Abstract
Objectives
To develop a rapid, dual-parameter, plate-based screening process to improve production and secretion rate of glucose oxidase simultaneously in Aspergillus niger.
Results
A morphology engineering based on CaCO3 was implemented, where the yield of GOD by A. niger was increased by up to 50%. Analysis of extracellular GOD activity was achieved in 96-well plates. There was a close negative correlation between the total GOD activity and its residual glucose of the fermentation broth. Based on this, a rapid, plate-based, qualitative analysis method of the total GOD activity was developed. Compared with the conventional analysis method using o-dianisidine, a correlation coefficient of −0.92 by statistical analysis was obtained.
Conclusion
Using this dual-parameter screening method, we acquired a strain with GOD activity of 3126 U l−1, which was 146% higher than the original strain. Its secretion rate of GOD was 83, 32% higher than the original strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose oxidase (GOD, β-d-glucose:oxygen-1-oxidoreductase) has diverse applications in the pharmaceutical, food, biotechnology and other industries (Ferri et al. 2011; Yu et al. 2017). It catalyzes the oxidation of β-d-glucose to d-glucono-δ-lactone and H2O2, using O2 as electron acceptor. Currently, the most widely-used microbial source for the fermentative production of GOD is Aspergillus niger which has generally-recognized-as-safe (GRAS) status for GOD production in the food industry (Guo et al. 2010).
Aspergillus niger efficiently produces GOD. Genes are present that encode one intracellular and three secreted glucose oxidases (Pel et al. 2007). Most of the commercially-produced GOD usually need cell disruption to release the enzyme (Ostafe et al. 2014). This means that strains with a high secretion ratio are of value because the separation and purification processes becomes more economic (Bankar et al. 2009; Taubert et al. 2000). To obtain improved strains, evolutionary approaches, such as genome shuffling or random mutagenesis, have been used (Li et al. 2014; Storms et al. 2005). But the success of any strain improvement mainly depends on the number of targets that can be screened after improvement. High-throughput screening plays a key role in searching for improved strains. Advances in high throughput and high-content screening (HTS and HCS) have been fostered by the development of specific routines that use robot- and computer-assisted technologies to automatize the tasks, allowing screening of a large number of compounds in a short time (Bellomo et al. 2017).
Herein, we have implemented morphology engineering using CaCO3, which increased GOD activity and the growth stability of Aspergillus niger in shake-flask culture. Further, we describe a rapid, dual-parameter, plate-based screening process to get strains with both higher GOD activity and higher GOD secretion from a mutant library. For extracellular GOD activity, we carried out a 96-well plate-based analysis. For total GOD activity, there was a close negative correlation between the total GOD activity and the residual glucose in the broth. Based on this, an efficient and qualitative analysis for total GOD was developed.
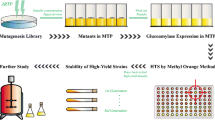
The technical route of this dual-parameter, plate-based screening strategy was illustrated in Fig. 1.
Rapid and plate-based screening process diagram. Firstly a mutant library was constructed using a combination of two mutagenesis methods—the NaNO2 and the atmospheric and room temperature plasma (ARTP). Then a plate-based screening process including cultivation of A. niger in 48-well microtiter plate and rapid analysis of both glucose oxidase and its secretion rates was carried out. The red arrow in the right corner represent the selected improved strain. After screening, to save room and labour, the chosen candidates were momentarily preserved in 48-well-plates. Furthermore, the morphology engineering in flasks using CaCO3 was also highlighted. Images of the terminal broth which initially contained 2 and 10% CaCO3 show that increasing the addition of CaCO3 can greatly decreased the size of the pellets and increased the GOD activity
Materials and methods
Strains and media
Aspergillus niger SDFY was kindly donated by Fuyang Corporation (Shandong, CHN). The seed agar medium was used for growing strains and harvesting spores. It contained (per liter): glucose 60 g, urea 0.2 g, KH2PO4 0.13 g, MgSO4·7H2O 0.02 g, corn steep liquor 1 g, pH 6.5–7.0. Then 10 g CaCO3, and 20 g agar were added. It was sterilized at 121 °C for 20 min at a pressure of 0.1 MPa. Cells were grown at 37 °C for 60 h. The spore suspension (1 to 3 × 108 spores ml−1) was transferred to flasks or micro-titer plates with screening medium which contained (per liter): glucose 200 g, KH2PO4 0.13 g, urea 0.2 g, MgSO4·7H2O 0.02 g, corn steep liquor 1 g, pH 6.5–7.0. 100 g CaCO3 was added before sterilization at 115 °C for 20 min.
Construction of mutant library
We constructed a mutant library using a combination of two mutagenesis procedures—the NaNO2 and the atmospheric and room temperature plasma (ARTP) methods. Aspergillus niger were suspended in 100 ml sterile normal saline in a 250 ml flask with about 150 glass beads (3 mm diameter). This suspension was manually shaken for 1 min to separate the spores from the mycelium and was filtered through sterile absorbent cotton to remove the mycelium. One ml of suspension was transferred to a sterile test-tube, mixed with equal volume of 0.1 M NaNO2 and 2 ml acetate buffer (0.1 M, pH 4.5). After incubation at 28 °C for 90 s, 20 ml Na2HPO4 (0.7 M, pH 8.6) was added to terminate the effect of NaNO2. In this case, the lethality was about 50%. Then 10 μl of the treated suspension was dipped onto the sterilized metal plate (5 mm diameter) and was treated with the atmospheric and room temperature plasma (ARTP mutagenesis breeding machine, CHN) for 90 s at a gas flow rate of 10 l min−1 and 10 mm irradiation distance. The final lethality was about 80%.
Culture conditions
Spores of Aspergillus niger growing on agar plates were washed with 100 ml sterile water and diluted to OD600 = 1. This suspension was transferred to flasks or MTPs (micro-titer plates) for further cultivation. The inoculation size was 2% (v/v). For MTPs, each well was filled with 700 μl sterile screening medium. The inoculated MTPs were cultured at the incubator with shaking at 37 °C, 220 rpm. For cultivation and validation with shake-flask culture, a 500 ml flask was loaded with 50 ml screening medium. Incubation was at 37 °C and 220 rpm.
After the screening, mutant candidates were temporarily preserved in 48-well plate with 1 ml ager medium as shown in Fig. 1—“Mutants in MTPs for further analysis”. The process was introduced in detail in our previous work (Zhu et al. 2017).
Analysis of glucose oxidase
GOD activity was measured using o-dianisidine was described in detail by Chu et al. (1996). Here, this method was improved to be conducted in 96-well plate to achieve an efficient detection process. The details were listed in the result section.
Analysis of glucose
Glucose was analyzed using a glucose kit. In this assay, glucose was specifically oxidized to generate a product which reacted with a dye to generate a color (λ = 500 nm) whose intensity was proportional to glucose concentration. The method was rapid, simple, sensitive, and suitable for high throughput.
Cell disruption
1.5 ml fresh fermentation broth was taken and put into a 2 ml EP tube. Each tube filled with 5 glass beads (3 mm diameter). The cells were disrupted using a broken crusher for 120 s. In the process, the sample tank was filled with crushed ice to keep a low temperature and reduce the loss of enzyme activity. After cell disruption, the EP tube was centrifuged at 50,000×g for 10 min in the cold. The supernatant was used for the determination of total enzyme activity.
Result and discussion
Morphology engineering for Aspergillus niger growth in 500 ml flasks
In the process of growing A. niger in 500 ml flasks, we encountered an absence of reproducibility of the same inocula. Hyphae of A. niger formed macroscopic mycelia or pellets which were large (~ 4 mm diameter) and inconformity in size, and, more pressingly, both the residual sugar and the GOD activity had a huge discrepancy (Fig. 2). In fact, one of the outstanding but often problematic characteristics of filamentous fungi is the complex morphology in submerged culture. Its morphological characteristics varied between freely dispersed mycelia and distinct pellets of aggregated biomass (Krull et al. 2010). Herein, we found a convenient strategy to effectively control the morphogenesis of A. niger cultivation by adding CaCO3 to the medium.
The morphology engineering of A. niger in shake-flask. Results derived from different mutants showed the good effects of CaCO3 in morphology engineering of A. niger. It increased both of the free GOD and total GOD activity (showed by the column) and the cultivation reproducibility (showed by the error bar). All the experiments were performed in triplicate, the error bar represented the standard deviation of the GOD activity
Initially, the medium already contained 2% (w/v) CaCO3 as an inducer of GOD and to neutralize the gluconic acid produced during the fermentation (Hatzinikolaou and Macris 1995). Before the depletion of the CaCO3, the pellet sizes were uniform and smaller than 1 mm in diameter. But when the CaCO3 was consumed at about 30 h of fermentation, the pellets grew bigger and their sizes became variable. So we believed that CaCO3 played a key role in the morphogenesis of A. niger cultivation. Based on this assumption, we stepwise increased the initial CaCO3, and found 10% (w/v) CaCO3 in the initial medium was the optimal. In this case, after 44 h fermentation, a little CaCO3 was left in the medium, the pellets sizes (~ 1 mm diameter) were uniform and much smaller than when 2% (w/w) CaCO3 was used (see Fig. 1). After optimization, shake-flask cultures in triplicate showed good reproducibility (Fig. 2). Furthermore, the final GOD activity increased by up to 50% (Fig. 2). Addition of CaCO3 did not add extra steps to subsequent enzyme activity analysis. So subsequent experiments involving shake-flask culture were with 10% (w/v) CaCO3.
Here we put forward two conceivable explanations of why CaCO3 can control the morphogenesis of A. niger. Aggregation has been described as the consequence of random encounters of conidia in suspension (Grimm et al. 2004). Hence we believed that the core shell pellets were initially taking shape on CaCO3 particles. Increasing the initial CaCO3 could provide more “cores” for the pellets. When CaCO3 was depleted, the newborn hyphae could only adhere to the existing pellets, leading to a large pellet. Driouch et al. (2012) revealed that the large pellets were only active in a 200 mm surface layer using fluorescence-based resolution of GFP expression. This matches with the critical penetration depth for nutrients and O2 typically observed for fungal pellets. Thus, GOD activity increased here mainly due to a reduced thickness of the biomass layer via smaller pellets as well as the core shell structure. Also, the pH control by the CaCO3 may also contribute to conidia aggregation which triggered by germination and hyphal length growth. As Krull et al. (2010) reported, in the early phase of cultivation the aggregation of A. niger conidia is dominantly affected by the pH and at pH 5.5 A. niger tends to form small pellets. Zeta potential measurements showed that the isoelectric point of A. niger spores is around pH 2. At higher pH values, the absolute zeta potential increases and cell walls of A. niger become negatively charged and electrostatic repulsion causes separation of the aggregated cells. This corresponds to the amount of aggregation decreasing at higher pH values.
Rapid analysis of the secretion rate of GOD
Rapid analysis of the extracellular GOD activity
GOD activity is usually detected using o-dianisidine. Oxidation of o-dianisidine forms a quinoneimine dye that is measured at 500 nm. However, this method is wasteful of time and materials: the time taken to analyse one sample is about 8 min. To achieve an efficient screening process, analysis was better when conducted in 96 MTPs (microtitre plates). However, the chemical equilibrium in MTP is poorer than that in the cuvette, which meant a series of optimizations related to the reagent dosages and reaction times were needed. Herein, we carried out the optimizations. In brief, 10 µl cell-free supernatant was diluted to keep the activity of GOD in the range of 1000–4000 U l−1, where the GOD activity had a linear relationship with the absorbance at 500 nm (Fig. 3a). Then the diluent was mixed with a blend of detection solution containing 50 μl 10% (w/w) glucose, 240 μl 0.21 M o-dianisidine and 10 μl 30 U horse radish peroxidase ml−1. Immediately, the kinetic parameter was detected by the microplate reader every 30 s UP TO 5 min. GOD activity was indicated by the maximum absorbance increment. Comparison of the results of the same samples using the spectrophotometer showed that the two methods had a good correlation at 0.95 (Fig. 3b).
Rapid analysis of GOD activity. a Relationship between GOD activity and the absorbance at 500 nm in 96-well-plate using o-dianisidine. The GOD activity had a linear relationship with the absorbance at 500 nm in the range of 1000–4000 U l−1; b correlation between the analysis in 96-well-plate and the spectrophotometer assay
Indirect analysis of the total GOD activity
The analysis of the total GOD activity which consists of extracellular and cell-bonded GOD, also needs complex procedures. Cell walls need to be broken by either grinding, homogenization, or chemical treatment. All of these methods are time-consuming and also filamentous fungi are resistant to these methods due to their specific morphologies.
Here, we found a correlation between the total GOD activity and the residual glucose. As shown in Fig. 4a, the concentration of glucose in broth decreased with the accumulation of GOD. This was probably because the host strain could continuously express GOD. Wherever the GOD was expressed, it catalyzed the oxidation of β-d-glucose to d-glucono-δ-lactone. For strains with different yields, this correlation was still effective. Figure 4b shows that the residual glucose of strains with different GOD production capacity still showed a strong negative correlation with their activity of the total GOD and the correlation R2 was −0.92. Based on this, it can be speculated that the residual glucose can be employed to indirectly characterize the yield of GOD of different strains. A glucose kit proved to be cost-effective and suitable for measurements in 96-well plates.
Establishing measurement priority and high-throughput analysis of the secretion rate of GOD activity
The purpose of the experiment was to screen a high-yield production strain with a high secretion. To make the screening more efficient, the extracellular GOD activity was detected as the first priority. Mutants with 1.5 times higher GOD activity than the mother strain were chosen for detection of higher secretion. For the first round, > 2500 mutant candidates were screened (Fig. 5a). With the threshold set at 1500 U l−1, 243 candidates were gated for secretion detection. In this process, glucose concentration, free GOD, and total GOD were denoted by G1, F1, and T1 respectively. Obviously, secretion was F1/T1. As we describe above that G1 has a negative correlation with T1, so that the secretion rate was positive correlation with F1 × G1 (denoted by Ratio in Fig. 5b).
Dual-parameter screening and characteristics of mutant candidates. a Exceeding 2500 mutant candidates (showed by the column) were screened. Mutants with 1.5 times higher GOD activity than the mother strain was chosen for secretion rate analysis; b 243 chosen candidates from the first round of screening were further screened for high secretion rate with the novel analysis method. The top 20 were showed in red column; c the further verification data in flask of the top 20 candidates. The value in each row was normalized to the corresponding value of the mother strain. A1, B1…D3 was the name of each candidate
After analyzing the ratio of 243 candidates, the top 20 (red column in Fig. 5b) were chosen for further verification. Free GOD, total GOD and the secretion rate (S. Rate) were detected (see Fig. 5c). The value in each row was normalized to the corresponding value of the mother strain. Except for the mutant H2, all candidates showed an increase in both of the activity of free GOD and total GOD. Mutant F1 had the highest free GOD activity, which was 2.2 times higher than the mother strain. The absolute free GOD activity of F1 was 2680 U l−1. Mutant D1 was the top one in aspect of total GOD activity—3180 U l−1. For the secretion of GOD, mutant G1 was especially high, with an absolute value of 96%, accompanied by a relative high GOD activity.
Except for mutant H2, the genetic stability of these mutants was verified by subculture for three successive generations. During this process, most mutants were sifted out. Finally, the mutant F1 was selected. As shown in Fig. 6a, subculture for three successive generations (G1, G2, G3), the free GOD, total GOD and the secretion value remained stable with respective average values of 2606, 3126 U l−1 and 83.3% and coefficients of variation of 3.7, 2.6 and 1.6%, respectively.
Characteristic of improved mutant F1. a The free GOD, total GOD and the secretion rate of strain F1. Subculture for three successive generations (G1, G2, G3), the free GOD, total GOD and the secretion rate of strain F1 kept stable; b the growth process of F1 compared with the mother strain. It showed an increase in the GOD activity and the glucose consumption
The growth process of F1 in flasks was tracked and compared with the mother strain. As is shown in Fig. 6b, mutant F1 had a significantly increase in the production of free GOD. Glucose consumption by F1 was also much higher than that of the mother strain.
Conclusion
High-content screening refers to any technique or process in which multiple measurements are obtained from a single well. Compared with single parameter assays, HCS provides richer contextual and concurring information that help better illustrate both the behavior and the mechanism of action of small molecules and genetic manipulations (An and Tolliday 2010). Herein, a dual-parameter high throughput screening process was carried out. A rapid and plate-based analysis method for GOD based on o-dianisidine was realized in 96-well-plates. A novel analysis method, based on a relationship between the activity of the total GOD and the residual glucose left in fermentation broth, was constructed for indirect analysis of total GOD. With the help of this method, a mutant with total GOD activity with an activity of 3200 U l−1, 2.2 times higher and 83% greater secretion was obtained. Morphology engineering using CaCO3 was efficient for GOD production in shake-flask culture. After the discovery of improved strains, expression patterns analysis or comparative genomics approach will be meaningful to conduct the directional optimization in aspect of genetic recombination and metabolic regulation (Mayr and Bojanic 2009).
References
An WF, Tolliday N (2010) Cell-based assays for high-throughput screening. Mol Biotechnol 45:180–186
Bankar SB, Bule MV, Singhal RS, Ananthanarayan L (2009) Glucose oxidase—an overview. Biotechnol Adv 27:489–501
Bellomo F, Medina DL, De Leo E, Panarella A, Emma F (2017) High-content drug screening for rare diseases. J Inherit Metab Dis 40:601–607
Chu J, Li Y, Yu J (1996) Study on glucose oxidase fermentation coupled with membrane dialysis. Appl Biochem Biotechnol 67:59–70
Driouch H, Hansch R, Wucherpfennig T, Krull R, Wittmann C (2012) Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng 109:462–471
Ferri S, Kojima K, Sode K (2011) Review of glucose oxidases and glucose dehydrogenases: a bird’s eye view of glucose sensing enzymes. J Diabetes Sci Technol 5:1068–1076
Grimm LH, Kelly S, Hengstler J, Gobel A, Krull R, Hempel DC (2004) Kinetic studies on the aggregation of Aspergillus niger conidia. Biotechnol Bioeng 87:213–218
Guo Y, Zheng P, Sun J (2010) Aspergillus niger as a potential cellular factory: prior knowledge and key technology. Sheng Wu Gong Cheng Xue Bao 26:1410–1418
Hatzinikolaou D, Macris BJ (1995) Factors regulating production of glucose oxidase by Aspergillus niger. Enz Microb Technol 17:530–534
Krull R, Cordes C, Horn H, Kampen I, Kwade A, Neu TR, Nortemann B (2010) Morphology of filamentous fungi: linking cellular biology to process engineering using Aspergillus niger. Biosyst Eng II 121:1–21
Li W, Chen G, Gu L, Zeng W, Liang Z (2014) Genome shuffling of Aspergillus niger for improving transglycosylation activity. Appl Biochem Biotechnol 172:50–61
Mayr LM, Bojanic D (2009) Novel trends in high-throughput screening. Curr Opin Pharmacol 9:580–588
Ostafe R, Prodanovic R, Nazor J, Fischer R (2014) Ultra-high-throughput screening method for the directed evolution of glucose oxidase. Chem Biol 21:414–421
Pel HJ, De Winde JH, Archer DB, Dyer PS et al (2007) Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol 25:221–231
Storms R, Zheng Y, Li H, Sillaots S, Martinez-Perez A, Tsang A (2005) Plasmid vectors for protein production, gene expression and molecular manipulations in Aspergillus niger. Plasmid 53:191–204
Taubert J, Krings U, Berger RG (2000) A comparative study on the disintegration of filamentous fungi. J Microbiol Method 42:225–232
Yu D, Shi Y, Wang Q, Zhang X, Zhao Y (2017) Application of methanol and sweet potato vine hydrolysate as enhancers of citric acid production by Aspergillus niger. Bioresour Bioproc 4:35
Zhu X, Arman B, Chu J, Wang Y, Zhuang Y (2017) Development of a method for efficient cost-effective screening of Aspergillus niger mutants having increased production of glucoamylase. Biotechnol Lett 39:739–744
Acknowledgements
This work was financially supported by National Major Scientific and Technological Special Project (2012YQ15008709) and National Key Research and Development Program (2017YFB0309302).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Sun, J. & Chu, J. High-content screening of Aspergillus niger with both increased production and high secretion rate of glucose oxidase. Biotechnol Lett 40, 103–110 (2018). https://doi.org/10.1007/s10529-017-2442-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2442-y