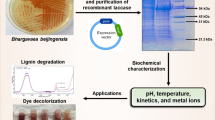
Abstract
Objective
To examine the potential for applications of TthLAC, a monomeric (~ 53 kDa) laccase encoded by the genome of Thermus thermophilus (strain HB 27) which can be produced at low cost in Escherichia coli.
Result
Functional, thermostable and mildly alkalophilic TthLAC of high purity (> 90%) was produced through simple heating of suspended (TthLAC overexpressing) E.coli cells at 65 °C. For reactions of short duration (< 1 h) the temperature for optimal activity is ~ 90 °C. However, TthLAC undergoes slow partial unfolding and thermal inactivation above 65 °C, making it unsuitable for long incubations above this temperature. With different substrates, optimal function was observed from pH 6 to 8. With the substrate, ABTS, catalytic efficiency (K m) and maximum velocity (Vmax) at 60 °C and pH 6.0 were determined to be 2.4 × 103 µM and 0.04 × 103 µM/min respectively. Ultra-pure, affinity-purified TthLAC was used to confirm and characterize the enzyme’s ability to oxidize known (laccase) substrates such as ABTS, syringaldazine and 4-fluoro-2-methylphenol. TthLAC decoloured up to six different industrial dyes, with or without the use of redox mediators such as ABTS.
Conclusions
Unlike versatile laccases from most other sources, which tend to be thermolabile as well as acidophilic, TthLAC is a versatile, thermostable, mildly alkalophilic laccase which can be produced at low cost in E.coli for various redox applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laccases (EC 1.10.3.2 p-diphenol: benzenediol oxygen oxidoreductase) are multi copper-containing phenol oxidases. They catalyze the oxidation of phenolic/non-phenolic and aromatic substrates concomitantly with reduction of oxygen to water (Alexandre and Zhulin 2000; Roberts et al. 2002). Most applications use laccases from fungi. However, thermostable or acid-/alkali-stable laccases of thermophilic origin are also of interest. Amongst laccases of bacterial origin (for a review see Sharma et al. 2007), thermostable laccases have been isolated from Bacillus subtilis (Martins et al. 2002), Aquifex aeolicus VF5(15) (Deckert et al. 1998) and Thermus thermophilus HB strain 27 (Miyazaki 2005).
The subject of this paper is Tth-laccase (TthLAC) from Thermus thermophilus HB 27. We cloned the gene encoding TthLAC, expressed, purified and characterized the enzyme in considerable detail. Chiefly, we find that TthLAC’s previously-reported apparent extreme structural (thermal) stability is only in part due to the thermodynamic stability but is mainly due to its kinetic stability (i.e., slow unfolding). Optimum activity is at ~ 65 °C under equilibrium conditions and not at 92 °C as reported previously (Miyazaki 2005); however, in assays involving short incubations at high temperatures, optimum activity does appear to be seen at ~ 90 °C (because the enzyme denatures slowly). Also, with the substrate, ABTS, the optimum pH of function (pH 6) was found by us to be higher than previously reported (pH 4.7). Further, the pH of optimal function was even higher for other substrates. This suggests that TthLAC works optimally on certain substrates under alkaline rather than acidic conditions.
TthLAC decolorizes a variety of dyes and requires no copper supplementation for folding or activity. We are attempting to fuse it genetically with a xylanase and a cellulase of bacterial origin to improve delignification of hydrolyzed plant biomass, and for bleaching of pulp for the paper industry. The main advantage of TthLAC, deriving from its thermostability, is likely to be a lower requirement of enzyme replenishment steps in large-scale processes.
Materials and methods
Materials
2,6-Dimethoxyphenol and 2,2′-azinobis (3-ethylbenzthiazoline-6-sulfonate) (ABTS), SGZ (syringaldazine) and 4-fluoro-2-methylphenol were from Sigma. Restriction enzymes and DNA ligase were from New England Biolabs (MA, USA). Agarose gel DNA extraction kit was from Qiagen, Hilden, Germany. Genomic DNA of Thermus thermophilus was from The Leibniz Institute DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH), Germany.
Cloning, expression, purification and verification of identity
The gene encoding TthLAC was amplified from the genomic DNA of Thermus thermophilus strain HB 27 through PCR reactions employing Deep Vent enzyme (New England Biolabs), with an annealing temperature of 64 °C, and primer oligonucleotides of the sequences specified below: 5′-ACACACATATGCAAGGCCCTTCATTCCCCGAGC-3′ (forward primer) and 5′-ATGTGAATTCACCCACCTCGAGTACTCCCATCATCCC-3′ (reverse primer). PCR-derived amplicon(s) of the gene incorporated restriction sites for NdeI and EcoRI (shown in bold letters within the primer sequences) at gene termini. Amplicons were double-digested, cloned into the pET28a vector, transformed into E.coli strain XLI Blue to produce plasmids for DNA sequence verification, and also transformed into E.coli strain BL21(DE3)pLysS under a promoter inducible by IPTG for production of TthLAC with an N-terminal 6xHis affinity tag. For production, cultures of 10 ml, and subsequently 1 l lysogeny broth supplemented with 2.5 µg kanamycin/ml and 3.5 µg chloramphenicol/ml were inoculated appropriately and induced by addition of IPTG (at OD600 of 0.6–0.8) with overnight growth at 37 °C. Cells were harvested through centrifugation at 7000×g for 7 min at 4 °C. Suspended cells were heated at 65 °C for 20 min (to lyse cells and precipitate other cytoplasmic proteins), and the supernatant was subjected to Ni–NTA affinity-based chromatography for purification of TthLAC according to standard protocols (Qiagen) under non-denaturing conditions. Purified TthLAC was dialysed against 20 mM Tris/HCl/HCl, pH 7 buffer. SDS–PAGE analysis of purity was performed according to standard methods. Glutaraldehyde crosslinking was used to examine whether TthLAC is monomeric or multimeric (and crosslinkable). TthLAC’s identity was verified through determination of protein intact mass, peptide mass fingerprinting (PMF), and sequencing of a tryptic peptide of mass 1936 Da, on a ESI-Q-TOF mass spectrometer (SYNAPT G2S-HDMS LC–MS-MS, WATERS Corporation), CD and fluorescence spectroscopy.
Circular dichroism (CD) measurements were carried out at 200–250 nm. Spectra were recorded using a cuvette with a path length of 0.1 cm, using protein concentrations of ~ 0.1 mg/ml with a monochromator scanning speed of 50 nm/min. Ellipticity data, θ, was converted into mean residue ellipticity data [θ] using standard methods. Intrinsic tryptophan fluorescence of TthLAC was monitored on a Cary Eclipse fluorimeter. Fluorescence spectra were obtained by excitation at 295 nm. Emission was monitored between 300 and 400 nm, using excitation/emission slit widths of 5 nm and a scanning speed of 100 nm/min. Alterations of structure due to heating (20 to 90 °C) were also studied by monitoring emission spectral changes (with or without normalization to the highest intensity).
Gel filtration chromatography and dynamic light scattering
The purified, concentrated protein was loaded onto a 24 ml Superdex-200 column (GE) on an AKTA Purifier-10 (GE) work-station pre-equilibrated with 20 mM Tris/HCl buffer, 50 mM NaCl (pH 7). TthLAC samples were 500 μl. Fractions were collected, pooled, concentrated, and analysed on SDS-PAGE. TthLAC was subjected to dynamic light scattering (DLS) analysis through measurement of time-dependent changes in scattering of light from molecules in solution to estimate translational diffusion coefficients and use these to infer shape-dependent (and, therefore, size-dependent) hydrodynamic radii using the Stokes–Einstein equation. A Zetasizer Nano ZS90 instrument (Malvern Instruments) was used. Samples were pre-filtered through a 0.2 µm PVDF filters. Solutions of 1 mg protein/ml were studied. Data from 100 acquisitions was averaged to generate intensity versus size, and volume fraction versus size curves.
Temperature- and denaturant-induced structural transitions (Tmid and Cmid)
The midpoints of structural transition for temperature and Gdm.HCl-induced unfolding were determined by CD spectroscopy. Samples (0.1 mg/ml) were heated (20–90 °C) within sealed quartz cuvettes at 0.1, 1, and 3 °C/min to examine scan-rate dependence of unfolding. Similarly, samples (0.3 mg/ml) were incubated overnight with Gdm.HCl (0–6 M), and urea (0–8 M). Circular dichroism signals for mean residue ellipticity were collected at 222 nm, to monitor reduction in intensity accompanying conversion of secondary structure into random-coils, and convert this into ‘fraction unfolded’ data. CD signals were plotted as a function of wavelength, and the sigmoidal unfolding curves were fitted using a sigmoidal Boltzmann function, on OriginPro 8.5, to permit calculation of Tmid and Cmid (to be distinguished from Tm and Cm., since complete unfolding was not achieved).
TthLAC enzyme activity, kinetic parameters and dye decolorization
Enzyme activity parameters were determined with ABTS as substrate. Reaction mixtures containing 0.2 ml 1.33 mM ABTS, 0.5 ml 20 mM Tris/HCl buffer (pH 6.0), and enzyme solution (0.12 µM) were incubated at 60 °C for 30 min. ABTS oxidation was monitored as increased absorbance at 420 nm (A420, ε = 36,000 M−1cm−1). One unit of laccase activity was defined as the amount of enzyme required to oxidize 1 μmol ABTS per min. Optimum pH of activity of TthLAC was determined using BR (Britton and Robinson) buffer system (buffer pH values in the range of 3–8). Effect of pH on TthLAC function upon different substrates was assessed after overnight incubation in buffers of different pH at room temperature, for ABTS (1.33 mM), SGZ (15 µM) and 4-fluoro-2-methylphenol (10 mM). Absorption was measured at 420, 536 and 500 nm, respectively, for ABTS, SGZ and 4-fluoro-2-methylphenol. Optimum temperature of activity was determined by measuring absorption at 420, and 536 nm, respectively, from 20 to 90 °C in 20 mM Tris/HCl buffer (pH 6.0) with ABTS (1.33 mM), and syringaldazine (SGZ; 15 µM). All parameters were plotted as mean of five measurements, with error bars from five independent preparations. Michaelis constant, K m(app), and maximum velocity (V max), were determined for all three substrates (SGZ, ABTS and 4-fluoro-2-methylphenol) at 5–25 µM for SGZ, 0.15–5 mM for ABTS, 0.15–25 mM for 4-fluoro-2-methylphenol. Data was turned into Lineweaver–Burk plots. Decolorizing ability of TthLAC (1.8 µM) was evaluated through overnight incubation, at 60 °C, with six azo and synthetic dyes with different absorption maxima: (i) Dye Orange λmax = 485 nm; (ii) Acid Red dye λmax = 512 nm; (iii) Green Dye λmax = 625 nm; (iv) Naphthol Brilliant Blue λmax = 618 nm; (v) Remazol Brilliant Blue λmax = 592 nm; and (vi) Congo Red λmax = 480 nm. Dyes were dissolved in Milli-Q water (0.1 mg/ml for Dye Orange, Acid Red dye, Green Dye, and 0.005 mg/ml for Naphthol Brilliant Blue (NBB), Remazol Brilliant Blue (RBB), and Congo Red). ABTS (5 mM) was used as a mediator for decolourization of NBB, RBB and Congo Red. The nature of the redox change in ABTS (i.e., whether reversible or not) was verified by passing oxidized (green) ABTS after enzymatic treatment on a desalting (PD-10, GE, Health Care Life Sciences) column and using it once more as a mediator for a fresh batch of decolourization of NBB dye. Effect of pH on dye decolourization was examined for different dyes in 0.1 M BR buffer from pH 3 to 8).
Results and discussion
A well-characterized, hyperthermophile-derived laccase with high thermostability, which is also produced easily at low-cost in ultra-pure form, could be useful in redox and oxidative applications involving dyes and colouring agents, and also in delignification of biomass. We describe work with such a laccase, TthLAC, derived from Thermus thermophilus and produced in Escherichia coli through heterologous overexpression.
Low-cost production and conformation of chemical identity
The gene encoding TthLAC (1.3 kb) was amplified from Thermus thermophilus genomic DNA (Supplementary Fig. 1a) with terminal sequences encoding an N-terminal 6×His affinity tag. The gene was inserted into the pET28a vector. Insertion was confirmed by verifying the restriction digestion-based release of the expected insert (1.3 kb) upon digestion with NdeI and EcoRI (Supplementary Fig. 1b). The vector was transformed into E. coli BL21(DE3)pLysS, and TthLAC was produced through induction with IPTG. Cells were harvested through centrifugation, resuspended and heated at 65 °C to undergo lysis. Heating resulted in the aggregation and precipitation of the bulk of all cytoplasmic proteins but in the survival in solution of the (thermal precipitation-resistant) TthLAC which remained in the supernatant. This TthLAC had > 90% purity, i.e., ready for application, with only three minor contaminating proteins visible on SDS-PAGE (data not shown). This indicates a potential for low-cost production of the enzyme for industrial applications without further purification. However, for detailed characterization studies we purified TthLAC to homogeneity through Ni–NTA-based IMAC affinity purification, as shown in Fig. 1a, which shows a single band on SDS-PAGE with the expected molecular weight of ~ 53 kDa, corresponding to the range of 50–70 kDa in which molecular weights of bacterial laccases generally fall (Sharma et al. 2007). The chemical identity of the purified protein was extensively confirmed by subjecting it to mass spectrometry-based (i) intact mass estimation (Fig. 1b), (ii) peptide mass fingerprinting (PMF) after trypsinolysis (Fig. 1c) and (iii) and sequencing of a tryptic peptide of mass 1936 Da (Supplementary Fig. 2). The intact mass was ~ 52 kDa, smaller than the expected mass of ~ 53.8 kDa, which could be due to either in vivo processing of the C-terminus or to insufficient accuracy of MALDI-TOF analysis. Within the PMF mass spectrum, 9 peptide masses exactly matched the expected trypic peptide masses for TthLAC. MS–MS fragmentation-based sequencing of a peptide of 1936 Da also yielded a sufficient match (Supplementary Fig. 2).
Beta sheet-dominated structure
The far-UV CD spectrum of TthLAC, shown in Supplementary Fig. 3a, reveals a distinct negative band at ~ 218 nm as well as an overall mean residue ellipticity (MRE) at this wavelength of ~ 6500 deg cm2 dmol−1 confirming a predominance of β-sheet-based secondary structure, consistent with the protein’s known crystal structure (Serrano-Posada et al. 2015). The quaternary structural status of TthLAC was confirmed to be a monomer by three methods: (i) Gel filtration chromatographyyielded an elution volume of ~ 17 ml on a ~ 24 ml Superdex-200 column, corresponding to a monomer as shown in Supplementary Fig. 3b. (ii) Glutaraldehyde treatment followed by SDS-PAGE analysis (12% gel) revealed no crosslinking of monomers into oligomers as shown in Supplementary Fig. 3c. Finally (iii) dynamic light scattering (DLS) analysisyielded a single distinct peak with a hydrodynamic radius of ~ 4.3 nm, as shown in Supplementary Fig. 3d consistent with what is expected for a monomeric ~ 50–55 kDa protein.
Ultra-high thermal structural stability
Owing to its origins in Thermus thermophilus, TthLAC is anticipated to be thermostable, assuming that folding occurs to native structure upon expression in E. coli. Heating of TthLAC at 0.1 °C/min between 20 and 90 °C accomplished 20% structural unfolding, reducing the MRE at ~ 222 nm from − 6500 to ~ 5250 deg cm2 dmol−1 as shown in Supplementary Fig. 4a, confirming that the protein is resistant to complete unfolding. Alteration of the heating rate to 1 °C/min (Supplementary Fig. 4b) and 3 °C/min (Supplementary Fig. 4c) reduced the extents of structural unfolding even further, to ~ 14 and ~ 8%, respectively, over the same range of rise in temperature. Since the thermal melting achieved was only ‘partial’, we use the term Tmid to describe the midpoint of the transition, instead of the usual term, Tm, which describes the temperature midpoint of complete thermal unfolding under equilibrium conditions. The Tmid was 73.7 °C for heating at 3 °C/min, 71.9 °C for heating at 1 °C/min and 58.96 °C for heating at 0.1 °C/min. The CD spectra of TthLAC collected at various temperatures between 20 and 90 °C, as shown in Fig. 2a establish that partial unfolding occurs slowly. Normalized fluorescence spectra, shown in, Fig. 2b establish that there is no heating-dependent change in the wavelength of maximal fluorescence emission of TthLAC, indicating negligible effects of heating to 90 °C upon the bulk of the enzyme’s tertiary structure. Such heating rate-dependent partial unfolding indicates a very high degree of thermal structural stability, i.e., the dependence upon heating-rate indicates that the structure is subject to significant kinetic stabilization, in addition to thermodynamic stabilization. Such high thermal stability is observed in proteins of thermophilic origin (Duy and Fitter 2005). Of particular interest was the observation (data not shown) that the otherwise soluble and monomeric protein becomes aggregated during cooling from 90 to 20 °C, for all three rates of heating, although no aggregation is observed at 90 °C. This indicates that aggregation owes not to hydrophobic interactions (since these would be enhanced at higher temperatures) but rather to intermolecular recognition-based interactions occurring between partially-unfolded sections of TthLAC polypeptides. Such aggregation can be easily reduced by reducing protein concentration; something which is easily done with enzymes which are, in any case, used at much lower concentrations that the 0.1 mg/ml used for these experiments. Furthermore, importantly, no aggregation is seen for cooling following heating up to 70 °C.
Panel A. Thermal structural perturbation of TthLAC monitored as change in the far-UV CD from 20 to 90 °C (where the symbols represents: solid square 20 °C, open square 30 °C, solid triangle 40 °C, open triangle 50 °C, solid circle 60 °C, open circle 70 °C, solid star 80 °C, open star 90 °C respectively). Panel B. Normalized fluorescence emission spectra of TthLAC from 20 to 90 °C at every 5 min step (where the symbols represents: solid square 20 °C, open square 25 °C, solid triangle 30 °C, open triangle 35 °C, solid circle 40 °C, open circle 45 °C, solid star 50 °C, open star 55 °C, solid hexagon 60 °C, open hexagon 65 °C, left solid triangle 70 °C, left open triangle 75 °C, sphere 80 °C respectively). Panel C. Denaturant-induced unfolding of TthLAC monitored as change in far-UV CD signal as a function of urea concentration (0 to 8 M). (where the symbols represents:- solid square native laccase (with no Gdm.HCl), open square 0.1 M, solid triangle 0.2 M, open triangle 0.3 M, solid circle 0.4 M, open circle 0.5 M, solid diamond 0.75 M, open diamond 1 M, solid star 1.25 M, open star 1.5 M, left solid triangle 1. 75 M, left open triangle 2 M, solid hexagon 2.5 M, open hexagon 3 M, asterisk 3.5 M, down solid triangle 4 M, down open triangle 4.5 M, solid sphere 5 M, horizontal line 5.5 M, cross(X) 6 M, half filled square 6.5 M, half filled circle 7 M, half filled triangle 7.5 M, half filled star 8 M respectively). Panel D. Denaturant-induced unfolding monitored as change in far-UV CD signal as a function of Gdm.HCl concentration (0 to 6 M). (where the symbols represents:- solid square native laccase (with no Gdm.HCl), open square 0.1 M, solid triangle 0.2 M, open triangle 0.3 M, solid circle 0.4 M, open circle 0.5 M, solid diamond 0.75 M, open diamond 1 M, solid star 1.25 M, open star 1.5 M, left solid triangle 1. 75 M, left open triangle 2 M, solid hexagon 2.5 M, open hexagon 3 M, asterisk 3.5 M, down solid triangle 4 M, down open triangle 4.5 M, solid sphere 5 M, open sphere 5.5 M, cross(×) 6 M respectively)
High chemical stability based primarily on electrostatic interactions
Upon overnight incubation with denaturants, incomplete unfolding was achieved even with 8 M urea, although another denaturant, Gdm.HCl, achieved complete unfolding. We use the term, Cmid, to describe the midpoint of the structural transitions seen with denaturants, i.e., Cmid is the denaturant concentration at which half of the molecular population is structurally altered (whether through a partial, or complete, structural unfolding). The data shown in Supplementary Fig. 5a and b suggest a Cmid of 2.79 M for TthLAC in urea, with a maximum of ~ 30% unfolding achieved even in 8 M urea. A Cmid of 1.19 M was obtained with Gdm.HCl for a complete unfolding transition. CD spectra at different denaturant concentrations are shown in Fig. 2c and d for both denaturants. Typically, Gdm.HCl is more effective at perturbing structure than urea because urea disrupts only hydrogen bonding interactions, whereas Gdm.HCl disrupts both hydrogen bonding and electrostatic interactions. As a rule of thumb, Gdm.HCl-induced unfolding concentrations are about half of the value for urea-induced unfolding transitions. With TthLAC, this rule is completely violated; not only is the Cmid for urea not 2.38 M (i.e., 2 × 1.19 M, which is the Cmid for Gdm.HCl), there is no unfolding even with 8 M urea. Thus, it can be concluded that the structure of TthLAC is stabilized much more significantly by electrostatic interactions than by hydrogen bonding interactions. This makes the enzyme vulnerable to high ionic-strength environments, but otherwise quite robust for applications.
Temperature and pH optima of TthLAC activity with different substrates
In assays of short duration, e.g., involving 10–30 min of incubation the optimum activity of TthLAC against the substrate ABTS was ~ 90 °C, close to the value of 92 °C given by Miyazaki (2005) using chromatographically purified Tth-laccase (TthLAC). For comparison, against the same substrate (ABTS), using enzymes from other sources (i) Martins et al. (2002) reported optimal activity at 75 °C for the B. subtilis CotA laccase; (ii) Reiss et al. (2011) reported optimal activity at 70 °C for Bacillus pumilus laccase; and (iii) Sherif et al. (2013) reported optimum activity at 60–70 °C for a multicopper oxidase from Streptomyces coelicolor. Therefore, Fig. 3 at first glance, TthLAC appears to be capable of functioning at much higher temperatures than laccases from mesophile organisms. However, we found that in assays of longer duration, e.g., involving 60–180 min of incubation, TthLAC shows optimum activity at ~ 65 °C. The data for 180 min of incubation is shown in Fig. 3. This suggests that TthLAC undergoes a slow, time-dependent, temperature-dependent, heating-rate dependent, unfolding or partial unfolding of some section(s) of its structure which are responsible for its enzymatic activity, with such unfolding resulting in slow thermal inactivation at temperatures above 65 °C. We have already observed that partial unfolding of TthLAC is achieved through heating, in a heating-rate dependent manner, owing to kinetic (rather than thermodynamic) thermal stabilization of some regions of TthLAC’s structure. It is this partial unfolding which is responsible for the lower observed temperature of optimum activity (65 °C) in assays of long duration. The original report by Miyazaki (2005) mentions only a duration of 10 min of incubation used in assays of thermal stability. It is possible, therefore, that this author overestimated the stability and optimum temperature of activity of TthLAC. The same conclusion could also potentially apply to various other studies of laccase activity, i.e., it is possible that the optimum temperatures of function of various mesophile-derived laccases could also have been correspondingly overestimated through assays involving 10 min of incubation.
Regarding the stability of the substrate(s) and the product(s) of reactions conducted at high temperature(s), TthLAC was observed to oxidize the substrate ABTS into a stable green coloured product at high temperature as shown in Supplementary Fig. 6a. The substrate, 4-fluoro-2-methyl phenol, is turned into a stable pale pink product (data not shown). However, with a different substrate, SGZ, an unstable pink product is obtained, which disappears quickly, with higher rates of fading of colour at higher temperatures. This suggests that SGZ is acted upon twice by two different copper centres in TthLAC (possessing four copper centres). With only appearance of colour being monitored, maximum activity was observed with SGZ at 80 °C using 5 min of incubation (data not shown; similar to the observation of an optimum of 90 °C with 10–30 min incubations for the substrate ABTS).
Different pH optima were determined with different substrates. With ABTS, as shown in Supplementary Fig. 6b, the pH optimum was ~ 6, although significant activity was also seen at mildly basic pH. The absorption spectrum of oxidized ABTS is shown in Supplementary Fig. 6C. With SGZ, the pH optimum was ~ 7. With 4-fluoro-2-methyl phenol, the pH optimum was ~ 8. The ability of TthLAC to function at mildly alkaline pH could cause it to have an advantage over fungal laccases that usually only operate at acidic pH (Margot et al. 2013). For example, the pH optima for ABTS oxidation at 37 °C by the B. subtilis CotA laccase, and the B. pumilis laccase, respectively, are ~ 3 (Martins et al. (2002) and ~ 4 (Reiss et al. 2011). Notably, the pH optimum against ABTS for TthLAC reported by Miyazaki (2005) is ~ 4.5, whereas we determined that the pH optimum is ~ 6. One possible reason for the difference is that the enzyme used by Miyazaki was a partially-purified enzyme, whereas our experiments were performed with affinity-purified enzyme of high purity.
Kinetic properties of purified TthLAC
Analyses were performed with the substrates ABTS, SGZ, and 4-fluoro-2-methyl phenol either at (or close to) their respective temperature and pH optima. The K m for ABTS was 2.4 × 103 µM at 60 °C, 3.8 × 103 µM at 55 °C, and 4.4 × 103 µM at 50 °C (Fig. 4a and b; Table 1). The K m for SGZ was 15.3 µM at 40 °C, and 16.7 µM at 35 °C (Fig. 4c and d; Table 2). The kcat was 4.8 s−1 for ABTS at 60 °C and it was 10.3 s−1 for SGZ at 40 °C. We used a lower temperature for experiments with SGZ because the developed colour fades rapidly at higher temperatures, making measurements difficult. For comparison, Martins et al. (2002) reported apparent K m values of 106 ± 11 and 26 ± 2 µM for ABTS and SGZ, respectively, with corresponding kcat values of 16.8 ± 0.8 and 3.7 ± 0.1 s−1 for the laccase from Bacillus subtilis endospore, at 37 °C. Interestingly, Miyazaki (2005) reported apparent K m and kcat of 0.9 mM and 24.6 s−1 for ABTS, and 1.88 mM and 6.47 s−1 for SGZ, respectively, for TthLAC, but this data does not mention the temperature. Reiss et al. (2011) reported apparent K m and kcat of 80 ± 4 μM and 291 ± 2.7 s−1 for ABTS 680 ± 27 μM and 11 ± 0.1 s−1 for 2,6-DMP and corresponding kcat value of 66 ± 1.5 s−1 for SGZ for B. pumulis laccase.
Panel A. Kinetic profiles of TthLAC acting upon ABTS. From highest peak to lowest one the concentration of substrate are 5 mM (sphere), 2.5 mM (open circle), 1.25 mM (plus), 0.625 mM (cross), 0.3125 mM (solid line), 0.15625 mM (filled circle). Panel B. Lineweaver–Burk plot of activity (ABTS). Panel C. Kinetic profiles of TthLAC acting upon SGZ From highest peak to lowest one the concentration of substrate are 25 µM (sphere), 20 µM (filled square), 18 µM (open square) 15 µM (open circle),12 µM (filled triangle) 10 µM (open triangle) 8 µM (vertical solid line) respectively. Panel D. Lineweaver–Burk plot of activity (SGZ)
Dye decolourization
There are various reports of the use of fungal laccases for decolourization of different dyes (Margot et al. 2013). In our studies, purified TthLAC was able to itself decolourize Green Dye, Orange Dye and Acid Red Dye. In contrast, TthLAC was unable to decolourize NBB, RBB, or Congo Red by itself; however, with ABTS (5 mM) acting as redox mediator, TthLAC decolourized NBB, RBB, or Congo Red, into clear solutions within a few hours of incubation (Fig. 5a–c), with the ‘already used’ ABTS proving to be reusable through reversal of its redox status. This suggests that TthLAC and ABTS can be used for ‘biobleaching’. As a redox mediator, ABTS acts like an ‘electron shuttle’ between laccases and compounds which are not natural substrates, promoting their oxidation (Bourbonnais and Paice 1996; Sharma et al. 2007). Different dyes are decolourized at different rates, based on the redox potential of the enzyme and the structure of the dye (Wong and yu 1999; Nyanhongo et al. 2002; Zhang et al. 2006). Figure 5d shows that the absorption of Green Dye is decreased by TthLAC over time. Maximum decolourization is seen with Congo Red in the pH range of 5–6; with Green Dye and Orange Dye in the pH range of 6–8; and with NBB and RBB in the pH range of 7–8 (data not shown). Evidently, thermostable TthLAC efficiently decolourizes a variety of dyes, with or without ABTS acting as a mediator (or as an electron shuttle) at temperatures of 60–65 °C.
Decolorization of different dyes using by purified TthLAC Panel A. Green dye, Panel B.Congo Red, Panel C. Naphthol Brilliant Blue Dye (where C is the control (i.e., without enzyme) R is the reaction mixture containing various dyes after enzymatic treatment with purified TthLAC. Panel D. Absorption spectrum for green dye after decolourization (solid line—control dye solution, dotted line- buffer solution and dashed line is decolorized dye by TthLAC
Conclusions
Specific laccases have specific uses in different industries. However, most are thermo-labile and function only at acidic pH. In contrast, TthLAC is thermostable. It is also thermophilic as well as mildly alkalophilic in its activity upon some substrates. Its thermostability allows it to be extracted easily from overexpressing E. coli cells, by simply heating bacterial cell suspensions at 65 °C, leading to > 90% purity because of the thermal aggregation and precipitation of all other proteins. We have reported details of the activity of the enzyme upon different substrates. After scale-up, TthLAC could potentially be used for oxidation of compounds, decolourization of dye pollutants, degradation of industrial effluents containing high concentrations of chlorides, detergents, sulfates, and metal ions, and delignification of biomass at acidic, neutral or mildly alkaline pH.
References
Alexandre G, Zhulin IB (2000) Laccases are widespread in bacteria. Trends Biotechnol 18:41–42
Bourbonnais R, Paice MG (1996) Enzymatic delignification of Kraft pulp using laccase and a mediator. Tech Assoc Pulp Pap Ind J 79:199–204
Deckert G, Warren PV, Gaasterland T, Young WG, Lenox AL, Graham DE, Overbeek R, Snead MA, Keller M, Aujay M, Huber R, Feldman RA, Short JM, Olson GJ, Swanson RV (1998) The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature 392:353–358
Duy C, Fitter J (2005) Thermostability of irreversible unfolding α-amylases analyzed by unfolding kinetics. J Biol Chem 280:37360–37365
Margot J, Bennati-Granier C, Maillard J, Blánquez P, Barry DA, Holliger C (2013) Bacterial versus fungal laccase: potential for micropollutant degradation. AMB Exp 3:63–77
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277:18849–18859
Miyazaki K (2005) A hyperthermophilic laccase from Thermus thermophilus HB 27. Extremophiles 6:415–425
Nyanhongo GS, Gomes J, G¨ubitz GM, Zvauya R, Read J, Steiner W (2002) Decolorization of textile dyes by laccase from a newly isolated strain of Trametes modesta. Water Res 36:1449–1456
Reiss R, Ihssen J, Thöny-Meyer L (2011) Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum. BMC Biotechnol 11:9–20
Roberts SA, Weichsel A, Grass G, Hazzard JT, Tollin G, Rensing C, Montfort WR (2002) Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc Natl Acad Sci USA 99:2766–2771
Serrano-Posada H, Centeno-Leija S, Patricia Rojas-Trejo S (2015) X-ray-induced catalytic active-site reduction of multicopper oxidase: structural insights into the proton-relay mechanism and O2-reduction states. Acta Cryst D 71:2396–2411
Sharma P, Goel R, Capalash N (2007) Bacterial laccases. World J Microbiol Biotechnol 23:823–832
Sherif M, Waung D, Korbec B, Mavisakalyan V, Flick R, Brown G, Abou-Zaid M, Yakunin AF, Master ER (2013) Biochemical studies of the multicopper oxidase (small laccase) from Streptomyces coelicolor using bioactive phytochemicals and site-directed mutagenesis. Microb Biotechnol 6:588–597
Wong L, Yu J (1999) Laccase-catalyzed decolorization of synthetic dyes. Water Res 33:3512–3520
Zhang M, Wu F, Wei Z, Xiao Y, Weimin Gong (2006) Characterization and decolorization ability of a laccase from Panus rudis. Enzym Microb Technol 39:92–97
Acknowledgements
AK and NK would like to thank the Centre of Excellence in Protein Science, Design and Engineering (CPSDE) funded by the Ministry of Human Resource Development (MHRD), Government of India, for financial support.
Supporting information
Supplementary Figure 1—(A) Amplification of the gene encoding TthLAC along with restriction sites and 6xHis affinity tag (1.3 kb). (B) Restriction-digestion based verification of the presence of the clone (gene) in the pET 28a Vector (size 5369 bp) through release of the insert of 1.3 kb upon digestion with NdeI and EcoRI.
Supplementary Figure 2—Fragmentation of a tryptic peptide of mass 1936 Da using ESI-TOF MS-MS mass spectrometery and analysis by the Biolynx tool of the Mass Lynx software of the WATERS G2SHDMS.
Supplementary Figure 3—(A) Far-UV circular dichroic (CD) spectrum of TthLAC. (B) Chromatographic profile of of TthLAC eluting from a Superdex-200 gel filtration column. (C) SDS-PAGE showing TthLAC incubated with glutaraldehyde. (D) Dynamic light scattering-derived hydrodynamic diameter data for TthLAC.
Supplementary Figure 4—Tmid values for the partial unfolding transitions of TthLAC.
Supplementary Figure 5—Denaturant-induced unfolding of TthLAC.
Supplementary Figure 6—(A) Oxidization of ABTS into a green product by TthLAC. (B) Variation of TthLAC activity with pH. (C) Absorption spectrum of ABTS after enzymatic treatment at pH 6.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumari, A., Kishor, N. & Guptasarma, P. Characterization of a mildly alkalophilic and thermostable recombinant Thermus thermophilus laccase with applications in decolourization of dyes. Biotechnol Lett 40, 285–295 (2018). https://doi.org/10.1007/s10529-017-2461-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2461-8