Abstract
Objectives
Since uptake of xylose limits its fermentation, we aimed to identify novel sugar transporters from Scheffersomyces stipitis that allow xylose uptake and fermentation by engineered Saccharomyces cerevisiae.
Results
An hxt-null S. cerevisiae strain, lacking the major hexose transporters (hxt1Δ-hxt7Δ and gal2Δ) but having high xylose reductase, xylitol dehydrogenase and xylulokinase activities, was transformed with a genomic DNA library from S. stipitis. Four plasmids allowing growth on xylose contained three genes encoding sugar transporters: the previously characterized XUT1 permease, and two new genes (HXT2.6 and QUP2) not previously identified as xylose transporters. High cell density fermentations with the recombinant strains showed that the XUT1 gene allowed ethanol production from xylose or xylose plus glucose as carbon sources, while the HXT2.6 permease produced both ethanol and xylitol, and the strain expressing the QUP2 gene produced mainly xylitol during xylose consumption.
Conclusions
Cloning novel sugar transporters not previously identified in the S. stipitis genome using an hxt-null S. cerevisiae strain with a high xylose-utilizing pathway provides novel promising target genes for improved lignocellulosic ethanol production by yeasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass, an abundant and renewable feedstock, is an attractive raw material for bioethanol production since it does not compete with food and feed production (Sarkar et al. 2011; Caspeta et al. 2013; Nogueira et al. 2013). The major fermentable sugars from hydrolysis of this lignocellulosic biomass are d-glucose and d-xylose, and to obtain an economically feasible industrial process for bioethanol production it is necessary to efficiently ferment both sugars into ethanol (Lin and Tanaka 2006; Stambuk et al. 2008; Kim et al. 2012). The development of robust microorganisms for xylose fermentation is required for efficient bioethanol production, and abundant research has been devoted to improve xylose utilization by recombinant strains of Saccharomyces cerevisiae, an industrial yeast that it is not able to ferment xylose (Matsushika et al. 2009; Young et al. 2010; Kim et al. 2012; Laluce et al. 2012; Cai et al. 2012; Nielsen et al. 2013; Sànchez Nogué and Karhumaa 2015).
A major focus in metabolic engineering for xylose fermentation has been to establish and improve an intracellular xylose catabolic pathway in S. cerevisiae. Two strategies are currently being used: overexpression of xylose isomerase (XI), or overexpression of xylose reductase (XR) and xylitol dehydrogenase (XDH). Since both pathways transform xylose into xylulose, it is also required to overexpress xylulokinase (XK) that will enhance the entrance of xylulose into the pentose-phosphate pathway. However, independently of the xylose utilizing pathway used, the uptake of xylose across the S. cerevisiae plasma membrane occurs through a large family of hexose transporters, encoded by the HXT1-HXT17 and GAL2 genes, that has been reported to have substantial metabolic flux control, especially when the intracellular pathway is optimized (Kötter and Ciriacy 1993; Hamacher et al. 2002; Lee et al. 2002; Gárdonyi et al. 2003; Sedlak and Ho 2004; Saloheimo et al. 2007; Bertilsson et al. 2008; Parachin et al. 2011; Gonçalves et al. 2014).
A strategy to overcome this problem has focused on the isolation of heterologous sugar transporters with better xylose-transporting properties for functional expression in xylose fermenting S. cerevisiae cells (Saloheimo et al. 2007; Hector et al. 2008; Katahira et al. 2008; Madhavan et al. 2009; Runquist et al. 2009, 2010; Young et al. 2011, 2014; Tanino et al. 2012; Diao et al. 2013). However, up to now very few heterologous xylose transporters have been characterized in S. cerevisiae, and recent surveys with over 34 heterologous known and putative sugar transporters from seven different organisms revealed that only half of the expressed permeases allowed significant utilization of xylose by S. cerevisiae cells (Young et al. 2011, 2014). Furthermore, most of these studies have just analyzed the capacity of the transformed cells to grow on xylose, and very few have verified the contribution of the permeases for xylose fermentation by the recombinant strains.
The yeast Scheffersomyces stipitis (formerly Pichia stipitis) is capable of fermenting a variety of sugars present in the lignocellulosic biomass, is one of the best xylose fermenting species found in nature, but unfortunately has several drawbacks for industrial fuel ethanol production mainly due to its low tolerance to the fermentation product (Toivola et al. 1984; Nigam 2001; Jeffries et al. 2007; Farias et al. 2014; Parambil and Sarkar 2014). Nevertheless, this yeast has been a major source of xylose-utilizing genes for genetic engineering of S. cerevisiae, including not only the necessary activities of the XR and XDH enzymes (encoded by the XYL1 and XYL2 genes), and other enzymes of the pentose-phosphate pathway (Kötter and Ciriacy 1993; Jin et al. 2005; Matsushika et al. 2009), but also many heterologous sugar transporters. The analysis of xylose transport by this yeast revealed complex kinetics, indicating the presence of several permeases with differing properties, a situation also found for hexose transport in S. cerevisiae (Kilian and Van Uden 1988; Does and Bisson 1989).
Up to now 15 monosaccharide transporters from S. stipitis have been identified and functionally analyzed in S. cerevisiae. These include the hexose transporters SUT1-SUT4, some of them able to mediate xylose transport in yeast cells (Weierstall et al. 1999; Katahira et al. 2008; Runquist et al. 2010; Moon et al. 2013), but also several other putative transporters encoded by the XUT1-XUT7, AUT1, HGT2, RGT2 and STL1 genes, of which few have been shown to allow significant xylose transport, fermentation and/or growth when expressed in S. cerevisiae recombinant strains (Du et al. 2010; Young et al. 2011, 2012; Moon et al. 2013).
We have recently developed a hxt-null S. cerevisiae strain, lacking the major hexose transporters (hxt1Δ-hxt7Δ and gal2Δ), with a high activity of the XR, XDH and XK enzymes due to overexpression (through an integrative plasmid) of the XYL1, XYL2 and XKS1 genes, and analyzed the impact that several S. cerevisiae HXT permeases have in anaerobic batch fermentations using xylose, glucose, or xylose plus glucose as carbon sources (Gonçalves et al. 2014). In the present report, a genomic library of S. stipitis was screened with this hxt-null strain to identify novel sugar transporters that allow xylose uptake and fermentation when expressed in S. cerevisiae, a required metabolic engineering approach to increase catabolic rates in recombinant yeasts for biofuel applications.
Materials and methods
Strains and media
The yeast strains used in this study are listed in Table 1. Escherichia coli strain DH5α was grown in lysogeny broth (1 % tryptone, 0.5 % yeast extract, 0.5 % NaCl) supplemented with ampicillin (at 100 mg l−1). Yeasts were grown in rich YP medium (1 % yeast extract, 2 % Bacto-peptone) or synthetic complete (SC) medium lacking uracil (0.67 % yeast nitrogen base without amino acids, supplemented with adequate auxotrophic requirements), containing 2 % maltose, glucose, fructose or xylose. The pH of the medium was adjusted to pH 5 with HCl. When required, 2 % Bacto-agar and 0.5 mg aureobasidin A l−1 (Takara Bio, Japan) were added to the medium.
Molecular genetic techniques
Standard methods for bacterial transformation, DNA manipulation and analysis were employed (Ausubel et al. 1995). The yeast S. stipitis (strain NBRC1687, Watanabe et al. 2011) genomic DNA library was constructed in the pPGK plasmid (Table 1). Genomic DNA was extracted using the MasterPure Yeast DNA purification kit (Epicenter, USA), digested with BamHI, and 3–5 kb fragments were isolated by gel electrophoresis. These DNA fragments were cloned into the BamHI site of plasmid pPGK. The genomic library was transformed into the S. cerevisiae hxt-null strain DLG-K1 (Table 1), which has high XR, XDH, and XK activities (Gonçalves et al. 2014), using the Yeastmaker yeast transformation kit (Clontech Laboratories, USA). The hxt-null DLG-K1 strain is available to the scientific community for research non-commercial purposes from the Collection of Microorganisms, DNA and Cells of Universidade Federal de Minas Gerais (member of the World Federation of Culture Collections, see http://www.wfcc.info/ccinfo/index.php/collection/by_id/1029/) under accession number UFMG-CM-Y5856. Strains transformed with the pPGK-derived plasmids were selected in SC medium lacking uracil and supplied with aureobasidin A and 2 % maltose or xylose. Plasmids were extracted from the recombinant yeast strains using the Zymoprep Yeast Plasmid Miniprep II kit (Zymo Research, USA). Plasmids recovered from yeast transformants were analyzed by restriction digestion and/or diagnostic PCR using primers pPGK_seq_F and pPGK_seq_R (Table 1). These same primers were used to sequence the 5′ and 3′ ends of the DNA fragments present in the recombinant pPGK vectors (ACTGene Analíses Moleculares Ltda, Brazil), and the sequences were used to identify the cloned genes by comparison with the known genome of S. stipitis (Jeffries et al. 2007).
Phylogenetic analysis
Amino acid sequences of S. stipitis sugar transporters were obtained from NCBI (www.ncbi.nlm.nih.gov/protein/) with the following accession numbers: AUT1 (GI:4836720); HGT2 (GI:4851832); HXT2.4 (GI:4850978); HXT2.6 (GI:4838414); QUP2 (GI:4840652); RGT2 (GI:4840859); STL1 (GI:4838168); SUT1 (GI:4851252); SUT2 (GI:4838413); SUT3 (GI:4839762); SUT4 (GI:4850775); XUT1 (GI:4839826); XUT2 (GI:4852047); XUT3 (GI:4851844); XUT4 (GI:4840896); XUT5 (GI:4840252); XUT6 (GI:4841106); and XUT7 (GI:4851701). Sequences were first aligned (see Supplementary Fig. 1) using Clustal Omega (Sievers et al. 2011), and then the phylogenetic tree was calculated and constructed using the neighbor joining method with Mega 5 (Tamura et al. 2011).
Growth and fermentations conditions
Yeast strains were pre-grown in SC medium containing 2 % (w/v) maltose for 36 h at 28 °C. The cells were collected by centrifugation at 6000×g for 5 min at 4 °C, washed twice with sterile water, and inoculated (0.1 ± 0.02 g of dry yeast l−1) into SC medium containing 2 % (w/v) maltose, xylose, glucose or fructose. Cells were grown aerobically at 28 °C with shaking (160 rpm) in Erlenmeyer flasks filled to 1/5 of the volume with medium. Cellular growth was followed from OD600 values and culture samples were harvested regularly, centrifuged (5000×g, 1 min), and their supernatants used for the determination of substrates and fermentation products as described below. For batch fermentations, cells were pre-grown as described above and inoculated at a high cell density (5.5 ± 0.5 g of dry yeast l−1) into 25 ml synthetic SC medium containing 2 % glucose or/and xylose. Anaerobic batch fermentations were performed at 30 °C in closed 50-ml bottles with a magnetic stir bar to allow mild agitation (100 rpm). Samples were collected regularly and processed as described above.
Determination of substrates and fermentation products
Glucose, xylose, ethanol, xylitol, and glycerol were determined by HPLC equipped with a refractive index detector using an Aminex HPX-87H column (Bio-Rad). HPLC was run at 40 °C using 5 mM H2SO4 as the mobile phase at a flow rate of 0.1 ml min−1 and 0.01 ml injection volume.
Results
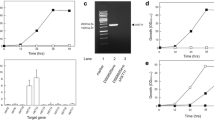
The genomic library of S. stipitis DNA was transformed into the S. cerevisiae hxt-null strain DLG-K1, which is deleted for its hexose transporter genes HXT1 to HXT7 and GAL2, and although it overexpresses the XYL1, XYL2 and XKS1 genes for efficient xylose fermentation, this hxt-null strain is unable to take up and grow on xylose, glucose or other monosaccharides as the sole carbon source (Gonçalves et al. 2014). Transformants were selected on a synthetic SC medium lacking uracil, and containing 2 % (w/v) xylose as carbon source and aureobasidin A. More than 90 transformant colonies grew up within 5 days at 28 °C, and after further inoculation into new SC-2 % xylose plates, eight transformants were selected by their capacity to grow in both xylose and glucose. Indeed, all these eight transformants were able to grow (to different extents) in the presence of several monosaccharides, including glucose, fructose, mannose, galactose and xylose (data not shown), indicating that the genes present on the pPGK plasmids encode for general monosaccharide transporters. These eight transformants able to grow on xylose were further analyzed by plasmid isolation and retransformation into the hxt-null strain DLG-K1. Four plasmids (pPGK-6, pPGK-24, pPGK-37 and pPGK-90, Table 1) were able to restore growth on SC medium containing xylose as a carbon source, and restriction enzyme analysis and partial sequencing showed that these four plasmids (Fig. 1a) contained three genes present in the known genome of S. stipitis (Jeffries et al. 2007).
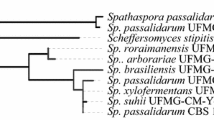
a Structure and restriction maps of the DNA fragments present in plasmids pPGK-6, pPGK-24, pPGK-37 and pPGK-90. The grey boxes indicate pPGK vector sequences, the lines indicate S. stipitis DNA sequences, and the black arrows indicate the identified open reading frames. b Maximum likelihood phylogeny with bootstrap values from alignment of sugar transporter-related proteins present in the S. stipitis genome, already characterized in S. cerevisiae (including the cellobiose transporter HXT2.4), with the three genes cloned in the present work in bold characters
Plasmid pPGK-6 has an insert of ~3 kb containing the HXT2.6 gene, present in chromosome 4 of S. stipitis, encoding for a transporter with 534 amino acid residues. Plasmid pPGK-24 contained a ~2.3 kb insert with the XUT1 gene, present in chromosome 6 of S. stipitis, encoding for a transporter with 561 amino acid residues. Plasmids pPGK-37 and pPGK-90 had two different inserts (they differ by the 5′ BamHI cleavage site that generated the DNA fragments), containing the same QUP2 gene (present in chromosome 7 of S. stipitis) that encodes for a transporter with 521 amino acid residues. Since the yeast strain transformed with these two plasmids containing the QUP2 had similar behavior, the results shown for them are the mean values of the two strains containing the pPGK-37 or pPGK-90 plasmids. All the three transporters mediate the uptake of glucose, fructose or xylose, allowing growth and fermentation of these sugars by strain DLG-K1 (Supplementary Table 1), although the specific growth rate, ethanol yield, and amount of sugar consumed by these strains with xylose as carbon source were lower, when compared to cell growth on glucose or fructose.
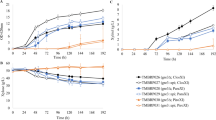
Figure 2 shows the kinetics of glucose or/and xylose consumption and fermentation by the recombinant yeast strains containing the cloned S. stipitis sugar transporters using high cell density fermentation conditions. The three genes expressed in the hxt-null DLG-K1 strain allowed glucose consumption in less than 3 h, showing high volumetric glucose consumption and volumetric ethanol production rates, as well as (with the exception of the QUP2 gene) high ethanol yields (Table 2). During glucose fermentations the major co-product found was glycerol, which accumulated up to 2.5 g l−1 when the hxt-null yeast strain contained the HXT2.6 gene, up to 1.5 g l−1 in the case of the XUT1 permease, and 2.6 g l−1 with the QUP2 gene (data not shown). Regarding xylose fermentation, the HXT2.6 gene allowed the consumption of this sugar, which during the first 10 h was fermented into ethanol but from that point on the cells stopped producing ethanol and the consumed xylose was reduced into xylitol, which accumulated in the media into the same levels as ethanol (Fig. 2). In contrast, the transporter that allowed the best xylose fermentation yields by the cells was XUT1 (Table 2), due to the production of very low levels of xylitol (Fig. 2). The permease encoded by the QUP2 gene, although allowed the uptake of xylose with considerable rates (Table 2), the volumetric ethanol production rates and yields were low as these cells converted the sugar mainly into xylitol, and not ethanol (Fig. 2).
Time course of glucose (SC-D), xylose (SC-X) or glucose plus xylose (SC-DX) fermentations by the hxt-null strain DLG-K1 expressing the HXT2.6 gene (strain BBY-6), XUT1 gene (strain BBY-24), or QUP2 gene (strains BBY-37 and BBY-90). The kinetics of glucose (black circles) or xylose (white circles) consumption, ethanol production during glucose (black triangles), xylose (white triangles) or glucose plus xylose (white squares) fermentations, and the production of xylitol (grey diamonds) or glycerol (inverted grey triangles) during xylose or glucose plus xylose fermentation, respectively, were determined as described in “Materials and Methods” section. Data are averages from two independent experiments, or the average of strains BBY-37 and BBY-90 for the QUP2 gene
During xylose and glucose co-fermentations the rates of glucose consumption were practically not affected (Table 3; Fig. 2) in the strains expressing the HXT2.6, XUT1, or QUP2 genes. From the data shown in Fig. 2 and Tables 2 and 3, it is evident that there is roughly a 40–50 % decrease in the rates of xylose consumption by these strains with equal amounts of both sugars present in the medium, when compared with the fermentations carried out with each sugar individually. In the case of the cells expressing the QUP2 permease, all the ethanol produced seems to come from glucose fermentation (Table 2, 3), and again high levels of xylitol (and glycerol) were produced during xylose consumption (Fig. 2). With the cells expressing the HXT2.6 and XUT1 permeases xylose consumption seemed to contribute to the ethanol yields (Fig. 2, Tables 2 and 3), but the amount of ethanol produced by the strain expressing the XUT1 gene was higher due to a very low production of xylitol and glycerol (Fig. 2). Finally, during glucose/xylose co-fermentations by the strain expressing the HXT2.6 permease, high quantities of glycerol accumulated in the media (Fig. 2).
Discussion
Scheffersomyces stipitis is of biotechnological interest as it ferments several sugars present in lignocellulosic hydrolysates and thus its sugar transporters have been the subject of extensive research. As mentioned in the Introduction, 15 monosaccharide transporter genes from this yeast (see Fig. 1b) have been analyzed in recombinant S. cerevisiae cells, and our cloning and screening approach with a hxt-null strain with high XR, XDH and XK activities allowed us to identify three genes that allow growth on and fermentation of xylose, of which two of them (HXT2.6 and QUP2) have not been identified before as capable of transporting xylose into yeast cells. [It should be noted that we were not expecting to clone many gene members of the S. stipitis sugar transporter family (e.g. SUT1, SUT2, XUT5, XUT6 and STL1), because they contain BamHI-cleaving sites in their ORFs that would impair their functional cloning from a genomic library made with DNA cleaved with this restriction enzyme.]
Based on amino acid sequence comparisons of these S. stipitis sugar transporter proteins studied in S. cerevisiae (Fig. 1b), many transporters (including SUT1-SUT4, RGT2, HGT2, XUT4 and XUT7) cluster together and are not closely related to the permeases cloned in this work. The XUT1 permease is closely related to XUT3, but distant to the HXT2.6 and QUP2 transporters that cluster with a distinct set of sugar transporters including XUT2, XUT5, and XUT6, a permease homolog to the STL1 glycerol-H+ symporter of S. cerevisiae, a putative arabinose-H+ symporter (AUT1), and the cellobiose transporter encoded by the HXT2.4 gene.
The XUT1 and XUT3 transporters have been characterized as permeases with moderate transport efficiency but higher xylose preference (Young et al. 2011, 2012). Both contain a conserved motive (G-G/F-x-x-x-G) at the end of the first transmembrane span that confers better xylose transport properties (Young et al. 2014). While this motif is absent in the HXT2.6 permease, the new QUP2 transporter identified in this work has a partial overlapping sequence (A-G-F-V-G-G) in this region of the protein. The three sugar transporters cloned in the present work also have the conserved PESPR motif right after the sixth transmembrane span, a common motif of sugar transporters that is required for efficient transport activity (Sun et al. 2012).
It is surprising that the transporter encoded by the HXT2.6 gene allows monosaccharide transport and fermentation by the hxt-null DLG-K1 strain, as this transporter has been included into another family of S. stipitis closely related sugar transporters (HXT2.1-HXT2.6) postulated to encode cellobiose permeases (Jeffries and van Vleet 2009; Ha et al. 2013). These genes are closely located with genes encoding endo-glucanases, α-glucosidases, or even other sugar permeases. While the recently characterized cellobiose transporter HXT2.4 (Ha et al. 2013) is found in a gene cluster with an endo-glucanase (EGC2) and a α-glucosidase (BGL5) in chromosome 1 of S. stipitis, the HXT2.6 permease is in close proximity with a α-glucosidase (BGL1) and the monosaccharide transporter SUT2 in chromosome 4. This HXT2.6 gene has been reported to be induced (together with BGL1) only after aerobic growth with cellobiose as the unique carbon source (Jeffries and van Vleet 2009). Thus, our results indicate that although this permease in S. stipitis is induced by cellobiose, its function is to transport the released monosaccharides after cellobiose (and maybe other oligosaccharides) cleavage by the α-glucosidase encoded by BGL1.
While we have not yet characterize the kinetic properties or sugar transport mechanism (facilitated diffusion or H+-symport) of the new cloned transporters, our results show that all three permeases allow not only growth on several monosaccharides, but also efficient xylose consumption and fermentation by the hxt-null DLG-K1 strain. It would also be nice to analyze the performance of these new transporters in a S. cerevisiae wild-type strain with all its native hexose transporters. Nevertheless, the hxt-null DLG-K1 strain with high XR, XDH and XK activities (Gonçalves et al. 2014) used in our genomic library screen to clone new S. stipitis xylose transporters is a promising strain platform to identify and characterize other new xylose permease genes from, for example, xylose-fermenting yeast species recently described (Cadete et al. 2009, 2013; Wohlbach et al. 2011; Lobo et al. 2014). The characterization of new xylose transporters can expand our understanding of permease function as well as suggest potentially useful classes of transport proteins or molecular engineering approaches for improving xylose utilization in recombinant S. cerevisiae for biofuel applications.
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1995) Short protocols in molecular biology, 3rd edn. Wiley & Sons, New York
Bertilsson M, Andersson J, Líden G (2008) Modeling simultaneous glucose and xylose uptake in Saccharomyces cerevisiae from kinetics and gene expression of sugar transporters. Bioprocess Biosyst Eng 31:369–377
Cadete RM, Santos RO, Melo MA, Mouro A, Golçalves DL, Stambuk BU, Rosa CA (2009) Spathaspora arborariae sp. nov., a d-xylose fermenting yeast species isolated from rotting wood in Brazil. FEMS Yeast Res 9:1338–1342
Cadete RM, Melo MA, Zilli JE, Vital MJ, Mouro A, Prompt AH, Gomes FC, Stambuk BU, Lachance MA, Rosa CA (2013) Spathaspora brasiliensis sp. nov., Spathaspora sushii sp. nov., Spathaspora roraimanensis sp. Nov. and Spathaspora xylofermentans sp. nov., four novel (d)-xylose-fermenting yeast species from Brazilian Amazonian forest. Anton Leeuw 103:421–431
Cai Z, Zhang B, Li Y (2012) Engineering Saccharomyces cerevisiae for efficient anaerobic xylose fermentation: reflections and perspectives. Biotechnol J 7:34–36
Caspeta L, Buijs NAA, Nielsen J (2013) The role of biofuels in the future energy supply. Energy Environ Sci 6:1077–1082
Diao L, Liu Y, Qian F, Yang J, Jiang Y, Yang S (2013) Construction of fast xylose-fermenting yeast based on industrial ethanol-producing diploid Saccharomyces cerevisiae by rational design and adaptive evolution. BMC Biotechnol 13:1–9
Does AL, Bisson LF (1989) Characterization of xylose uptake in the yeasts Pichia heedie and Pichia stipitis. Appl Environ Microbiol 55:159–164
Du J, Li S, Zhao H (2010) Discovery and characterization of novel d-xylose-specific transporters from Neurospora crassa and Pichia stipitis. Mol BioSyst 6:2150–2156
Farias DR, de Andrade R, Maugeri-Filho F (2014) Kinetic modeling of ethanol production by Scheffersomyces stipitis from xylose. Appl Biochem Biotechnol 172:361–379
Gárdonyi M, Jeppsson M, Lidén G, Gorwa-Grauslund MF, Hahn-Hägerdal B (2003) Control of xylose consumption by xylose transport in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng 82:818–824
Gonçalves DL, Matsushika A, Sales BB, Goshima T, Bon EPS, Stambuk BU (2014) Xylose and xylose/glucose co-fermentation by recombinant Saccharomyces cerevisiae strains expressing individual hexose transporters. Enzyme Microb Technol 63:13–20
Ha SJ, Kim H, Lin Y, Jang UM, Galazka JM, Kim TJ, Cate JHM, Jin YS (2013) Single amino acid substitutions in Hxt2.4 from Scheffersomyces stipitis lead to improved cellobiose fermentation by engineered Saccharomyces cerevisiae. Appl Environ Microbiol 79:1500–1507
Hamacher T, Becker J, Gardonyi M, Hahn-Hägerdal B, Boles E (2002) Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiology 148:2783–2788
Hector RE, Qureshi N, Hughes SR, Cotta MA (2008) Expression of a heterologous xylose transporter in a Saccharomyces cerevisiae strain engineered to utilize xylose improves aerobic xylose consumption. Appl Microbiol Biotechnol 80:675–684
Jeffries TW, Van Vleet JRH (2009) Pichia stipitis genomics, transcriptomics, and gene clusters. FEMS Yeast Res 9:793–807
Jeffries TW, Grigoriev IV, Grimwood J, Laplaza JM, Aertis A, Salamov A, Schmutz J, Lindquist E, Dehal P, Shapiro H, Jin YSU, Passoth V, Richardson PM (2007) Genome sequence of the lignocellulose-bioconverting and xylose-fermenting yeast Pichia stipitis. Nat Biotechnol 25:319–326
Jin Y, Alper H, Yang Y, Stephanopoulos G (2005) Improvement of xylose uptake and ethanol production in recombinant Saccharomyces cerevisiae through an inverse metabolic engineering approach. Appl Environ Microbiol 71:8249–8256
Kang Y, Kane J, Kujan J, Stadel JM, Tipper DJ (1990) Effects of expression of mammalian GeL and hybrid mammalian-yeast Ga proteins on the yeast pheromone response signal transduction pathway. Mol Cell Biol 10:2582–2590
Katahira S, Ito M, Takema H, Fujita Y, Tanino T, Tanaka T, Fukuda H, Kondo A (2008) Improvement of ethanol productivity during xylose and glucose co-fermentation by xylose-assimilating S. cerevisiae via expression of glucose transporter Sut1. Enzyme Microb Technol 43:115–119
Kilian SG, Van Uden N (1988) Transport of xylose and glucose in the xylose fermenting yeast Pichia stipitis. Appl Microbiol Biotechnol 27:545–548
Kim SR, Ha SJ, Wei N, Oh EJ, Jin YS (2012) Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol. Trends Biotechnol 30:274–282
Kötter P, Ciriacy M (1993) Xylose fermentation by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 38:776–783
Laluce C, Schenberg ACG, Gallardo JCM, Coradello LFC, Pombeiro-Sponchiado SR (2012) Advances and developments in strategies to improve strains of Saccharomyces cerevisiae and processes to obtain the lignocellulosic ethanol—a review. Appl Biochem Biotechnol 166:1908–1926
Lee WJ, Kim MD, Ryu YW, Bisson LF, Seo JH (2002) Kinetic studies on glucose and xylose transport in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 60:186–191
Lin S, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642
Lobo FP, Gonçalves DL, Alves SL Jr, Gerber AL, Vasconcelos ATR, Basso LC, Franco GR, Soares MA, Cadete RM, Rosa CA, Stambuk BU (2014) Draft genome sequence of the d-xylose-fermenting yeast Spathaspora arborariae UFMG-HM19.1AT. Genome Announc 2:e01163-13
Madhavan A, Tamalampudi S, Srivastava A, Fukuda H, Bisaria VS, Kondo A (2009) Alcoholic fermentation of xylose and mixed sugars using recombinant Saccharomyces cerevisiae engineered for xylose utilization. Appl Microbiol Biotechnol 82:1037–1047
Matsushika A, Inoue H, Kodaki T, Sawayama S (2009) Ethanol production from xylose in engineered Saccharomyces cerevisiae strains: current state and perspectives. Appl Microbiol Biotechnol 84:37–53
Moon J, Liu ZL, Ma M, Slininger PJ (2013) New genotypes of industrial yeast Saccharomyces cerevisiae engineered with YXI and heterologous xylose transporters improve xylose utilization and ethanol production. Biocatal Agric Biotechnol 2:247–254
Nielsen J, Larsson C, van Maris A, Pronk J (2013) Metabolic engineering of yeast for production of fuels and chemicals. Curr Opin Biotechnol 24:398–404
Nigam JN (2001) Ethanol production from wheat straw hemicelluloses by Pichia stipitis. J Biotechnol 87:17–27
Nogueira LAH, Moreira JR, Schuchardt U, Goldemberg J (2013) The rationality of biofuels. Energy Policy 61:595–598
Parachin NS, Bergdahl B, van Niel EW, Gorwa-Grauslund MF (2011) Kinetic modelling reveals current limitations in the production of ethanol from xylose by recombinant Saccharomyces cerevisiae. Metab Eng 13:508–517
Parambil LK, Sarkar D (2014) Probing the bioethanol production potential of Scheffersomyces (Pichia) stipitis using validated genome-scale model. Biotechnol Lett 36:2443–2451
Runquist D, Fonseca C, Radstrom P, Spencer-Martins I, Hahn-Hägerdal B (2009) Expression of the Gxf1 transporter from Candida intermedia improves fermentation performance in recombinant xylose-utilizing Saccharomyces cerevisiae. Appl Microbiol Biotechnol 82:123–130
Runquist D, Hahn-Hägerdal B, Radstrom P (2010) Comparison of heterologous xylose transporters in recombinant Saccharomyces cerevisiae. Biotechnol Biofuels 3:5
Saloheimo A, Rauta J, Stasyk OV, Sibirny AA, Penttilä M, Ruohonen L (2007) Xylose transport studies with xylose-utilizing Saccharomyces cerevisiae strains expressing heterologous and homologous permeases. Appl Microbiol Biotechnol 74:1041–1052
Sànchez Nogué V, Karhumaa K (2015) Xylose fermentation as a challenge for commercialization of lignocellulosic fuels and chemicals. Biotechnol Lett 37:761–772
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2011) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–23
Sedlak M, Ho NWY (2004) Characterization of the effectiveness of hexose transporters for transporting xylose during glucose and xylose co-fermentation by a recombinant Saccharomyces yeast. Yeast 21:671–684
Sievers F, Wilm A, Dineen DG, Gibson TJ, Karplus K, Li W, Lopez R, Mcwilliam H, Remmert M, Soding J, Thomson JD, Higgins D (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:1–6
Stambuk BU, Eleutherio ECA, Florez-Pardo LM, Souto-Maior AM, Bon EPS (2008) Brazilian potential for biomass ethanol: challenge of using hexose and pentose cofermenting yeast strains. J Sci Ind Res 67:918–926
Sun L, Zeng X, Yan C, Sun X, Gong X, Rao Y, Yan N (2012) Crystal structure of a bacterial homologue of glucose transporters GLUT1–4. Nature 490:361–368
Tamura K, Peterson D, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tanino T, Ito T, Ogino C, Ohmura N, Ohshima T, Kondo A (2012) Sugar consumption and ethanol fermentation by transporter-overexpressed xylose-metabolizing Saccharomyces cerevisiae harboring a xylose isomerase pathway. J Biosci Bioeng 114:209–211
Toivola A, Yarrow D, Van Den Bosch E, Van Dijken JP, Scheffers WA (1984) Alcoholic fermentation of d-xylose by yeasts. Appl Environ Microbiol 47:1221–1223
Watanabe T, Watanabe I, Yamamoto Y, Ando A, Nakamura TA (2011) UV-induced mutant of Pichia stipitis with increased ethanol production from xylose and selection of a spontaneous mutant with increased ethanol tolerance. Bioresour Technol 102:1844–1848
Weierstall T, Hollenberg CP, Boles E (1999) Cloning and characterization of three genes (SUT1-3) encoding glucose transporters of the yeast Pichia stipitis. Mol Microbiol 31:871–883
Wohlbach DJ, Kuo A, Sato TK, Potts KM, Salamov AA, Labutti KM, Sun H, Clum A, Pangilinan JL, Lindquist EA, Lucas S, Lapidus A, Jin M, Gunawan C, Balan V, Dale BD, Jeffries TW, Zinkel R, Barry KW, Grigoriev C, Gash AP (2011) Comparative genomics of xylose-fermenting fungi for enhanced biofuel production. Proc Natl Acad Sci USA 108:13212–13217
Young E, Lee SM, Alper H (2010) Optimizing pentose utilization in yeast: the need for novel tools and approaches. Biotechnol Biofuels 3:24
Young E, Poucher A, Comer A, Bailey A, Alper H (2011) Functional survey for heterologous sugar transport proteins, using Saccharomyces cerevisiae as a host. Appl Environ Microbiol 77:3311–3319
Young ME, Comer AD, Huang H, Alper HS (2012) A molecular transporter engineering approach to improving xylose catabolism in Saccharomyces cerevisiae. Metab Eng 14:401–411
Young E, Tong A, Bui H, Spofford C, Alper HS (2014) Rewiring yeast sugar transporter preference through modifying a conserved protein motif. Proc Natl Acad Sci USA 111:131–136
Acknowledgments
This work was supported in part by grants and fellowships from the Brazilian agencies CNPq (Process Nos. 490029/2009-4, 551392/2010-0, 307290/2012-3, and 478841/2013-2), FINEP (Process No. 01.09.0566.00/1421-08) and FAPESC (Process No. 17293/2009-6), and by the Japanese International Cooperation Agency (JICA).
Supporting information
Supplementary Table 1—Growth rates and ethanol yields of the recombinant yeast strains grown on synthetic SC medium with the indicated sugars.
Supplementary Fig. 1—Amino acid sequence alignment of S. stipites sugar transporters.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Sales, B.B., Scheid, B., Gonçalves, D.L. et al. Cloning novel sugar transporters from Scheffersomyces (Pichia) stipitis allowing d-xylose fermentation by recombinant Saccharomyces cerevisiae . Biotechnol Lett 37, 1973–1982 (2015). https://doi.org/10.1007/s10529-015-1893-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1893-2