Abstract
After 150 years of introduction of the brown trout Salmo trutta in Himalaya, the native species’ response to this globally pervasive invader, is still unknown. Here, we investigate the effects of invasion of brown trout on native snow trout Schizothorax richardsonii, one of the most primitive species that co-evolved with the Himalayan orogeny. We contrast two natural river systems which harbour snow trout in the absence (allopatry) and presence (sympatry) of brown trout. We put forth that sympatric snow trout adapted to a ‘fast’ life history with maturation at a smaller length, greater fecundity and smaller egg diameter to cope with brown trout invasion. However, investment in a reproductive-somatic trade off was evident with a disrupted size structure and reduced abundance as compared to the allopatric population. Although the fast life history adaptations of snow trout might increase their competitive ability with invasive brown trout, trading off the somatic fitness in the process seemingly acts as a deterrent to longevity. We attribute the plastic responses of snow trout to their plausible inherent potential of sustenance and recovery from high invasion pressures. The popularity of brown trout as a sport fish in Himalaya however poses extraneous propagule pressure on the snow trout, which warrants quantification through future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brown trout Salmo trutta, historically native to Europe and parts of Asia, have been introduced outside their native range owing to their popularity as a sport fish. Although the initial introductions aimed at angling leisure opportunities, they gained notoriety for ubiquitous regional spread post initial transplants worldwide (McIntosh et al. 2012). The global distribution of this invader is driven by their large fundamental niche, aggressive territoriality, effective dispersal abilities and an incredible phenotypic plasticity to adapt to novel habitats, heightened further by their repeated stockings in the wild (Budy et al. 2013; Lobón‐Cerviá and Sanz 2017). Spreading out to all the continents but Antarctica, the brown trout has been identified as one of the top 30 worst freshwater invaders of the globe (McIntosh et al. 2012).
Recent decades have seen burgeoning research documenting the effects of invasion of brown trout on the native fish species in the form of altered food webs, reduced native fish abundance and escalated competition for space and food (Budy and Gaeta 2017; Trego 2017; Al‐Chokhachy and Sepulveda 2019; Jones et al. 2019). Native-invasive interactions across globe are being continually monitored to aid development of effective conservation measures for the native fish fauna (Garman and Nielsen 1982; Townsend 1996; Kitano 2004; McIntosh et al. 2012; Wagner et al. 2013; Trego 2017). Research evidencing negative implications of the brown trout invasion have also favoured experiments on their manual removal from the system, resulting in native population revivals (Fausch and White 1981; Hoxmeier and Dieterman 2012, 2016). Being cold-water specialists, their initial establishment was predicted to be successful in the high altitudes and latitudes of the world (McIntosh et al. 2012). Himalaya is the highest mountain chain on Earth and was thus, a favoured stocking site for this species during the 1860s (Sehgal 1999; Belica 2007; McIntosh et al. 2012). Research has since been conducted on the brown trout across the Himalayan watersheds mainly on their distribution and habitat ecology (Zhang and Wang 1962; Hao et al. 2006; Bhatt et al. 2008; Hao and Chen 2009; Rawat et al. 2011, 2017; Rasool and Jan 2013; Chen et al. 2020). Surprisingly however, over 150 years post-introductions in the Himalaya, the effects of their invasion on the native fish species still remain unknown.
The brown trout overlap their range with an endemic Alwan snow trout Schizothorax richardsonii of the Himalaya, which is worrying. Most primitive amongst all the Schizothoracines, the snow trout is specialized for torrential streams and has co-evolved with the changing patterns of Himalayan geomorphology (He and Chen 2006; Sharma et al. 2019). Distantly related to the typical ‘trout’ (Salmoniformes), the snow trout are in fact minnows (Cypriniformes) (Regmi 2019). Categorized as Vulnerable on the IUCN Red List (Vishwanath 2010), currently the species is facing serious threat due to the ongoing hydropower developments (Rajvanshi et al. 2012), flow alterations (Grumbine and Pandit 2013) and brown trout introductions (Sharma et al. 2019). However, the mechanisms by which the snow trout might be affected by brown trout invasion in their native range is still unclear.
Although the brown trout have adapted to varied habitats, their documented high establishment rates in the Himalayan headwaters and superior competitiveness at extremely low temperatures could be taxing for the snow trout, which typically prefer the same habitats (Behnke 2002; Belica 2007). Globally, the aggressive territoriality of brown trout regardless of the extreme cold temperatures, has resulted in displacement and reduced abundance of many native fish species with limited access to food and space (Garman and Nielsen 1982; Wagner et al. 2013; Trego 2017).
Apart from the investigations of brown trout invasion on the riverine ecosystems, some rare, albeit classic studies have investigated the invasion-induced ‘life history’ responses of the native fish fauna (Näslund et al. 1998; McHugh and Budy 2005; Öhlund et al. 2008; Hoxmeier and Dieterman 2012; Al-Chokhachy et al. 2016; Al‐Chokhachy and Sepulveda 2019). Life-history traits like fecundity, egg diameter, size and age at maturity show major variation across the biotic and abiotic stressors (Stearns 1992), and are idiosyncratic to the environmental setup a species is placed in. Given such tremendous role of life history traits in strengthening a species’ ability to tackle threats, its understanding can have major conservation implications for the native fishes under invasion pressures. Various management decisions have been made globally based on the life history responses of the threatened native species (Goldstein et al. 2018). Regardless, the studies discussing invasion threats on Himalayan fishes are more or less anecdotal (Gupta and Everard 2019; Gupta et al. 2020) lacking empirical evidences on the native fish species’ responses to invasion. Robust scientific analysis and trait-based research on the effects of invasion on the Himalayan native fish fauna is thus strongly warranted for effective restoration and conservation measures. Many facets of the brown trout invasion in Himalaya are uncertain. That, whether the brown trout invasion has actually altered the native population, or that the presumptions are rather unfounded, is yet not verified. Regardless, the brown trout habitat preferences hint its range to be strongly coalescing with that of the endemic snow trout.
The current lack of knowledge on the trait-based responses of snow trout co-occurring with the brown trout in Himalaya, impede the understanding on the invasion potential of brown trout and the resilience of native snow trout to cope with these invasions. Until the life-history responses of the native snow trout are better understood, initiation of effective conservation strategies would stay at an unsolicited halt. We thus initiated the first ever study on the brown trout invasion in Himalaya to investigate a sympatric (snow trout co-occurring with brown trout) and an allopatric (snow trout in absence of brown trout) population. We set out to answer the question: do the snow trout show any reproductive and/or size structure plasticity in response to the brown trout invasion? Through our study, we ultimately bolster the current understanding on the probable responses of snow trout to biological invasions in high-altitude Himalayan streams.
Methodology
Study area
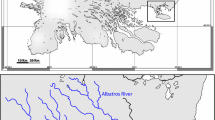
Sympatric and allopatric associations of snow trout with introduced brown trout were investigated in Tirthan and Asiganga rivers in the west and north-western Himalaya, India (Fig. 1). Both the rivers are undammed and anthropogenically unaltered in structure and environmental settings. Tirthan traverses through the protected bounds of the Great Himalayan National Park Conservation Area (GHNPCA), which is a United Nations Educational, Scientific and Cultural Organization (UNESCO) world heritage site giving it an outstanding biodiversity conservation significance. It flows south-westerly to join River Beas, a primary tributary of River Indus. Asiganga, on the other hand, flows south-easterly to join Bhagirathi, which is a primary tributary of River Ganga. The total river stretch inclusive of the tributaries is 120 km for Tirthan and 36 km for Asiganga.
Both the rivers are typical high-altitude lotic ecosystems of Himalaya, with a similar elevational coverage of 990–4880 masl (Tirthan) and 1158–4400 masl (Asiganga). We also checked whether they share similar climatic profiles for which we compared mean annual temperatures (30 s resolution) extracted from the Worldclim Climate Database (version 2.1) (Fick and Hijmans 2017) for both the basins. The mean annual temperature was chosen for comparison as it has been widely reported to be a prime factor governing the metabolism and hence the distribution of organisms on earth (Pearson and Dawson 2003; Brown et al. 2004).
Historically, brown trout occurrence had been reported for Asiganga until the year 2013, when a major cloudburst induced flash-floods resulted in complete extirpation of the non-native salmonids from the river. Priority effects resulted in the retention of just the natives post the extirpation of the invaders (Sharma et al. 2019). Asiganga thus acted as a natural control setup to study allopatric snow trout populations. Tirthan, on the other hand, had never been investigated before this study.
Fish sampling
Fish sampling was conducted throughout the year in Tirthan (2017 and 2018) and Asiganga (2018). Owing to its long stretch, which could only be accessed through trekking along the river bed, a two-year sampling in Tirthan was necessitated. Sampling was conducted at an interval of 500 m for the higher-order streams (4th and higher) while a 200 m interval was chosen for the lower order streams (3rd and lower) to ensure an equivalent representation as they often had a length of less than 500 m. This extensive survey resulted in a total of 109 sampling points in River Tirthan and 22 in River Asiganga. Owing to inaccessibility of most of the sampling area due to heavy snowfall in peak winter months, we further selected 22 accessible sampling sites in Tirthan and 15 in Asiganga, representative of the upper, lower and middle stretch for monthly sampling. Cast nets of 10 and 30 mm mesh sizes were used for sampling. The catch per unit effort (CPUE) was deduced by dividing the catch of each sampling site by number of hours fished. All of the snow trout and brown trout captured were measured for total length (to the nearest mm) and weight (to the nearest g) in both the rivers.
Fish life history traits
Weight-length relationship (WLR)
WLRs for all the populations were deduced using the length and weight data of fishes following Clark (1928). As most of the fishes showed greater variabilities and data dispersion on the length axis of a weight-length relationship, a two-parameter power function with a multiplicative error term was used to fit the non-linear model (Ogle 2016) with the equation:
where W and L refer to the weight and length, α and β are the parameters of the WLR, while \({10}^{{\varepsilon }_{i}}\) is the multiplicative error term specific to the ith fish. Regressions were performed using the logarithmically transformed WLR to eliminate non-linearity.
The multiplicative term for the logarithmic WLR was additive allowing constant variability around the line of best fit. Slope and intercept here, are estimated by the parameters \(\beta\) and \(\alpha\) respectively.
The significance of the relationship between the response (log10W) and predictor (log10L) variable was statistically confirmed based on comparing two models; one with predictor variable and intercept term and the second with just the intercept term, using F-test statistic and p values of the analysis of variance (ANOVA). To further test the significance of relationship, we checked if the slope of the linear regression model was significantly different from zero using the two-tailed t-test statistics. The normality and homoscedasticity assumptions of the models were checked through the residual plots while linearity assumptions were checked using histogram of model residuals.
WLR comparisons for sympatry-allopatry
Differences in length-frequency data amongst the snow trout populations in allopatry and sympatry with the brown trout were tested using the Kolmogorov–Smirnov (KS) two-sample test (Neumann and Allen 2007) on the empirical cumulative distribution function (ECDF) with 500 bootstrap iterations. To test for differences in parameters of WLR amongst the two populations, a dummy variable regression (DVR) was performed. A single equation constituting the differences in parameters amongst the two populations (Ogle 2016) was transformed from the WLR equation as:
where \(fTir\) is the factor dummy variable for Tirthan snow trout, \(\alpha\) and \(\beta\) are intercept and slope parameters of the DVR-WLR for the Asiganga population. The \(\alpha +\delta\) and \(\beta +\gamma\) are the intercept and slope for the Tirthan population DVR-WLR, respectively. The transformed DVR model was built to avoid formulation of two separate sub-models. The parameter \(\delta\) is the intercept difference while \(\gamma\) is the slope difference amongst the two populations. Significant differences of \(\gamma\) and \(\delta\) from 0 were used to determine the variability in the two WLRs based on ANOVA F-test statistic and p values.
Condition factor
Relative condition factor (Kni; Le Cren 1951) was used to investigate the condition of the individual fishes. Kni was deduced by the deviation of an individual from the average weight for the population at a given length using the equation:
where \({{W}}_{i}\) is the recorded weight of the ith fish and \({\widehat{W}}_{i}\) is the predicted mean weight calculated by anti-logging the predicted log weights of the fitted regression. A value of Kni < 1 is indicative of a below average fish weight in comparison to the expected weight of a same length fish in the population. The Kn for the entire population was calculated by the averaging out Kni for all individuals. The differences in the inter-population mean Kn were tested with the Welch’s unequal variances t-test. Simple linear regression was fitted for month-wise Kn and tested for statistical assumptions. Temporal variation in Kn were then tested with one-way ANOVA for sympatric while Kruskal–Wallis Test (with Holm’s p-adjustment) for allopatric snow trout. Monthly patterns of Kn were analysed to understand the somatic growth of snow trout as a response to brown trout, as we expected variations in Kn of the allopatric and sympatric populations specifically during the pre-spawning months (owing to variation in energy allocated to gonadal development under competition). As the Kn is known to be correlated with gonadal development, we then compared the Kn with gonado-somatic index as a measure of the reproductive investments of the sympatric snow trout (Le Cren 1951).
Gonado-somatic indices
Fishes sampled for estimation of reproductive parameters were euthanized on site with a 2 ml solution of clove oil: 95% ethanol (1:10) per 350 ml river water (Wong et al. 2014). Given a small population of the wild snow trout available for the study, especially in the River Tirthan, we restricted ourselves to a subsample of the population assessed for the WLR, maintaining the same relative representation from each size class. Reproductive investment in the form of gonado-somatic Index (GSI) was calculated as a quotient of gonadal weight by the total body weight. A percentage conversion of the quotient was then used as the GSI value denoted by Ig (%) as:
The GSI values across the months and populations were compared with the Type III sum of squares two-way ANOVA post assumption checks. Significantly differing months within and between the basins were identified based on Tukey’s Honestly Significant Difference (HSD) adjusted p values (Tukey 1953).
Length at maturity (L50)
Length at maturity of fishes were determined by visual observations of the gonads classifying them from I to VI (Fontana 1969). A logistic regression approach was used to model the length at which 50% of the population matures. The maturity stages were binomial transformed classifying I–II as immature, III–V as mature and VI as spent. Using total length as the allometric explanatory variable and state of maturity (mature: 1, immature: 0), as the binomial response variable, the response was linearized by transforming the maturity probability (p) into odds \((p/1-p))\) of success and failure. A logarithm of the odds was then computed to complete the linearization (log (\(p/1-p))\). Bayesian regression using posterior distribution probabilities was used with a total of 999 metropolis iterations to compute the L50 with the following equation:
The statistical calculations were made using the SizeMat package in R (Torrejon-Magallanes 2019).
Fecundity and egg diameter
Fecundity was calculated from the Stage V females. Sub-samples from front, middle and rear sections of the ovary were taken and weighed. Subsample fecundity (Fn) was calculated by counting the yolk-laden oocytes for all the subsections (Shoko et al. 2015) using the formula:
where n = 1–3. A mean of all three F values was then made to estimate the absolute fecundity (AF). The AF is a measure to estimate the total number of eggs in an individual fish
Eggs preserved in 4% formaldehyde were brought to the laboratory and diameter measurements were made to the nearest mm under microscope using stage and ocular micrometer. The diameters of five eggs were taken randomly and averaged to get the mean.
Results
Both the rivers Asiganga and Tirthan were found to be climatically similar. The comparisons of mean annual temperatures across Asiganga and Tirthan basins revealed similar thermal gradients (Supplementary material: Fig. S1), with the values at the occurrence locations of snow trout ranging between 13.2–18.7 °C in Asiganga and 11.9–18.9 °C in Tirthan, respectively. Furthermore, the Welch’s t-test performed on mean annual temperatures at the occurrences failed to find evidence for any difference in the two basins at an alpha of 0.05 (Welch's t-test statistic = − 2.5584, p = 0.02).
The sampling resulted in a total of 602 fishes which were used for the WLRs including 240 snow trout from Asiganga (allopatry) and 133 from Tirthan (sympatry). While Asiganga showed complete absence of brown trout a total of 229 brown trout were captured from the Tirthan basin. The average estimated CPUE for the snow trout in Asiganga was 1.75 ± 1.90 kg h–1 (0.22–6.14 kg h–1) while that for Tirthan was distinctly lower at 0.48 ± 0.30 kg h–1 (0.14–1.03 kg h–1). For brown trout, the estimated CPUE was 0.86 ± 0.45 kg h–1 (0.14–2.36 kg h–1) in the Tirthan River. All the populations sampled showed a female biased sex ratio (57.5% for allopatric and 59.4% for sympatric female snow trout). Total length ranged from 113–360 mm in allopatric and 60–359 mm in sympatric snow trout. Brown trout showed a similar length range of 145–350 mm. The sympatric snow trout showed two distinct (small; n = 19 and large; n = 114) length stanzas, with an inflexion point at about 120 mm total length unlike the allopatric snow trout with a single growth stanza (Fig. 2).
A log–log plot showing the weight length relationships (WLRs) for snow trout in allopatry (Asiganga) and sympatry (Tirthan). A distinct small length stanza (sympatric-small length stanza) in the sympatric snow trout is discernible with a near isometric b (2.936) than the larger stanza which showed negative allometry (b = 2.569). The top and right panels show respectively, the superimposed density plots of log Length and log Weight for all the populations investigated in the study
WLR parameters
Two distinct growth stanzas were observed for the sympatric snow trout population, with the smaller-stanza (n = 19) individuals having a significantly different WLR (r2 = 0.981, 95% CI of b = 2.756 to 3.116, ANOVA F-test statistic = 1187.9; p < 2.2e−16) than the larger-stanza (n = 114) individuals (r2 = 0.946, 95% CI of b = 2.455 to 2.684, ANOVA F-test statistic = 1968.5; p < 2.2e−16) with an inflexion point at about 120 mm (Table S1). This was quite different from the allopatric population, which showed a single growth stanza. Despite the similar sampling effort for both the populations, smaller-stanza in the catch of sympatric snow trout might have resulted from greater percentage of small individuals. Regardless, we removed the smaller group stanza from the final analysis of WLRs to remove a parameter bias.
Model fitting for all the populations indicated that log10(length) significantly predicted the log10(weight) (ANOVA F-test statistic 1188–2928; p < 0.001). The slopes differed from zero for all the WLRs indicating a significant relationship between log10(length) and log10(weight) (two tailed t-test statistic 34.47–54.11; p < 0.001). A high coefficient of determination was recorded for all the WLRs (R2 > 0.9) (Table S1).
WLR comparisons for sympatric and allopatric snow trout populations
The assumption checks for both the simple linear regressions and DVRs indicated that the data sets were normal, linear and homoscedastic. The KS two-sample tests for the sympatric and allopatric snow trout populations showed significantly different ECDFs (bootstrap p value = 0.002, full sample statistic = 0.18972). The length frequency histograms for the sympatric and allopatric populations (Fig. 3) showed a distinct coercing in the same length range (170–210 mm). The coefficient of allometry b equalled 3.23 (95% CI: 3.11; 3.35) and 2.56 (95% CI: 2.45; 2.68) for allopatric and sympatric snow trout indicating positive and negative allometry respectively. The DVR model showed a significant difference (F = 54.629, p < 0.001) in slopes of both the populations with the slope \((\upgamma )\) for sympatric population 0.661 (95% CI: 0.837; 0.485) lower than that of the allopatric population. The predicted weight-at-length was found to be lower for the sympatrics than the allopatrics for almost all the length classes (Fig. S2).
Condition factor
In the case of condition factor (Kn), the Welch’s t-test failed to find evidence for any difference in Kn at an alpha of 0.05 (Welch's t-test statistic = − 1.321, p = 0.189) between the sympatric (mean Kn = 1.015) and allopatric populations (mean Kn = 1.061) of snow trout. While there were no differences in Kn observed among the two snow trout populations, a temporal pattern in variation were distinct (Fig. 4). A distinct variability was found in the Kn trends across the months for the allopatric snow trout (Kruskal–Wallis rank sum test p value < 0.001), with highest differences evident in the months preceding (Holm-adjusted p value < 0.05, November–August) and following (Holm–adjusted p values: < 0.001, November–January; < 0.05, November–February) the completion of spawning in November. On the contrary, no significant temporal trend in Kn was observed for the snow trout in sympatry with the brown trout (one-way ANOVA; p = 0.512).
Gonado-somatic indices, fecundity and egg diameters
Out of the total samples used for the WLRs, we chose a subset of 110 and 85 snow trout individuals from Asiganga and Tirthan. We ensured that the fish retained in both the subsets represented similar size classes for better comparisons. The GSI for the sympatric population differed significantly (ANOVA type III SS; p = 0.001) from that of the allopatric snow trout (Fig. 5). A post hoc analysis showed maximum disparity in the major (December: Tukey’s HSD; p < 0.001) and minor (July and August: Tukey’s HSD; p < 0.01) spawning bouts for the snow trout. The GSI in the non-spawning months nevertheless, remained largely similar for the allopatric and sympatric snow trout (April, May and June: Tukey’s HSD; p = 1) (Fig. 6a, b). The sympatric snow trout in their highest maturity (stage V) had a higher GSI (11.95 ± 5.21) as compared to the same-stage allopatrics (7.67 ± 1.37). The GSI increased substantially as the snow trout transitioned from stage III to V (Fig. 7a) when in sympatry with the brown trout. The allopatric snow trout however showed a gradual rise in GSI across stages.
Comparative density ridgeline visualisation of the gonadosomatic indices (Ig (%)) for allopatric and sympatric snow trout populations. The peaks of the ridgelines correspond to the higher density distributions of the gonadosomatic index for all the months across the year where January to December are represented by 1 to 12
Stacked density visualizations for the temporal distribution of maturity stages (I–V) in snow trout where band sizes for all maturity stages reflect the frequency of observation per month for a River Tirthan (sympatric) showing higher representation of fecund fishes (stage V) across the year unlike that in b River Asiganga (allopatric) which do not show the presence of fecund individuals throughout the year
Box whiskers parallelly jittered to visualise the maturity stages (I–V) of the allopatric (River Asiganga) and sympatric (River Tirthan) snow trout in relation to a gonadosomatic index (Ig (%)): differs significantly between the two snow trout populations (ANOVA type III SS; p = 0.0003) with the fecund fishes (stage V) in the sympatrics showing higher Ig (11.95 ± 5.21) than that of the allopatrics (7.67 ± 1.37), b absolute fecundity: higher at stage IV and V for the sympatrics (5492 ± 1291.08) than the allopatrics (3089 ± 1708.54). Both the populations show a decline in absolute fecundity as they transition from stage IV to V and c egg diameter: transition from stage IV to V shows a plummet in the sympatrics (1.843 ± 0.504 to 1.347 ± 0.417) unlike the allopatrics which show an increase (1.339 ± 0.558 to 1.836 ± 0.409)
The absolute fecundity estimates for the spawning (V) stage were higher for the sympatric (5492 ± 1291.08, max = 8124) than the allopatric (3089 ± 1708.541, max = 6143) snow trout. The transition from stage IV to V witnessed a sudden decline in fecundity for both the sympatric (5611 to 5492) and allopatric (4088 to 3089) populations (Fig. 7b). The mean egg diameter dropped from stage IV to V in the sympatric population (1.843 ± 0.504 to 1.347 ± 0.417), unlike the allopatric population which showed a significant increase in egg diameter as they enter the spawning stage (1.339 ± 0.558 to 1.836 ± 0.409) (Fig. 7c). Fully fecund fishes (stage V) started to appear at as low as 102 mm total length in the sympatric snow trout unlike a length of 224 mm for the allopatric snow trout (Fig. S3).
Length at first maturity
The Bayesian length ogives for the snow trout predicted 50% of the allopatric snow trout population in Asiganga to mature at the length of 248.9 mm with the model best fit at parameters A = − 5.63 and B = 0.02. However, a much smaller length at first maturity 177.9 mm (A = − 1.21, B = 0.01) was predicted for the snow trout in the presence of brown trout in Tirthan (Fig. 8a, b), which matured at 190.3 mm (A = − 4.11, B = 0.02) (Fig. S4 a and b).
Discussion
The comparison of the allopatric and sympatric snow trout life history shows striking evidences of effects of brown trout invasion. Our investigations reveal a characteristic plasticity in size structure and reproductive traits of the snow trout co-occurring with the brown trout. Further, it is clearly evident that the escalated reproductive investments through early maturity, higher fecundity and reduced egg size in the sympatric snow trout indicate an invasion-induced ‘fast’ life history adaptation unlike the allopatric population. The skewed length-frequency distribution after attainment of length at first maturity in the sympatrics, indicates a somatic-reproductive trade off. Our study reveals that the snow trout seems to forcibly invest in reproductive efficiency, thereby reducing its somatic growth, as a plausible effort to cope with the brown trout invasion pressures.
We strongly attribute the plastic responses of sympatric snow trout to be a resultant of its biotic interactions with brown trout, rather than abiotic and harvest-related differences of Asiganga (allopatric) and Tirthan (sympatric) basins. This being so, as we did not document any differences in elevational or thermal gradients for the two basins. In addition, both the rivers provide similar potential bioclimatic niches for snow trout as underscored by Sharma et al. (2021). Furthermore, harvest is not a potential factor translating into the life-history traits differences as commercial fishing is completely prohibited in Asiganga as well as Tirthan. Considering the fact that snow trout in Asiganga co-existed with brown trout until the flash floods in 2013 (Sharma et al. 2019), its transition to allopatry is rather recent. A population which transitions from sympatry to allopatry, often retains the effects of sympatric association for long (Reznick et al. 1997, 2008), which might also have been the case for Asiganga snow trout. However, the oldest individual that we record from Asiganga in 2018 was of the age 4 (Johal et al. 2020 submitted manuscript) through which we can ascertain that none of the snow trout individuals in 2018 were alive during the sympatry (2013) and that the population has undergone a complete renewal.
An invasive species rarely extirpates any native species in entirety, as such reduced abundance or altered community structure are often the prime indications of the negative effects of invasions (Theoharides 2007; Powell et al. 2011). Low abundance of the sympatric snow trout in comparison with the allopatrics in our study indicated a demographic disruption. There is substantial research evidencing the native fish population decline due to brown trout invasions (Moyle 1976; Garman and Nielsen 1982; Wagner et al. 2013; Al-Chokhachy et al. 2016; Trego 2017; Al‐Chokhachy and Sepulveda 2019). On the contrary, experimental removals of brown trout from rivers have recorded a spike in the native fish species abundance (Fausch and White 1981; Hoxmeier and Dieterman 2012, 2016), further cementing the impact of brown trout invasion on native ecosystems.
Studies like those in the Middle Provo River, tested the effects of increased habitat complexity and maintenance of natural flow regime on the co-existence of native and invasive fishes and reported that restoration of habitat complexity led to their non-competitive co-existence and expansion in the distribution of native fish species (Belk et al. 2016). Although the River Tirthan similarly provides a natural habitat complexity with unaltered flow, yet contrary to Belk et al (2016), our study recorded a competitive co-existence of the native snow trout with introduced brown trout, evident with the altered size-structure and reduced abundance of the natives in sympatry. We contemplate that the suggestions made by Belk et al. (2016) and others (Belk and Johnson 2007; Billman et al. 2011; Hanisch et al. 2012), emerge from natural setups where invasive trout numbers were not anthropogenically enhanced via stocking. The case of Tirthan however, entails a brown trout propagule pressure due to its frequent stocking in high numbers aimed at promoting sport fishing, thereby generating livelihood opportunities for the local communities. Research has stressed on the overwhelming inabilities of the native taxa to cope with effects of invasion buttressed by anthropogenic propagule pressure (Leprieur et al. 2008; Holle and Simberloff 2005), which might be the case in our study, however further research is warranted to confirm this across Himalayan rivers where stocking pressures are minimal.
The present study on the sympatric snow trout population, recorded a higher number of small length individuals (< 170 mm), which is contrary to other areas which report small length individuals in comparatively lower numbers (Peterson et al. 2004; Al‐Chokhachy and Sepulveda 2019). An increased piscivory in the brown trout outside its native range (Budy et al. 2013), seemingly is the most explicable reason for reduced numbers of small-length individuals in native fish populations. However, we observed piscivory in just two of the 238 brown trout individuals dissected. Furthermore, our aim was not to investigate diet overlaps per se owing to contrasting feeding strategies of herbivory in the snow trout and carnivory in the brown trout (Sharma 1984; Froese and Pauly 2011). The higher number of small length individuals in our study reflect the investment of snow trout in increasing the number of individuals for expansion of territories, as is observed in other species competing for space with an aggressive competitor (Hôrková and Kováč 2014). Behavioural studies on the brown trout in invaded streams concur with its increased aggressiveness and resultant reduction in dominance of the native species (Sorensen et al. 1995, Stradmeyer et al. 2008). The snow trout and brown trout in Tirthan show similar habitat preferences, however their spatial distribution in the basin indicates a relegation of snow trout from the main channel towards tributaries (Sharma et al. 2020 submitted manuscript), unlike Asiganga where snow trout is uniformly distributed in main channel as well as tributaries. The sympatric snow trout in our study, thus seem least effected by the predation pressures of the brown trout and it is rather interference (competition for space), than exploitative (predator–prey interaction) competition which is altering the size structure of snow trout. Additionally, the greater fecundity in conjunction with the minimal predation pressures which we document in the sympatric snow trout, presumably increases the number of small-length individuals.
We further conducted the WLR investigations on the two snow trout populations. Unlike expected, we observed two distinct growth stanzas in the sympatric population indicating a bi-phasic growth. As per our knowledge, no other snow trout populations have been reported with two growth phases. Despite studies attempting investigation of the WLRs for snow trout populations across Himalaya (Tyagi et al. 2014; Lohani and Ram 2018), none of them checks for linearity in double logarithmic plots to detect multiple growth stanzas. The inflexion point indicating separation of the two growth stanzas was thus most likely overlooked. Furthermore, the evidently low sample size in these studies fails to build strong WLRs for the snow trout, refraining us to compare our results with theirs.
Multiple growth stanzas have been observed in some fish species as a natural ontogenic drift in life history owing to limitations in niche availability or predation pressures (Froese 2006). The b parameter in the WLR for each growth stanza informs us often more than just the isometric (b = 3) or allometric (b ≠ 3) growth. Smaller length individuals have been reported with a higher b parameter to avoid predation pressures (Dadzie et al. 2008; Wang et al. 2013). On the contrary, a higher b observed in the larger individuals of a population can be indicative of a shift in niche or feeding habits (Stergiou and Fourtouni 1991). The sympatric snow trout in this study albeit, showed diminutive growth in weight for the higher length stanza (b = 2.57) in contrast to a near isometric growth for the lower length individuals (b = 2.94). Two growth stanzas in the sympatric snow trout seemingly are a resultant trade-off associated with their substantial energy investments in reproduction, leading to a reduced somatic growth as evident with a low b value for the larger stanza. Our results on the reproductive traits further corroborate this trade-off with a greater fecundity and GSI ensuing a strikingly reduced length at first maturity.
The size structure of a species cueing early reproduction as reported in other minnows (Danylchuk and Tonn 2001) is a cyclical event, as is indicated by the cumulative inferences we draw from our results of length at first maturity, size structure and reproductive parameters of the snow trout in Tirthan. The bioenergetic trade-offs compromising somatic fitness are evident through the sudden drop in the length-frequency histogram of the sympatric snow trout. Noteworthily, the numbers drop down soon after the first maturity length is attained, which is the point whence the sympatric size structure diverges from the allopatrics. A conjunctive inference drawn from our results on the early maturity and size structure are strongly indicative of the overall demography of the sympatrics being structured by the early length of maturity.
Trade-offs between early maturity and increased fecundity is a plastic response idiosyncratic to many invasive fishes (Olden et al. 2006). Interestingly, the plasticity in the native snow trout in our study is possibly an indication of its effort to compete with the brown trout. A depauperate population structure of the sympatric snow trout as compared to a stable allopatric population, further is suggestive of the brown trout dominance. A ‘maturity-induced mortality’ is known largely from studies on fish species coping with competition pressures (Myers and Doyle 1983; Myers 1984; Stearns 1992; Öhlund et al. 2008; Christie et al. 2018). Such reproduction-maturity trade-offs result in a lower muscle mass due to increased surplus energy directed to reproduction and thus a small post-maturational growth (Myers and Doyle 1983). Our results of reduced post-maturation abundance in the sympatric snow trout seemingly are in line with Myers and Doyle (1983) explanation where an early reproducing snow trout population might be entailing a maturity-induced mortality risk. The maturity-mortality trade-off, as in the sympatric snow trout, is often linked to the ‘live fast, die early’ strategy favoured by evolution to enhance species survival success associated with a fast life-history (Depczynski and Bellwood, 2005). In fact, the fish species naturally displaying a fast life have been reported to benefit in the early life stages with higher offspring number, but trade off their longevity (Wang et al. 2020). In addition to the trade-offs entailing a fast-life history, snow trout are potamodromous and migrate from the mainstream to the warmer spring-fed tributaries for spawning (Sharma 1989; Sehgal 1999; Joshi 2004; Mohan 2005) possibly expending additional energy during the spawning events. Considering the bioenergetics of the sympatrics, we speculate the individuals might be weak enough for a swim back to the mainstream, further reducing their survivability after an early maturation at 175 mm. Although we did not investigate behavioural traits, being beyond the scope of our present goals, yet understanding the snow trout behaviour and migration through future mesocosm experiments would be interesting.
Different native fishes in sympatry have been documented to show variable responses to invasion pressures in terms of condition factor. Condition factor is a measure of ‘fatness’ which assumes a heavier fish at a particular length to be under better conditions (Froese 2006), thus hints on the food available for the individual. The sympatric and allopatric fishes however failed to show any significant differences in their mean condition. Our results corroborate some of the other investigations (Novinger and Rahel 2003; McGrath and Lewis 2007; McHugh and Budy 2005; Hoxmeier and Dieterman 2012) where brown trout sympatry did not affect the native fish condition. It is interesting to note that if the connectivity in the river is maintained and natives are free to emigrate to safer and favourable areas, the mean condition of the fish remains undisturbed (Anderson and Neumann 1996). The sympatric snow trout in Tirthan is mostly benefited by a protected and unaltered river structure, enabling availability of ample food thus showing a ‘good’ condition. We do not compare the condition factors of the allopatric and sympatric in detail though, as the Le Cren’s condition factor doesn’t allow for inter-population comparisons. Relative weight in relation to the species mean weight across all known records could have given us a good picture of the well-being of the snow trout populations (Froese 2006). It is of particular concern though, that most of the available WLR investigations on snow trout are based on different sampling strategies, biased towards specific seasons and with small sample sizes not explicitly representative of a population, thus making inter-population comparisons unlikely. We however, draw clear inferences on the specific population condition as good or bad based on the mean condition factor. Noteworthily, comparing the monthly variations in condition factor across the allopatric and sympatric populations provides a better understanding of the reproductive investments and somatic trade-offs associated with a fast life history strategy of sympatric snow trout population.
Since gonadal development prior to spawning, results in a significantly raised total body weight, the condition factor of a fish is also often used to determine its reproductive activity (Ba et al. 2016), specifically when inferred in conjunction with the reproductive traits. We found the allopatric population with evident differences in condition factor for the month of November as compared to those of peak monsoon (August) and winter (January and February) months. Our results on highest condition factor in monsoon and winter months indicate that snow trout spawn in two bouts across the year when free from brown trout competition pressures. Similar results have widely been reported for snow trout in other Himalayan Rivers, with two spawning bouts, one in monsoons and the other in winters (Baloni and Tilak 1985; Mohan 2005; Joshi et al. 2016). While some studies record a single spawning event (Badola and Singh 1984; Sharma 1984; Singh and Sharma 1995), almost all the existent records do report a spawning peak in monsoons. Furthermore, we report that the allopatric snow trout record a lowest condition factor in November indicating that maximum number of individuals might have already spawned by this month. In the presence of the brown trout though, we observed no distinct peaks for the snow trout, showing a rather uniform condition factor across all months. Research has documented monthly fluctuation in the condition factor reflecting the fish energy budgets (Guy and Willis 1991; Pangle and Sutton 2005) as a function of body lipid reserves at various reproductive stages (Herbinger and Friars 1991). Our results indicate that the presence of aggressive territorial competitor like the brown trout might be resulting in higher expenditure of energy reserves by the sympatric snow trout in Tirthan, probably as an effort to find sites of refuge and rest (Dieterman and Mitro 2019). With mature individuals recorded in high percentage across all months, we discern the sympatrics investing significantly higher energy in reproduction, with an understandable lack of distinct plummet in condition factor. The condition factor results from our study thus strongly indicate a ‘multiple-spawning’ reproductive plasticity (Matthews 2012) in the sympatric snow trout as a probable life-history response to brown trout invasion. Our results on the GSI, fecundity and egg diameter further concur with those of the condition factor. The sympatric snow trout involve in a temporally continuous investment in reproductive energy, as evident by the GSI, clearly hinting a heightened investment in reproduction parcelled out across the year. The question arises what benefit entail for the snow trout investing in a higher GSI? A high GSI is indicative of enhanced reproductive effort in terms of an increase in either the egg numbers or size. Any species would choose to commit for greater energy investments with early reproduction and higher reproductive effort only when the current profits more than outweigh the loss of rejecting a late reproduction with reduced energy investments (Williams 1966a, b). Furthermore, an ensured nutrition-rich environment for the offspring to grow post-hatching strengthens the choice of increased reproductive effort (Magnan et al. 2005). The sympatric snow trout in our study escalating its reproductive energy investments is in line with other studies where interspecific competition for refugia (Mittelbach 1988) and food (Osenberg et al. 1992) escalated the reproductive investments in fishes. We recorded mature individuals in every month for the sympatric snow trout with highest numbers in December followed by July–August as opposed to the allopatrics, indicating a peak spawning in winters with a second albeit smaller spawning bout in monsoons for the sympatric snow trout. A lengthened spawning period in the sympatric snow trout may be providing ample opportunity for producing more offspring, thus enhancing the establishment success, as recorded for other competing species (For instance, Gozlan et al. 2010 and Hôrková and Kováč 2014). This is cemented by the spawning patterns we reported through the condition factor investigations.
A sudden gain in GSI when the population transitions from stage III to V in the sympatric snow trout seemingly indicates the zone where trade-off between somatic and reproductive growth is heightened. That the growth in length was affected to enhance reproductive outputs is evident with small length of highly fecund individuals (stage V) in the sympatric snow trout. Minnows under competitive pressures have been documented to be governed more by their ongoing demographic state than by the seasonal cues for reproduction (Danylchuk and Tonn 2001). An insignificant variation in GSI across the year for the sympatric snow trout is explicit of a state-dependent reproductive activity like what Danylchuk and Tonn (2001) reported in their study, thus indicating that the reproductive life-history in the sympatrics is governed by the demographic state of the population rather than being cued through the seasons alone.
The snow trout in sympatry with brown trout appear to engage in an egg number-egg size trade off in their attempt to reproductively compete with the brown trout. Interestingly, while the sympatric snow trout produce an evidently greater number of eggs than the allopatrics, a confined body cavity at a particular fish length forces a decrease in egg size with increasing fecundity (Klibansky 2006). Trading off egg size for egg number has often been recorded to be a not so fruitful plastic trait as smaller eggs produce relatively smaller juveniles which are low at energy post-hatching putting their survivability at a stake (Einum and Fleming 2000; Skaala et al. 2012, 2019). A greater egg size proffers increased yolk reserve for the offspring to increase its survival rate pre and post hatching, also ensuring a larger offspring size than what a smaller egg would have produced (Sargent et al. 1987; Mousseau and Fox 1998). The snow trout accordingly are at a greater risk considering the competing population is the brown trout which has been reported with a markedly large offspring size directly correlated to the egg size (Dahl 1919; Duarte and Alcaraz 1989; Karjalainen et al. 2016). If the smaller eggs incur costs on the snow trout, why do they choose to lay them?
Larger eggs take longer to hatch than the smaller ones (Rombough 1985). The offsprings from the smaller eggs thus get a change to claim their territories before their larger competitors hatch, thus giving them an advantage of better resource utilization with lesser competition (Cutts et al. 1999). Smaller offspring have been reported to be transported to farther distances downstream with the flow of the river, unlike the larger individuals which tend to stay near the hatching site with their ability to swim against the river current (Jones and Closs 2017). Our results showing a considerably larger egg size in the brown trout than the snow trout in sympatry is a befitting trait for the snow trout juvenile growth. While the snow trout have reported to emerge in about 5.1 days under normal conditions (Thapliyal et al. 2011), the brown trout take a considerably longer duration of about 380–degree days to hatch (Vandeputte and Labbé 2012). Although we report similar spawning bouts for the snow trout and brown trout in sympatry, their non-overlapping emergence timings, owing partially to the differences in their egg sizes seem a beneficial setup for the snow trout with low foraging-space competition with the highly territorial brown trout. The parental care in brown trout with highly yolk laden eggs post-hatching result in alevins with a yolk sac to aid their sustenance without foraging for a few days, further reducing competition for the snow trout. The brown trout fingerlings are aggressive territorials post hatching, thus might raise competitive pressures on the snow trout (Bagenal 1969). It thus seems a profitable strategy for the snow trout to invest greater energy in producing a greater number of smaller offspring which can occupy nutritionally beneficial areas before the brown trout hatch.
Our study is the first of its kind elucidating snow trout life-history plasticity as a response to the brown trout invasion pressures, and should encourage other ichthyologists to test the generality of these findings for snow trout across Himalayan rivers. Overall, we conclude that (1) snow trout respond to brown trout invasion by investing higher energy in reproductive efficiency trading off somatic fitness, thus resulting in a disrupted demography with reduced number of individuals post maturation. Also, (2) the fast life history adaptation by snow trout might benefit its competitive ability for an enhanced probability of survival under invasion threats. Furthermore, we do not contest the contention that the plastic life history responses are uniform for all the sympatric snow trout populations in Himalaya. Idiosyncratic investigations are therefore warranted for the sympatric snow trout in other Himalayan rivers to understand the envelope of responses, which snow trout can exhibit to such invasions. Cessation of brown trout stocking in Tirthan is a prudent recommendation, barring which, brown trout might most likely suppress the native snow trout population to a probable local extinction in the near future.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Code availability
All the R codes used for this study are available from the corresponding author on reasonable request.
References
Al-Chokhachy R, Schmetterling D, Clancy C, Saffel P, Kovach R, Nyce L, Liermann B, Fredenberg W, Pierce R (2016) Are brown trout replacing or displacing bull trout populations in a changing climate? Can J Fish Aquat Sci 73(9):1395–1404
Al-Chokhachy R, Sepulveda AJ (2019) Impacts of nonnative Brown Trout on Yellowstone Cutthroat Trout in a tributary stream. N Am J Fish Manag 39(1):17–28
Anderson R, Neumann R (1996) Length, weight, and associated structural indices. In: Murphy BR, Willis DW (eds) Fisheries techniques, 2nd edn. American Fisheries Society, Bethesda, pp 447–481
Ba K, Thiaw M, Lazar N, Sarr A et al (2016) Resilience of Key Biological parameters of the Senegalese flat Sardinella to overfishing and climate change. PLoS ONE 11(6):e0156143
Badola SP, Singh HR (1984) Spawning of some important coldwater fish of the Garhwal Himalaya. J Bombay Nat Hist Soc 81(1):54–58
Bagenal TB (1969) Relationship between egg size and fry survival in brown trout Salmo trutta L. J Fish Biol 1(4):349–353
Baloni SP, Tilak R (1985) Ecological observations on Schizothorax richardsonii (Gray). J Bombay Nat Hist Soc 82(3):581–585
Behnke RJ (2002) Trout and salmon of North America, 1st edn. The Free Press Simon and Schuster Inc, New York
Belica L (2007) Brown Trout (Salmo trutta): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. http://www.fs.fed.us/r2/projects/scp/assessments/browntrout.pdf. Accessed 09 March 2020
Belk MC, Billman EJ, Ellsworth C, McMillan BR (2016) Does habitat restoration increase coexistence of native stream fishes with introduced Brown Trout: a case study on the Middle Provo River, Utah, USA. Water 8(4):121
Belk MC, Johnson JB (2007) Biological status of leatherside chub: a framework for conservation of western freshwater fishes. In: American Fisheries Society Symposium, vol 53, American Fisheries Society, Bethesda, Maryland, p 67
Bhatt JP, Bhaskar A, Pandit MK (2008) Biology, distribution and ecology of Didymosphenia geminata (Lyngbye) Schmidt an abundant diatom from the Indian Himalayan rivers. Aquat Ecol 42(3):347–353
Billman EJ, Tjarks BJ, Belk MC (2011) Effect of predation and habitat quality on growth and reproduction of a stream fish. Ecol Freshw Fish 20(1):102–113
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85(7):1771–1789
Budy P, Gaeta JW (2017) Brown trout as an invader: A Synthesis of problems and perspectives in North America. In: Lobón-Cerviá J, Sanz N (eds) Brown trout: biology, ecology and management. Wiley, Hoboken, pp 525–534
Budy P, Thiede GP, Lobón-Cerviá J et al (2013) Limitation and facilitation of one of the world’s most invasive fish: an intercontinental comparison. Ecology 94(2):356–367
Chen X, Wang J, Yue W, Lei S, Dobjay S, Li Z, Wang C (2020) Integrated transcriptome provides resources and insights into the adaptive evolution of colonized brown trout (Salmo trutta fario) in the Tibetan plateau. J World Aquacult Soc 51:763–774
Christie MR, McNickle GG, French RA, Blouin MS (2018) Life history variation is maintained by fitness trade-offs and negative frequency-dependent selection. PNAS 115(17):4441–4446
Clark FN (1928) The weight–length relationship of the California sardine (Sardina caerulea) at San Pedro. Division of Fish and Game, Fish Bull. 12, pp 59
Cutts CJ, Metcalfe NB, Taylor AC (1999) Competitive asymmetries in territorial juvenile Atlantic salmon, Salmo salar. Oikos 86:479–486
Dadzie S, Abou-Seedo F, Manyala JO (2008) Length–length relationship, length–weight relationship, gonadosomatic index, condition factor, size at maturity and fecundity of Parastromateus niger (Carangidae) in Kuwaiti waters. J Appl Ichthyol 24(3):334–336
Dahl K (1919) Studies of trout and trout waters in Norway. Salm Trout Mag 18:16–33
Danylchuk AJ, Tonn WM (2001) Effects of social structure on reproductive activity in male fathead minnows (Pimephales promelas). Behav Ecol 12(4):482–489
Depczynski M, Bellwood DR (2005) Shortest recorded vertebrate lifespan found in a coral reef fish. Curr Biol 15(8):288–289
Dieterman DJ, Mitro MG (2019) Stream habitat needs for brook trout and brown trout in the Driftless Area. In: A look back at driftless area science to plan for resiliency in an uncertain future. Special Publication of the 11th Annual Driftless Symposium, La Crosse, Wisconsin, pp 29–44
Duarte CM, Alcaraz M (1989) To produce many small or few large eggs: a size-independent reproductive tactic of fish. Oecologia 80(3):401–404
Einum S, Fleming IA (2000) Selection against late emergence and small offspring in Atlantic salmon (Salmo salar). Evolution 54(2):628–639
Fausch KD, White RJ (1981) Competition between brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta) for positions in a Michigan stream. Can J Fish Aquat Sci 38(10):1220–1227
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. Int J Climat 37(12):4302–4315
Fontana A (1969) Étude de la maturité sexuelle des sardinelles Sardinella eba (Val) et Sardinella aurita (c. etv.) de la région de Pointe-Noire. Cahier ORSTOM Série Océanographie 7:101–113
Froese R (2006) Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22(4):241–253
Froese R, Pauly D (eds) (2011) Some data on Salmo trutta trutta and S. trutta fario . FishBase. World Wide Web electronic publication. www.fishbase.org. version (08/2019) Accessed 6 Mar 2020
Garman GC, Nielsen LA (1982) Piscivority by stocked brown trout (Salmo trutta) and its impact on the nongame fish community of Bottom Creek. Virginia Can J Fish Aquat Sci 39(6):862–869
Goldstein EA, Merrick MJ, Koprowski JL (2018) Low survival, high predation pressure present conservation challenges for an endangered endemic forest mammal. Biol conserv 221:67–77
Gozlan RE et al (2010) Pan-continental invasion of Pseudorasbora parva: towards a better understanding of freshwater fish invasions. Fish Fish 11(4):315–340
Grumbine RE, Pandit MK (2013) Threats from India’s Himalaya dams. Science 339(6115):36–37
Gupta N, Everard M (2019) Non-native fishes in the Indian Himalaya: an emerging concern for freshwater scientists. Int J River Basin Manag 17(2):271–275
Gupta N, Everard M, Nautiyal P, Kochhar I, Sivakumar K, Johnson JA, Borgohain A (2020) Potential impacts of non-native fish on the threatened mahseer (Tor) species of the Indian Himalayan biodiversity hot spot. Aquat Conserv 30(2):394–401
Guy CS, Willis DW (1991) Seasonal variation in catch rate and body condition for four fish species in a South Dakota natural lake. J Freshwater Ecol 6(3):281–292
Hanisch JR, Tonn WM, Paszkowski CA, Scrimgeour GJ (2012) Complex littoral habitat influences the response of native minnows to stocked trout: evidence from whole-lake comparisons and experimental predator enclosures. Can J Fish Aquat Sci 69(2):273–281
Hao F, Chen Y (2009) The reproductive traits of brown trout (Salmo trutta fario L.) from the Yadong River. Tibet Environ Biol Fishes 86:89–96
Hao FH, Chen YF, Cai B (2006) Embryonic development of Salmo trutta fario from Yadong River, Tibet. J Fish China 30(3):289–296
He D, Chen Y (2006) Biogeography and molecular phylogeny of the genus Schizothorax (Teleostei: Cyprinidae) in China inferred from cytochrome b sequences. J Biogeogr 33:1448–1460
Herbinger CM, Friars GW (1991) Correlation between condition factor and total lipid content in Atlantic salmon, Salmo salar L., parr. Aquacult Res 22(4):527–529
Holle BV, Simberloff D (2005) Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86(12):3212–3218
Hôrková K, Kováč V (2014) Different life-histories of native and invasive Neogobius melanostomus and the possible role of phenotypic plasticity in the species’ invasion success. Knowledge Manag Aquat Ecosys 412:01
Hoxmeier RJH, Dieterman DJ (2012) Demographic responses of brook trout to removal of brown trout from a driftless area stream in Minnesota. Final Report STUDY 677. https://files.dnr.state.mn.us/areas/fisheries/lanesboro/research/677report.pdf. Assessed 16 Mar 2020.
Hoxmeier RJH, Dieterman DJ (2016) Long-term population demographics of native brook trout following manipulative reduction of an invader. Biol Invasions 18(10):2911–2922
Johal MS, Sharma A, Dubey VK, Johnson JA, Rawal YK, Sivakumar K (2020) Invasive brown trout Salmo trutta induce differential growth strategies in the native snow trout Schizothorax richardsonii of Himalaya: are natives in unaltered rivers better at picking the gauntlet of invasion? Manuscript submitted for publication
Jones P, Closs G (2017) The introduction of brown trout to New Zealand and their impact on native fish communities. In: Lobón-Cerviá J, Sanz N (eds) Brown trout: biology, ecology and management. Wiley, Hoboken, pp 556–851
Jones DA, Akbaripasand A, Nakagawa S, Closs GP (2019) Landscape features determine brown trout population structure and recruitment dynamics. Ecol Freshw Fish 28(4):554–562
Joshi KD (2004) Artificial breeding and rearing of Schizothorax richardsonii (Gray). Indian J Fish 51(2):233–237
Joshi KD, Das SCS, Khan AU, Pathak RK, Sarkar UK (2016) Reproductive biology of snow trout, Schizothorax richardsonii (1832) in a tributary of River Alaknanda, India and their conservation implications. Int J Zool Investig 2(1):109–114
Karjalainen J, Urpanen O, Keskinen T, Huuskonen H, Sarvala J, Valkeajärvi P, Marjomäki TJ (2016) Phenotypic plasticity in growth and fecundity induced by strong population fluctuations affects reproductive traits of female fish. Ecol Evol 6(3):779–790
Kitano S (2004) Ecological impacts of rainbow, brown and brook trout in Japanese inland waters. Glob Environ Res 8(1):41–50
Klibansky NP (2006) Differences in fecundity and egg size of cod on Georges Bank and in the Gulf of Maine. Dissertation, University of Massachusetts Amherst
Le Cren ED (1951) The length–weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Ecol 20:201–219
Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S (2008) Fish invasions in the world’s river systems: when natural processes are blurred by human activities. PLoS Biol 6(2):404–410
Lobón-Cerviá J, Sanz N (eds) (2017) Brown trout: biology, ecology and management. Wiley, Hoboken, pp 523–640
Lohani V, Ram RN (2018) Length–weight relationship and condition factor based assessment of growth pattern of a cold water fish Schizothorax richardsonii from different habitats of Himalayan region. J Entomol Zool Stud 6(6):765–770
Magnan P, Proulx R, Plante M (2005) Integrating the effects of fish exploitation and interspecific competition into current life history theories: an example with lacustrine brook trout (Salvelinus fontinalis) populations. Can J Fish Aquat Sci 62(4):747–757
McGrath CC, Lewis WM Jr (2007) Competition and predation as mechanisms for displacement of greenback cutthroat trout by brook trout. Trans Am Fish Soc 136(5):1381–1392
McHugh P, Budy P (2005) An experimental evaluation of competitive and thermal effects on brown trout (Salmo trutta) and Bonneville cutthroat trout (Oncorhynchus clarkii utah) performance along an altitudinal gradient. Can J Fish Aquat Sci 62(12):2784–2795
McIntosh AR, McHugh P, Budy P (2012) Salmo trutta L. (brown trout). In: Francis RA (ed) A handbook of global freshwater invasive species. Earthscan, Oxon, pp 285–299
Mittelbach GG (1988) Competition among refuging sunfishes and effects of fish density on littoral zone invertebrates. Ecology 69(3):614–623
Mohan M (2005) Spawning biology of snow trout, Schizothorax richardsonii (gray) from River Gaula (Kumaon, Himalayas). Indian J Fish 52(4):451–457
Mousseau TA, Fox CW (eds) (1998) Maternal effects as adaptations. Oxford University Press, Oxford
Moyle PB (1976) Fish introductions in California: history and impact on native fishes. Biol Conserv 9(2):101–118
Myers RA (1984) Demographic consequences of precocious maturation of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 41(9):1349–1353
Myers RA, Doyle RW (1983) Predicting natural mortality rates and reproduction–mortality trade-offs from fish life history data. Can J Fish Aquat Sci 40(5):612–620
Näslund I, Degerman E, Nordwall F (1998) Brown trout (Salmo trutta) habitat use and life history in Swedish streams: possible effects of biotic interactions. Can J Fish Aquat Sci 55(4):1034–1042
Neumann RM, Allen MS (2007) Size structure. In: Guy CS, Brown ML (eds) Analysis and interpretation of freshwater fisheries data. American Fisheries Society, Bethesda, pp 375–421
Novinger DC, Rahel FJ (2003) Isolation management with artificial barriers as a conservation strategy for cutthroat trout in headwater streams. Conserv Biol 17(3):772–781
Ogle DH (2016) Introductory fisheries analysis with R. Chapman and Hall/CRC Press, Boca Raton
Öhlund G, Nordwall F, Degerman E, Eriksson T (2008) Life history and large-scale habitat use of brown trout (Salmo trutta) and brook trout (Salvelinus fontinalis)—implications for species replacement patterns. Can J Fish Aquat Sci 65(4):633–644
Olden JD, Poff NL, Bestgen KR (2006) Life-history strategies predict fish invasions and extirpations in the Colorado River Basin. Ecol Monogr 76(1):25–40
Osenberg CW, Mittelbach GG, Wainwright PC (1992) Two-stage life histories in fish: the interaction between juvenile competition and adult performance. Ecology 73(1):255–267
Pangle KL, Sutton TM (2005) Temporal changes in the relationship between condition indices and proximate composition of juvenile Coregonus artedi. J Fish Biol 66:1060–1072
Pearson RG, Dawson TP (2003) Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol Biogeograp 12(5):361–371
Peterson DP, Fausch KD, White GC (2004) Population ecology of an invasion: effects of brook trout on native cutthroat trout. Ecol Appl 14(3):754–772
Powell KI, Chase JM, Knight TM (2011) A synthesis of plant invasion effects on biodiversity across spatial scales. Am J Bot 98(3):539–548
Rajvanshi A, Arora R, Mathur VB, Sivakumar K, Sathyakumar S, Rawat GS, Johnson JA, Ramesh K, Dimri N, Maletha A (2012) Assessment of cumulative impacts of hydroelectric projects on aquatic and terrestrial biodiversity in Alaknanda and Bhagirathi Basins. Wildlife Institute of India, Technical Report, Uttarakhand, pp 203
Rasool N, Jan U (2013) Study on the fecundity of Salmo trutta fario (Brown trout) in Kashmir. J Biol Life Sci 4(1):181–193
Rawat MS, Bantwan B, Singh D (2017) Study on the fecundity of brown trout (Salmo trutta fario L.) in River Asiganga, Uttarkashi (Uttarakhand), India. Int J Fish Aquat Stud 5(1):167–217
Rawat MS, Bantwan B, Singh D, Gusain OP (2011) Status of brown trout (Salmo trutta fario L) in Garhwal Himalaya with a note on its morphometric characteristics. Environ Conserv J 12(3):47–52
Regmi B (2019) Phylogenomics and geometric morphometrics define species flocks of snow trout (Teleostei: Schizothorax) in the Central Himalayas. Dissertations, University of Arkansas, Fayetteville
Reznick DN, Ghalambor CK, Crooks K (2008) Experimental studies of evolution in guppies: a model for understanding the evolutionary consequences of predator removal in natural communities. Mol Ecol 17(1):97–107
Reznick DN, Shaw FH, Rodd FH, Shaw RG (1997) Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275(5308):1934–1937
Rombough PJ (1985) Initial egg weight, time to maximum alevin wet weight, and optimal ponding times for chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 42(2):287–291
Sargent RC, Taylor PD, Gross MR (1987) Parental care and the evolution of egg size in fishes. Am Nat 129(1):32–46
Sehgal KL (1999) Coldwater fish and fisheries in the Indian Himalayas: rivers and streams. Fish and fisheries at higher altitudes: Asia. Food and Agriculture Organization of the United Nations Technical Paper 385:41–63
Sharma A, Dubey VK, Nautiyal P, Johnson JA, Rawal YK, Sivakumar K (2019) When nature decides who stays and who goes: Priority effects extirpating the non-native brown trout Salmo trutta fario L. Population from a Himalayan river. Curr Sci 117(2):186–187
Sharma A, Dubey VK, Johnson JA, Rawal YK, Sivakumar K (2020) Spatial assemblage and interference competition of introduced Brown Trout (Salmo trutta) in a Himalayan river network: implications for native fish conservation. Manuscript submitted for publication
Sharma A, Dubey VK, Johnson JA, Rawal YK, Sivakumar K (2021) Is there always space at the top? Ensemble modeling reveals climate-driven high-altitude squeeze for the vulnerable snow trout Schizothorax richardsonii in Himalaya. Ecol Indic 120:106900
Sharma BP (1989) Status of Schizothorax species in the Indian-Chinese sub-continent. In Papers Contributed to the Workshop on the Use of Cyprinids in the Fisheries Management of Larger Inland Water Bodies of the Indo-Pacific, Kathmandu, Nepal, 8–10 September 1988; And, Country Reports Presented at the Fourth Session of the Indo-Pacific Fishery Commission Working Party of Experts on Inland Fisheries, Kathmandu, Nepal, 8–14 September 1988 Food & Agriculture Organisation. No. 405, pp 90
Sharma RC (1984) Tropic dynamics of snow-trout, Schizothorax richardsonii (Gray) of Garhwal, Himalayas. Indian J Anim Sci 54(7):66–69
Shoko AP, Limbu SM, Mrosso HDJ, Mgaya YD (2015) Reproductive biology of female Nile tilapia Oreochromis niloticus (Linnaeus) reared in monoculture and polyculture with African sharptooth catfish Clarias gariepinus (Burchell). SpringerPlus 4(275):1–9
Singh D, Sharma RC (1995) Age and growth of a Himalayan teleost Schizothorax richardsonii (Gray) from the Garhwal Hills (India). Fish Res 24(4):321–329
Skaala Ø, Besnier F, Borgstrøm R et al (2019) An extensive common-garden study with domesticated and wild Atlantic salmon in the wild reveals impact on smolt production and shifts in fitness traits. Evol appl 12(5):1001–1016
Skaala Ø, Glover KA, Barlaup BT et al (2012) Performance of farmed, hybrid, and wild Atlantic salmon (Salmo salar) families in a natural river environment. Can J Fish Aquat Sci 69(12):1994–2006
Sorensen PW, Essington T, Weigel DE, Cardwell JR (1995) Reproductive interactions between sympatric brook and brown trout in a small minnesota stream. Can J Fish Aquat Sci 52(9):1958–1965
Stradmeyer L, Höjesjö J, Griffiths SW, Gilvear DJ, Armstrong JD (2008) Competition between brown trout and Atlantic salmon parr over pool refuges during rapid dewatering. J Fish Biol 72(4):848–860
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stergiou KI, Fourtouni H (1991) Food habits, ontogenetic diet shift and selectivity in Zeus faber Linnaeus, 1758. J Fish Biol 39(4):589–603
Thapliyal M, Bahuguna SN, Thapliyal A (2011) Egg and early larval development of snow trout, Schizothorax richardsonii (cyprinidae), an important food fish of Garhwal Himalaya, Uttarakhand, India. J Mt Res 6:1–8
Theoharides KA (2007) Plant invasions across space and time: factors affecting non-indigenous plant species success during four stages of invasion. Dissertation, University of Massachusetts, Boston
Torrejon-Magallanes J (2019) sizeMat: an R package to estimate size at sexual maturity. CRAN R-Project
Townsend CR (1996) Invasion biology and ecological impacts of brown trout Salmo trutta in New Zealand. Biol Conserv 78(1–2):13–22
Trego CT (2017) Salmonid response to habitat restoration in a high-elevation West Virginia watershed. Dissertation, West Virginia University. https://researchrepository.wvu.edu/etd/6829
Tukey JW (1953) The problem of multiple comparisons. Unpublished manuscript, Princeton University
Tyagi LK, Gupta BK, Pandey A, Bisht AS, Lal KK, Punia P, Singh RK, Mohindra V, Jena JK (2014) Length–weight relationships and condition factor of snow trout, Schizothorax richardsonii (Gray, 1832) from different rivers of the Himalayan region in India. PNAS India Sect B Biol Sci 84(2):299–304
Vandeputte M, Labbé L (2012) Cultured Aquatic Species Information Programme. Salmo trutta. Cultured Aquatic Species Information Programme. In: FAO Fisheries and Aquaculture Department. Rome. http://www.fao.org/fishery/culturedspecies/Salmo_trutta/en Assessed 16 Mar 2020
Vishwanath W (2010) Schizothorax richardsonii (errata version published in 2018). The IUCN red list of threatened species 2010. https://doi.org/10.2305/IUCN.UK.2010-4.RLTS.T166525A6228314.en. Assessed 16 Mar 2020
Wagner T, Deweber JT, Detar J, Sweka JA (2013) Landscape-scale evaluation of asymmetric interactions between brown trout and brook trout using two-species occupancy models. Trans Am Fish Soc 142(2):353–361
Wang HY, Shen SF, Chen YS, Kiang YK, Heino M (2020) Life histories determine divergent population trends for fishes under climate warming. Nat comm 11(1):1–9
Wang X, Xue Y, Ren Y (2013) Length–weight relationships of 43 fish species from Haizhou Bay, central Yellow Sea. J Appl Ichth 29(5):1183–1187
Williams GC (1966a) Adaptation and natural selection. Princeton University Press, Princeton
Williams GC (1966b) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100(916):687–690
Wong D, von Keyserlingk MA, Richards JG, Weary DM (2014) Conditioned place avoidance of zebrafish (Danio rerio) to three chemicals used for euthanasia and anaesthesia. PLoS ONE 9(2):e88030
Zhang CL, Wang WB (1962) A preliminary report on the fishes from Tibet. Acta Zool Sin 14(4):529–536
Acknowledgements
We thank the Department of Science and Technology (DST), Govt. of India for funding this study under the National Mission for Sustaining the Himalayan Ecosystem (NMSHE) project. The authors are grateful to Director and Dean WII for their encouragement. Special thanks are due to Dr. S. Sathyakumar, Nodal Scientist, NMSHE, WII for his advice and guidance throughout this work. We thank the Head, Department of Zoology, Panjab University for support. We acknowledge the Himachal Pradesh and Uttarakhand forest departments for granting permission and supporting the fieldwork. The authors are immensely thankful to the anonymous reviewers and the handling editor, late Professor Olaf Weyl for their constructive comments that greatly improved the paper.
Funding
This study was funded by DST, Govt. of India (Grant Number: DST/SPLICE/CCP/NMSHE/TF-2/WII/2014[G]).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethics approval
Fish sampling permissions were obtained from concerned state fisheries department via letter No. FSH-F-(4)-33/2012-D-IV-4801.
Consent to participate
Not applicable.
Consent for publication
All authors provide their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, A., Dubey, V.K., Johnson, J.A. et al. Introduced, invaded and forgotten: allopatric and sympatric native snow trout life-histories indicate brown trout invasion effects in the Himalayan hinterlands. Biol Invasions 23, 1497–1515 (2021). https://doi.org/10.1007/s10530-020-02454-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02454-8