the Patagonian Traful system sport catches were primarily of Atlantic salmon in the 1980s but the dominant species is brown trout only 20 years later (Fig. 5).
Discussion
In the Traful and Blanco river systems, brown trout adapted better than Atlantic salmon (e.g. Macchi et al. 2008; Valiente et al. 2010), as was also demonstrated in other even more remote ecosystems like the virgin Subantarctic French territory of the Kerguelen Islands (Ayllon et al. 2004a, b). Decline of Salmo salar in Northern Patagonia is a well known phenomenon (Macchi et al. 2008; Vigliano and Alonso 2007), and has been attributed to difficulties in adaptation to landlocked situations, dam construction, and generalized habitat change in the Traful system. Brown trout, however, overcome those disadvantageous circumstances and seem to be well adapted to the new ecosystems. The better adaptation of brown trout over Atlantic salmon in the Andean Argentine Patagonia and its higher invasiveness is highly evident from the existing bibliography on distribution and abundance of these two species (Pascual et al. 2002, 2007; Macchi et al. 2008; Aigo et al. 2008). This bibliography covers a geographic range extending from Northern Neuquen Province to Tierra del Fuego. Our results confirm it in the two systems studied.
Other lessons can also be learned from this study. Our results point out that the degree of domestication is important for adaptation and eventual invasiveness in wild habitats. The outcome of introductions of domestic and wild stocks into this exotic system was a final colonization by a majority or exclusiveness of undomesticated individuals belonging to wild populations, even if domestic releases were initially more abundant (Table 5). In the wild, domestic salmon exhibit lower fitness than wild salmon and aquaculture escapes can endanger natural populations compromising their viability (Clifford et al. 1998; McGinnity et al. 1998, 2003, 2009). Domestication encompasses genetic changes in life-history traits, which can be subjected to directional selection in farm conditions (Fleming and Einum 1997; Hindar et al. 2006) and also in behavioral components such as aggressiveness, mate choice and others (Fleming and Gross 1993; Fleming et al. 2000, 2002; Mignon-Grasteau et al. 2005; Orlov et al. 2006; Castillo et al. 2008). As an indicator of such altered behavior, individuals of domestic origin provide most hybrid crosses between Atlantic salmon and brown trout in southern Europe (Castillo et al. 2008).
As the whole history of salmonid introductions in South America over the past 100 years is incomplete, it is possible that the number and origin of real founders deviates somewhat from the published information and the geographic lineages of the donors are more complex than presented here. From the available information, it seems that the geographic origin of the donor stocks was not relevant for adaptation, at least in comparison with the effect of domestication status. Wild (or recently domesticated) southern European stocks adapted to Patagonian systems better than domestic central European trout. However, successful adaptation of brown trout to different exotic regions has occurred irrespective of their geographic origin; for example, although in this study we demonstrated that German brown trout did not perform well in the studied systems within the Argentinean Patagonia, they adapted perfectly to and colonized varied systems in different latitudes, from Chile to North America (Fausch et al. 2001; Pascual et al. 2002; Macchi et al. 2007; Soto et al. 2006). The explanation for their low success in Patagonia was probably the presence of conspecific competitors (other donors) of wild origin.
Life-history traits likely contribute to explain adaptation to new environments much better than genetics (Valiente et al. 2007, 2010). Growth is crucial for adaptation. Early growth and therefore capacity of a fast biomass increase has been cited as key for invasiveness (Townsend 2003, Valiente et al. 2010). Our results are relatively concordant with that hypothesis: trout grew faster than salmon during the first year of life in the River Traful, but in the River Blanco salmon grew faster (Fig. 2). But, Not only growth during the first year is important. Higher growth rate during the entire lifespan (Fig. 3) is likely the decisive factor that determines invasiveness. The habitat requirements of year classes of salmon and trout overlap in all their freshwater life stages (Heggenes et al. 1999; Armstrong et al. 2003), including spawning. Exhibiting a bigger size is advantageous when competing for space, food and spawning sites, and brown trout tend to out-compete Atlantic salmon in most situations (e.g. Armstrong et al. 2003).
These results may have important implications for management. Aquaculture is an activity in expansion worldwide (Reilly and Kaeferstein 1999; Naylor et al. 2000; Vergara 2003; James 2009), but encompasses risks like potential invasions from accidental escapes (e.g. Linde et al. 2008; Krishnakumar et al. 2009). Total containment of cultured fish is not possible or is very difficult and total sterilization of all farm fish may not be feasible at this point (Piferrer et al. 2009). In this situation, some recommendations for brown trout and Atlantic salmon producers and managers can be suggested. The first and most logical is to avoid farming of these species in exotic regions, as it encompasses a risk of escapes. But in some cases economic reasons are prevalent and these fish species, of high commercial value, are cultivated for enhancing the economy of regions in development. In such cases, selection of long-term domesticated individuals can be a possibility of some value for conservation. Total prevention of invasions if escapes are produced can not be guaranteed, but their capacity of naturalization and therefore invasiveness is likely lower than that of recently domesticated farm stocks.
References
Aigo J, Cussac V, Peris S et al (2008) Distribution of introduced and native fish in Patagonia (Argentina): patterns and changes in fish assemblages. Rev Fish Biol Fisheries 18:387–408
Armstrong JD, Kemp PS, Kennedy GJA, Ladle M, Milner NJ (2003) Habitat requirements of Atlantic salmon and brown trout in rivers and streams. Fish Res 62:143–160
Astorga MP, Valenzuela C, Arismendi I, Iriarte JL (2008) Naturalized Chinook salmon in the northern Chilean Patagonia: do they originate from salmon farming? Rev Biol Mar Oceanogr 43:669–674
Ayllon F, Martinez JL, Davaine P, Beall E, Garcia-Vazquez E (2004a) Interspecific hybridization between Atlantic salmon and brown trout introduced in the subantarctic Kerguelen Islands. Aquaculture 230:81–88
Ayllon F, Davaine P, Beall E, Martinez JL, Garcia-Vazquez E (2004b) Bottlenecks and genetic changes in Atlantic salmon (Salmo salar L.) stocks introduced in the Subantarctic Kerguelen islands. Aquaculture 237:103–116
Ayllon F, Davaine P, Beall E, Garcia-Vazquez E (2006) Dispersal and rapid evolution in brown trout colonizing virgin Subantarctic ecosystems. J Evol Biol 19:1352–1358
Baigún C, Ferriz R (2003) Distribution patterns of native freshwater fishes in Patagonia (Argentina). Org Divers Evol 3:151–159
Baigún C, Quirós R (1985) Introducción de peces exóticos en la República Argentina. Inf Téc 2, Inst Invest y Desarr Pesq: 1–90
Becker LA, Pascual MA, Basso NG (2007) Colonization of the Southern Patagonia ocean by exotic chinook salmon. Cons Biol 21:1347–1352
Braithwaite VA, Salvanes AGV (2005) Environmental variability in the early rearing environment generates behaviourally flexible cod: implications for rehabilitating wild populations. Proc R Soc B 272:1107–1113
Burge C, Karlin S (1997) Prediction of complete gene structures in human genomic DNA. J Mol Biol 268:78–94
Busacker GP, Adelman IR, Goolish EM (1990) Growth. In: Schreck CB, Moyle PB (eds) Methods for fish biology. American Fisheries Society, Bethesda, pp 363–387
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of nonindigenous marine organisms. Science 261:78–82
Castillo AGF, Ayllon F, Moran P, Izquierdo JI, Martinez JL, Beall E, Garcia-Vazquez E (2008) Interspecific hybridization and introgression are associated with stock transfers in salmonids. Aquaculture 278:31–36
Ciancio JE, Pascual MA, Lancelotti J, Riva Rossi CM, Botto F (2005) Natural colonization and establishment of a Chinook salmon (Oncorhynchus tshawytscha) population in the Santa Cruz River, an Atlantic basin of Patagonia. Environ Biol Fish 74:217–225
Clifford SL, McGinnity P, Ferguson A (1998) Genetic changes in Atlantic salmon (Salmo salar) populations of Northwest Irish rivers resulting from escapes of adult farm salmon. Can J Fish Aquat Sci 55:358–363
Cornuet JM, Piry S, Luikart G et al (1999) New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genet 153:1989–2000
Correa C, Gross MR (2008) Chinook salmon invade southern South America. Biol Inv 10:615–639
Davaine P, Beall E (1982) Introduction des salmonides dans les Terres Australes et Antarctiques Francaises. Comité Natl. Français des Recherches Antarctiques 51:289–300
Davaine P, Beall E (1997) Salmonid introductions into virgin ecosystems (Kerguelen Islands, Subantarctic): stakes, results, prospects. Bulletin Français de la Pêche et la Pisciculture 344(345):93–110
de Buen F (1959) Los peces exóticos en las aguas dulces de Chile. Investigaciones Zoológicas Chilenas 5:103–137
Estoup A, Largiader CR, Perrot E et al (1996) Rapid one-tube DNA extraction for reliable PCR detection of fish polymorphic markers and transgenes. Mol Marine Biol Biotechnol 5:295–298
Fausch KD, Taniguchi Y, Nakano S, Grossman GD, Townsend CR (2001) Flood disturbance regimes influence rainbow trout invasion success among five holarctic regions. Ecol Appl 11:1438–1455
Fleming IA, Einum S (1997) Experimental tests of genetic divergence of farmed from wild Atlantic salmon due to domestication. J Mar Sci 54:1051–1063
Fleming IA, Gross MR (1993) Breeding success of hatchery and wild coho salmon (Oncorhynchus Kisutch) in competition. Ecol Applic 3:230–245
Fleming IA, Hindar K, Mjolnerod IB, Jonsson B, Balstad T, Lamberg A (2000) Lifetime success and interactions of farm salmon invading a native population. Proc R Soc Lond Biol 267:1517–1523
Fleming IA, Agustsson T, Finstad B, Johnsson JI, Björnsson BT (2002) Effects of domestication on growth physiology and endocrinology of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 59:1323–1330
Fofonoff PF, Steves B, Miller WA, Ruiz G, Carlton J (2003) In ships or on ships? Unravelling the relative contribution of ballast tanks vs hull fouling to ship-mediated invasions of North America by marine species. In: Ruiz GM, Carlton JT (eds) Invasive species: vectors and management strategies. Island Press, Washington, pp 152–182
Ford JS, Myers RA (2008) A global assessment of salmon aquaculture impacts on wild salmonids. PLoS Biol 6:411–417
GENETIX (2000) GENETIX logiciel sous WindowsTM pour la génetique des populations. Montpellier: Laboratoire Genome et Populations, CNRS UPR 9060, Université de Montpellier II
Gollasch S (2006) Overview on introduced aquatic species in European navigational and adjacent waters. Helgol Mar Res 60:84–89
Heggenes J, Baglinière JR, Cunjak RA (1999) Spatial niche variability for young Atlantic salmon (Salmo salar) and brown trout (S. trutta) in heterogeneous streams. Ecol Freshw Fish 8:1–21
Hindar K, Fleming IA, McGinnity P, Diserud O (2006) Genetic and ecological effects of salmon farming on wild salmon: modelling from experimental results. J Mar Sci 63:1234–1247
Inoue M, Miyata H, Tange Y, Taniguchi Y (2009) Rainbow trout (Oncorhynchus mykiss) invasion in Hokkaido streams, northern Japan, in relation to flow variability and biotic interactions. Can J Fish Aquat Sci 66:1423–1434
Izquierdo JI, Castillo AGF, Ayllon F, de la Hoz J, Garcia-Vazquez E (2006) Stock transfers in Spanish brown trout populations: a long-term assessment. Environ Biol Fish 75:153–157
James SD (2009) Aquaculture production and biodiversity conservation. Bioscience 59:27–38
Jensen P (2002) Behaviour genetics, evolution and domestication. In: Jensen P (ed) The ethology of domestic animals. CABI Publishing, Wellingford, pp 13–30
Kinnison M, Unwin M, Boustead N et al (1998) Population-specific variation in body dimensions of adult Chinook salmon (Oncorhynchus tshawytscha) from New Zealand and their source population, 90 years after introduction. Can J Fish Aquat Sci 55:554–563
Krishnakumar K, Raghavan R, Prasad G et al (2009) When pets become pests–exotic aquarium fishes and biological invasions in Kerala, India. Curr Sci 97:474–476
Linde AR, Izquierdo JI, Moreira JC, Garcia-Vazquez E (2008) Invasive tilapia juveniles are associated with degraded river habitats. Aquat Conservat Mar Freshwat Ecosyst 18:891–895
Lura H, Saegrov H (1991) Documentation of successful spawning of escaped farmed female Atlantic salmon, Salmo salar, in Norwegian rivers. Aquaculture 98:151–159
Macchi PJ, Pascual MA, Vigliano PH (2007) Differential piscivory of the native Percichthys trucha and exotic salmonids upon the native forage fish Galaxias maculatus in Patagonian Andean lakes. J Limno 37:76–87
Macchi PJ, Vigliano PH, Pascual M, Alonso MF, Denegri MA, Milano D, Garcia Asorey M, Lippolt GE (2008) Historical policy goals for fish management in Northern Continental Patagonia, Argentina: a structuring force of actual fish assemblages? In: Nielsen JL, Dodson JJ, Friedland K, Hamon TR, Musick J, Verspoor E (eds), Reconciling fisheries with conservation: Proceedings of the Fourth World Fisheries Congress. American Fisheries Society, Symposium 49, Bethesda, Maryland, pp 331–348
MacCrimmon HR, Gots BL (1979) World distribution of Atlantic salmon, Salmo salar. J Fish Res Board Can 36:422–457
Martinez JL, Moran P, Garcia-Vazquez E (1999) Dinucleotide repeat polymorphism at the SS4, SS6 and SS11 loci in Atlantic salmon (Salmo salar). Anim Genet 30:462–478
McDowall RM (1990) When galaxiid and salmonid fishes meet—a family reunion in New Zealand. J Fish Biol 37:35–43
McDowall RM (2003) Impacts of introduced Salmonids on native Galaxiids in New Zealand upland streams: a new look at an old problem. Trans Am Fish Soc 132:229–238
McDowall RM (2006) Crying wolf, crying foul, or crying shame: alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Rev Fish Biol Fish 16:233–422
McGinnity P, Stone C, Taggart JB et al (1998) Genetic impact of escaped farmed Atlantic salmon (Salmo salar L.) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural river environment. J Mar Sci 54:998–1008
McGinnity P, Prodöhl P, Ferguson A et al (2003) Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc Biol Sci 270:2443–2450
McGinnity P, Jennings E, DeEyto E et al (2009) Impact of naturally spawning captive-bred Atlantic salmon on wild populations: depressed recruitment and increased risk of climate-mediated extinction. Proc R Soc B 276:3601–3610
McIntosh AR (2000) Habitat- and size-related variations in exotic trout impacts on native galaxiid fishes in New Zealand streams. Can J Fish Aquat Sci 57:2140–2151
McMeel OM, Hoey EM, Ferguson A (2001) Partial nucleotide sequences, and routine typing by polymerase chain reaction-restriction fragment length polymorphism, of the brown trout (Salmo trutta) lactate dehydrogenase, LDH-C1*90 and *100 alleles. Mol Ecol 10:29–34
Mignon-Grasteau A, Boissy J, Boix JM et al (2005) Genetics of adaptation and domestication in livestock. Livest Prod Sci 93:3–14
Moran P, Pendas AM, Garcia-Vazquez E, Izquierdo JI (1991) Failure of a stocking policy of hatchery reared brown trout, Salmo trutta L., in Asturias, Spain, detected using LDH-5* as a genetic marker. J Fish Biol 39:117–121
Naylor RL, Goldburg RJ, Primavera JH et al (2000) Effect of aquaculture on world fish supplies. Nature 405:1017–1024
Naylor R, Hindar K, Fleming IA et al (2005) Fugitive salmon: assessing the risks of escaped fish from net-pen aquaculture. Bioscience 55:427–437
Orlov AV, Gerasimov YV, Lapshin OM (2006) The feeding behaviour of cultured and wild Atlantic salmon, Salmo salar L., in the Louvenga River, Kola Peninsula, Russia. J Mar Sci 63:1297–1303
Pascual MA, Macchi PJ, Urbanski J, Marcos F, Riva-Rossi C, Novara M, Dell’Arcipreste P (2002) Evaluating potential effects of exotic freshwater fish from incomplete species presence-absence data. Biol Inv 4:101–113
Pascual MA, Cussac V, Dyer B, Soto D, Vigliano P, Ortubay S, Macchi P (2007) Freshwater fishes of Patagonia in the 21st century after a hundred years of human settlement, species introductions, and environmental change. Aquat Ecosys Health Manag 10:212–227
Perez JE, Alfonsi C, Salazar SK, Macsotay O, Barrios J, Martinez Escarbassiere R (2007) Especies marinas exóticas y criptogénicas en las costas de Venezuela. Bol Inst Oceanogr Venezuela 46:79–96
Perez-Rubin J (2006) Mariano de la Paz Graells y Agüera (1809–1898): Entre la pesca “científica” y la ciencia pesquera en España. Sociedad Española de las Ciencias y de las Técnicas. Tomo II: 1045–1055
Piferrer F, Beaumont A, Falguière JC, Flajšhans M, Haffray P, Colombo L (2009) Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293:25–156
Reilly A, Kaeferstein F (1999) Food safety and products from aquaculture. J Appl Microbiol 85:249–257
Riva Rossi CM (2008) Origen y desarrollo de historias de vida alternativas en poblaciones introducidas de trucha arco iris (Oncorhynchus mykiss) en Patagonia. Tesis Doctoral. Universidad Nacional del Comahue, Bariloche, Argentina
Ruiz GM, Lorda J, Arnwine A, Lion K (2006) Shipping patterns associated with the panama canal: effects on biotic exchange? In: Springer (Ed) Bridging divides maritime canals as invasion corridors. Netherlands, pp 113–126
Säisä M, Koljonen MJ, Gross R, Nilsson J, Tähtinen J, Koskiniemi J, Vasemägi A (2005) Population genetic structure and postglacial colonization of Atlantic salmon (Salmo salar) in the Baltic Sea area based on microsatellite DNA variation. Can J Fish Aquat Sci 62:1887–1904
Slettan A, Olsaker I, Lie Ø (1995) Atlantic salmon, Salmo salar, microsatellites at the SSOSL25, SSOSL85, SSOSL311, SSOSL417 loci. Anim Genet 26:281–282
Smith LD, Wonham MJ, McCann LD, Ruiz GM, Hines AH, Carlton JT (1999) Invasion pressure to a ballast-flooded estuary and an assessment of innoculant survival. Biol Inv 1:66–87
Soto D, Arismendi I, Gonzalez J et al (2006) Southern Chile, trout and salmon country: invasion patterns and threats for native species. Rev Chil Hist Nat 79:97–117
Sušnik S, Snoj A, Pohar J et al (1997) The microsatellite marker (BRFO 002) characteristic for different geographically remote brown trout, Salmo trutta L., populations. Anim Genet 28:370–383
Townsend CR (1996) Invasion biology and ecological impacts of brown trout Salmo trutta in New Zealand. Biol Conservat 78:13–22
Townsend CR (2003) Individual, population, community and ecosystem consequences of a fish invader in New Zealand streams. Cons Biol 17:38–47
Tulian EA (1908) Acclimatization of American fishes in Argentina. Bull Bureau Fish USA 18:957–965
Valiente AG, Juanes F, Nuñez P, Garcia-Vazquez E (2007) Is genetic variability so important? Adaptation of non-native salmonids in South America. J Fish Biol 71:136–147
Valiente AG, Juanes F, Nuñez P, Garcia-Vazquez E (2010) Brown trout invasiveness: plasticity in life-history is more important than genetic variability. Biol Inv 12:451–462
Vergara M (2003) La acuicultura en Chile. Ediciones Tecno-Press SA, Santiago
Vigliano PH, Alonso MF (2007) Salmonid introductions in Patagonia: a mixed blessing. In: Bert TM (Ed) Ecological and genetic implications of aquaculture activities. Methods and technologies in fish biology and fisheries, vol 6. Berlin, pp 315–331
Vitousek PM, D’Antonio CM, Loope LL, Rejmánek M, Westbrooks R (1997) Introduced species: a significant component of human-caused global change. New Zealand J Ecol 21:1–16
Acknowledgments
This study has been supported by the Spanish project CGL2009-08279. Ivan G. Pola helped with laboratory tasks. We are grateful to D. Simberloff and two anonymous reviewers who made very helpful comments and contributed to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valiente, A.G., Ayllon, F., Nuñez, P. et al. Not all lineages are equally invasive: genetic origin and life-history in Atlantic salmon and brown trout acclimated to the Southern Hemisphere. Biol Invasions 12, 3485–3495 (2010). https://doi.org/10.1007/s10530-010-9746-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9746-3