Abstract
Objectives
To develop an efficient cost-effective screening process to improve production of glucoamylase in Aspergillus niger.
Results
The cultivation of A. niger was achieved with well-dispersed morphology in 48-deep-well microtiter plates, which increased the throughput of the samples compared to traditional flask cultivation. There was a close negative correlation between glucoamylase and its pH of the fermentation broth. A novel high-throughput analysis method using Methyl Orange was developed. When compared to the conventional analysis method using 4-nitrophenyl α-D-glucopyranoside as substrate, a correlation coefficient of 0.96 by statistical analysis was obtained.
Conclusion
Using this novel screening method, we acquired a strain with an activity of 2.2 × 103 U ml−1, a 70% higher yield of glucoamylase than its parent strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucoamylase (EC 3.2.1.3) is widely used in food industries (Kumar and Satyanarayana 2009). It hydrolyzes α-1,4- and α-1,6-glucosidic bonds to release glucose from non-reducing ends of starch and related poly- and oligosaccharides. Aspergillus niger, which has generally recognized as safe (GRAS) status, has an excellent ability to produce glucoamylase (Tang et al. 2015). However, A. niger with improved production of glucoamylase is still required. At present, numerous evolutionary approaches, such as genome shuffling or random mutagenesis, have been used to procure improved strains, but the success of any strain improvement program mainly depends on the number of targets that can be screened after mutagenic treatment (Wahler and Reymond 2001).
An integrated high-throughput screening strategy, which includes high-throughput cultivation and the matched high-throughput analysis, has a key role in search for improved strains. Microtiter plates (Geddie et al. 2004) are attractive for high-throughput cultivation due to their small working volume and high degree of parallelization (Long et al. 2014). For A. niger, two limiting factors hamper the high-throughput screening process: (1) A. niger grows poorly in the microtiter plates because its thallus covers the surface of liquids and adheres to the wall of the well. (2) Glucoamylase assay is typically determined by a spectrophotometric method using 4-nitrophenyl α-D-glucopyranoside as a substrate (Lu et al. 2011), which is hydrolysed into glucose and p-nitrophenol. After adjusting to alkaline pH, the p-nitrophenol is determined at 405 nm to measure enzyme activity. However, this method is less economical when used for screening very large number of mutants with the cost of 4-nitrophenyl α-D-glucopyranoside at $200 per 103 strains. Furthermore, this method is labor intensive and time consuming.
To overcome these two bottlenecks and to obtain A. niger strains with high activity of glucoamylase, a novel high-throughput screening process was developed. By using microtiter plates for cultivation, this significantly increased the throughput of samples compared to the cultivation in flasks. Further, a novel analysis method using Methyl Orange has decreased the cost to $0.1 and 1 h for 103 samples assay. The technical route of this high-throughput screening strategy is illustrated in Fig. 1.
High throughput screening process diagram. This high-throughput process included high-throughput cultivation of A. niger in 48-well microtiter plate and high-throughput analysis with Methyl Orange. The process of mutants cultivation in 48-well microtiter plate was also highlighted. It was convenient and fast to trace back to high-yield mutants when clone-picker is not available
Materials and methods
Strains and glucoamylase production
Aspergillus niger DS03043 was kindly donated by DSM Corporation (Delft, The Netherlands). Potato/dextrose/agar (PDA) was used for harvesting spores after growth for 72 h at 34 °C. Malto-dextrose medium (MDO3) (pH 5.6) was used for propagation and glucoamylase production. It contained: 70 g maltose monohydrate l−1, 25 g peptone l−1, 12.5 g yeast extract l−1, 2 g K2SO4 l−1, 1 g KH2PO4 l−1, 0.5 g MgSO4·7H2O l−1, 0.03 g ZnCl2 l−1, 0.02 g CaCl2 l−1, 0.009 g MnSO4·H2O l−1, 0.3 g FeSO4·7H2O l−1. The strain was grown in a 500 ml baffled flask containing 50 ml MDO3 at 30 °C for 30 h with shaking at 180 rpm.
Construction of mutant library
Aspergillus niger were suspended in 100 ml sterile normal saline in a 250 ml flask with about 150 glass beads (3 mm diam). This was manually shaken for 1 min to separate the spores from the mycelium and was filtered through sterile absorbent cotton to remove the mycelium. 10 μl of the filtered suspension (OD600 = 1) was dipped onto the sterilized metal plate (5 mm diam) and was treated with the atmospheric and room temperature plasma (ARTP Mutagenesis Breeding Machine, CHN) for 100 s at a gas flow rate of 10 lmin−1 and 10 mm irradiation distance (Lu et al. 2011), which showed about 80% lethality. The candidate mutants on the metal plate were washed with 990 μl sterilized saline solution and then were cultivated in 48-well microtiter plates as follows.
Growth of mutants in 48-well microtiter plates
48-well microtiter plates with 1 ml PDA in each well were used for growth of the mutants. Mutagenic spores suspension (20 μl) was equally allotted into the wells of the microtiter plates. This volume theoretically contained 2–3 clones for each well after dilution (Tan et al. 2013). Cultivation for 72 h at 30 °C, one single clone in the majority of wells could be obtained. The spores grown on the surface of PDA were washed later with 700 µl sterile MDO3 medium. This suspension was cultured in another sterile empty 48-well microtiter plates at 34 °C, 170 rpm.
Analysis of glucoamylase using 4-nitrophenyl α-D-glucopyranoside as a substrate
Enzyme activity is expressed in AGI units, which is related to an officially assigned glucoamylase standard. One AGI unit is defined as the amount of enzyme that produces 1 μM glucose per min at 60 °C and at pH 4.3 from soluble starch substrate. The cell-free supernatant (1.65 × 104 g for 10 min at 4 °C) was diluted to ensure the enzyme activity was in the range of 10–40 AGI ml−1. 20 μl supernatant (prewarmed for 5 min at 37 °C) was mixed with 230 μl p-NPG substrate (2 g 4-nitrophenyl α-D-glucopyranoside l−1 acetate buffer pH 4.3). After incubation at 37 °C for 20 min, the reaction was stopped by adding 100 μl 0.3 M Na2CO3 with immediate absorbance reading at 405 nm on a plate reader. The standard sample (E.C 3.2.1.2, 10, 115) was used to make a standard curve with R2 > 0.999. All the off-line measurements were performed in triplicate.
Rapid analysis the pH of broth using Methyl Orange
90 μl cell-free supernatant (1.65 × 104 g for 10 min at 4 °C) was mixed with 10 μl of 1% (w/w) Methyl Orange. The absorbance reading at 507 nm was obtained on a plate reader after shaking in situ for 5 min.
Results and discussion
High-throughput screening using a microtiter plate is based on robotic handling of small amounts of materials, and equivalent advances in the detection of relevant signals and interpretation of the data (Persidis 1998). In aspect of the strains screening, high-throughput cultivation and high-throughput analysis procedures constitute a complete screening process (Dorr et al. 2016).
Optimization of condition for Aspergillus niger growth
Initially, A. niger grew poorly in 48-well plates (Fig. 2a). Growing thallus covered the surface of the broth and adhered to the wall, which led to inhomogeneous growth of each well. After 72 h fermentation, the medium remaining was insufficient for analysis and the activity of glucoamylase was low as well.
Optimization of micro-culture in 48-well microtiter plate. a Morphology of strains growing in microtiter plate before (left) and after (right) optimization; b glucoamylase activity for each combination of shaking speed and medium volume set out in Table 1; c glucoamylase activity of 10 strains cultivated for 36, 48, 72 h respectively
Based on the conditions of the fermentation in shaking flask and the empirical optimizing presets used in microtiter plate, variables like inoculum age, inoculum size and addition of glass beads (1–4 mm diam) (Table 1) were taken into consideration to accomplish the optimization of the cultivation in microtiter plate (Liccioli et al. 2011).
Glass beads were added to each well and were anticipated to increase the aeration and decrease the adherent growth. But this eventually proved useless by using different numbers and size. It was also found that the inoculum age and size influenced the yield insignificantly but the condition of 18 h (inoculum age) and 3% (inoculum size) worked well in reducing the adherent growth.
For the shaking speed and the volume of medium in each well, 150/180/220/250 rpm and 500/600/700/800 µl were chosen for optimization. The results in Fig. 2b showed that the optimal combination pair was 150 rpm and 800 μl. To address the issue of insufficient medium for analysis, we decreased the culture time from 72 to 48 h, as the glucoamylase activity at 48 h is correlated with that of 72 h at a correlation coefficient of 0.972 (Fig. 2c).
To summarize, the optimized conditions of the micro-culture were 800 μl medium in each well at 150 rpm, for 48 h at predetermined 34 °C.
Construction of high-throughput analyzing method
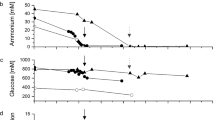
During the fermentation of Aspergillus niger, the pH of broth negatively correlated with the activity of glucoamylase (Fig. 3a). The initial pH was 6, then it decreased to around pH 3.5 along with the glucoamylase accumulation. For strains with different yields this correlation was still proved to be effective (Fig. 3b). That was to say, the pH could indirectly characterize the yield of glucoamylase. Herein, a pH indicator Methyl Orange was chosen to denote the ultimate pH of the broth as its color changes at pH 3.1–4.4. As shown in Fig. 3c, the absorbance of Methyl Orange at 507 nm showed a correlation of −0.995 with the pH of the buffer at the range of 3–4.
Construction of high throughput analyzing method. a The growth curve of Aspergillus niger. The pH of broth was negative correlation with the activity of glucoamylase; b strains with different yields showed a negative correlation between their pH and glucoamylase activity as well; c the standard curve of the absorbance of Methyl Orange at 507 nm and the pH of phosphate buffer solution. The absolute values of the Methyl Orange were 1, 2, 5 and 10 μl for 1, 2, 5 and 10% (v/v) respectively; d correlation between the Methyl Orange method and the 4-nitrophenyl α-D-glucopyranoside assay
An experiment to find the appropriate dosage of Methyl Orange was also carried out. As shown in Fig. 3c, 90 μl supernatant of fermentation broth mixed with 10 μl 1% (w/w) Methyl Orange showed the best correlation and highest resolving ability.
The reliability of the Methyl Orange method was verified by comparison with 4-nitrophenyl α-D-glucopyranoside assay. After cultivation for 48 h in 48-well plates, samples were randomly selected and detected by both methods and a correlation coefficient of 0.96 was obtained by statistical analysis.
High-throughput screening of Aspergillus niger with high yield of glucoamylase
Using the novel high-throughput culture and analysis methods, 1.2 × 103 mutants obtained by atmospheric and room temperature plasma were screened for high-producing strains. The top 20 high-yield mutants were then selected for genetic stability analysis in the way of subculture for three successive generations. During this process, most of them were sifted out. Finally, three genetic stable mutants with more than 50% increase in yield were picked. Compared to the parent strain with a activity of 1.31 × 103 AGI ml−1 in flasks, the glucoamylase activity of mutants VII-F-6 (2.23 × 103 AGI ml−1), III-F-2 (2.09 × 103 AGI ml−1) and IV-D-1 (1.97 × 103 AGI ml−1) were increased by 70, 59 and 50% respectively (Fig. 4b).
Mutants screened by novel high-throughput method. a 1.21 × 103 mutants by atmospheric and room temperature plasma were screened with the Methyl Orange method, and the top 100 mutants were chosen for further verification; b mutants III-F-2, VII-F-6, IV-D-1 with activity of 2.09 × 103 AGI ml−1, 2.23 × 103 AGI ml−1, and 1.97 × 103 AGI ml−1 showed the best activity and genetic stability among all mutants
Conclusion
High-throughput technology is enthusiastically applied to drugs, genes, protein, interactions and microorganisms screening (Mayr and Bojanic 2009). Here, it was used for strains screening. A relationship between the activity of glucoamylase and its pH of the fermentation broth was discovered. And a high-throughput process including cultivation and screening for improved A. niger with high yield of glucoamylase was established. With the aid of this platform, an improved A.niger mutant with 70% higher yield than its parent strain was attained and proved genetically stable. The results also emphasize the importance of high-throughput strains screening and its extensive applicability (An and Tolliday 2010). After the discovery of upgraded strains, the analysis of different expression patterns and comparative genomics approach will be useful to conduct the directional optimization in aspect of genetic recombination and metabolic regulation.
References
An WF, Tolliday N (2010) Cell-based assays for high-throughput screening. Mol Biotechnol 45:180–186
Dorr M, Fibinger MP, Last D, Schmidt S, Santos-Aberturas J, Bottcher D, Hummel A, Vickers C, Voss M, Bornscheuer UT (2016) Fully automatized high-throughput enzyme library screening using a robotic platform. Biotechnol Bioeng 113:1421–1432
Geddie ML, Rowe LA, Alexander OB, Matsumura I (2004) High throughput microplate screens for directed protein evolution. Protein Eng 388:134–145
Kumar P, Satyanarayana T (2009) Microbial glucoamylases: characteristics and applications. Crit Rev Biotechnol 29:225–255
Liccioli T, Tran TMT, Cozzolino D, Jiranek V, Chambers PJ, Schmidt SA (2011) Microvinification-how small can we go? Appl Microbiol Biotechnol 89:1621–1628
Long Q, Liu X, Yang Y, Li L, Harvey L, McNeil B, Bai Z (2014) The development and application of high throughput cultivation technology in bioprocess development. J Biotechnol 192:323–338
Lu Y, Wang L, Ma K, Li G, Zhang C, Zhao H, Lai Q, Li H-P, Xing X-H (2011) Characteristics of hydrogen production of an Enterobacter aerogenes mutant generated by a new atmospheric and room temperature plasma (ARTP). Biochem Eng J 55:17–22
Mayr LM, Bojanic D (2009) Novel trends in high-throughput screening. Curr Opin Pharmacol 9:580–588
Persidis A (1998) High-throughput screening. Advances in robotics and miniturization continue to accelerate drug lead identification. Nat Biotechnol 16:488–489
Tan J, Chu J, Hao Y, Guo Y, Zhuang Y, Zhang S (2013) High-throughput system for screening of cephalosporin C high-yield strain by 48-deep-well microtiter plates. Appl Biochem Biotechnol 169:1683–1695
Tang W, Pan A, Lu H, Xia J, Zhuang Y, Zhang S, Chu J, Noorman H (2015) Improvement of glucoamylase production using axial impellers with low power consumption and homogeneous mass transfer. Biochem Eng J 99:167–176
Wahler D, Reymond JL (2001) High-throughput screening for biocatalysts. Curr Opin Biotechnol 12:535–544
Acknowledgements
This work was financially supported by Royal DSM (Delft, The Netherlands) and partially supported by National Basic Research Program (973 Program 2013CB733600), NWO-MoST Joint Program (2013DFG32630).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Arman, B., Chu, J. et al. Development of a method for efficient cost-effective screening of Aspergillus niger mutants having increased production of glucoamylase. Biotechnol Lett 39, 739–744 (2017). https://doi.org/10.1007/s10529-017-2291-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2291-8