Abstract
Objectives
The availability of self-targeting and low immunogenic therapeutic agents is critical to efficient cancer therapy. Therefore, the development of humanized therapeutic antibodies is particularly appealing.
Results
A humanized single-chain variable fragment (scFv) antibody that can target human epidermal growth factor receptor-2 (HER2)-overexpressing cancer cells was designed and produced via expression in Pichia pastoris. The expression gave a high yield of 8 mg protein/l (with a purity of 92 %) using shake-flask cultures. Functional studies also revealed that the purified recombinant anti-HER2 scFv exhibited anti-proliferative activity and could bind efficiently to HER2-overexpressing human breast cancer cell line SKBR3.
Conclusion
The recombinant scFv offers promising therapeutic and binding efficiencies that are desirable for targeted cancer therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a major cause of death in the world. For pharmacotherapy, targeted therapy has become increasingly critical for efficient cancer treatments since it minimizes side effects and enhances the availability of active agents to the cancer sites (Gerber 2008). Among the variety of targeting agents examined, humanized monoclonal antibodies are especially desired. One important cancer-targeting antibody is trastuzumab (herceptin), which has been widely examined for treatment of cancer cells overexpressing human epidermal growth factor receptor-2 (HER2) (Colombo et al. 2010).
Compared to typical production of antibodies via higher animals or mammalian cell cultures, microbial expression of single-chain variable fragments (scFv) consisting of the variable regions of both the heavy chain (VH) and light chain (VL) provides several advantages. In addition to the convenience of production with the well-developed microbial processes (Spadiut et al. 2014), scFv are generally of low molecular weights that improve their penetrability into tumors while retaining specific affinity and low immunogenicity (Liu et al. 2011). In particular, anti-HER2 scFv can enable the efficient targeting of therapeutical proteins, drugs, and nanoparticles toward cancer cells (Afshar et al. 2009; Reynolds et al. 2012; Zdobnova et al. 2012).
Murine instead of humanized anti-HER2 scFv has been expressed in systems such as Escherichia coli, Pichia pastoris, etc. (Cheng et al. 2003; Galeffi et al. 2005, 2006; Hu et al. 2006; Lombardi et al. 2005; Sommaruga et al. 2011). Although these studies produced scFv with the desired targeting affinity, the yield was low (0.1–0.3 mg/l) (Cheng et al. 2003; Lombardi et al. 2005). Alternative production via transgenic plants has also been reported, yet that suffered from similar limitations (Galeffi et al. 2005). Improved yields could be obtained with cell-free translational systems; however, the increase in production costs can be daunting (Galeffi et al. 2006). Murine anti-HER2 scFv (scFv800E6) produced in P. pastoris KM71H offered higher yields, yet the product appeared to suffer from impurities (Sommaruga et al. 2011). In addition, the immunogenicity of the murine antibody fragment was a major obstacle for its further development as a therapeutic agent (Muzard et al. 2009; Pavlinkova et al. 2001).
To achieve efficient production of humanized anti-HER2 scFv, the P. pastoris expression system is appealing because it offers several advantages including mature protein processing technologies and favorable post-translational modifications (e.g. glycosylation) (Ahmad et al. 2014). Accordingly, we explore in this work the expression and production of humanized anti-HER2 scFv, other than murine scFv variants, in P. pastoris. Results showed that this approach gave yields in the order of 8 mg/l, and the product displayed the desired specificity and activity in recognizing and binding to HER2-overexpressed human breast cancer cells.
Materials and methods
Materials
Pichia pastoris GS115 (his4) and plasmid pPIC9 K were kindly provided by t Professor Xiangshan Zhou. Human breast cancer cell line SKBR3 was purchased from Cell Bank of Committee on Type Culture Collection of Chinese Academy of Sciences and was grown in McCoy’s 5A (modified) medium (Gibco) with 10 % (v/v) fetal bovine serum, 100 U penicillin/ml, and 100 μg streptomycin/ml. A similar cultivation was applied to normal breast cell Hs 578Bst (ATCC) except the replacement of McCoy’s 5A (modified) medium with MEM medium (Gibco). Cells were maintained at 37 °C with 5 % (v/v) CO2. Geneticin (G418) was purchased from Majorbio Biotech (Shanghai, China). All other reagents and chemicals were of analytical grade.
Construction of expression plasmid and transformation of P. pastoris
The sequence of anti-HER2 scFv was customer synthesized by Generay Biotech (Shanghai, China) and cloned into the XhoI/EcoRI sites of the pPIC9 vector. From the resulted recombinant plasmid, product obtained by digesting with BamHI/EcoRI was then inserted into the BamHI/EcoRI sites of pPIC9 K to generate plasmid pXDC2. About 5 μg pXDC2 DNA was linearized with SalI and electrotransformed into P. pastoris strain GS115 using a micropulser following the standard procedure as suggested by the supplier. The transformants were then plated onto minimal glycerol (MGY) agar plates.
Identification of high-level expression transformants
In order to screen for overproducers with multiple inserts, ~105 cells of selected transformants were grown on YPD (yeast extract peptone dextrose) agar plates containing 3 mg G418 l/ml and 4 mg G418/ml at 28 °C for 2–5 days. Ten colonies were chosen from the G418-resistant colonies and were grown in 50 ml buffered complex glycerol (BMGY) medium in baffled flasks and were grown at 30 °C until OD600 reached 2–6. The cells were then collected and resuspended in 100 ml BMMY medium for continued cultivation till OD600 reached ~1. For induction, pure methanol was added every 24 h to give 0.5 % (v/v). Samples were taken every 24 h for SDS-PAGE analysis after being precipitated with trichloroacetic acid to determine the expression level. The genomic DNA of these colonies were isolated using TIANamp Yeast DNA Kit (Tiangen Biotech Beijing, China) and the integration of gene encoding anti-HER2 scFv was confirmed by PCR with primers specific to 5′ AOX1/3′ AOX1: 5′-GACTGGTTCCAATTGACAAGC-3′ and 5′-GCAAATGGCATTCTGACATCC-3′.
Purification of humanized anti-HER2 scFv
The culture of the best transformant was centrifuged (10,000×g, 5 min, 4 °C) for collection. The supernatant was dialyzed against binding buffer (20 mM sodium phosphate, 500 mM NaCl, pH7.2) using an Ultracel PL membrane (Millipore, cutoff 5 kDa) in a stirred cell module (Merck Millipore) and mixed with 1 ml Ni–NTA resin (Yeli, Shanghai, China) for 1 h. The supernatant-Ni–NTA mixture was loaded into a column and the column flow-through was collected. The column was then washed with a binding buffer containing 10 mM imidazole and the protein was eluted with a binding buffer with 100 mM imidazole. Ten μl of each fraction was analyzed by using SDS-PAGE (12 %). The scFv was then dialyzed against PBS in dialysis tubing (Mw cutoff 8–14 kDa) and detected by western blot analysis. The membrane was blocked by using 5 % (w/v) skimmed milk in PBST solution (0.1 % v/v Tween-20 in PBS) for 1 h at room temperature and incubated overnight with the rabbit anti-His tag antibody (Beyotime, Jiangsu, China) at 4 °C. After washing with PBST for several times, the membrane was incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Merck) at room temperature for 1 h and was detected by using a Pro-light HRP Chemiluminescent Kit (Tiangen Biotech, Beijing, China).
Cell proliferation assay
SKBR3 cells (5 × 105/ml) were grown with different concentrations of anti-HER2 scFv overnight in 96-well plates. Cells without the addition of the antibody were used as controls. Cell viability was determined by using the MTT assay. The absorbance of the suspension was measured at 490 nm using a plate reader.
Binding of anti-HER2 scFv to SKBR3 cell
Cells of cancer cell line SKBR3 and normal cell Hs 578Bst were grown in 12-well plates to reach confluency, and were then treated with 20 μg anti-HER2 scFv/ml at 37 °C for 2 h. The same amount of PBS buffer was added to cell cultures to be used as controls. The cells were subsequently washed with ice cold PBS three times, fixed in 4 % (w/v) paraformaldehyde at room temperature for 10 min, and were permeabilized with 0.2 % (v/v) Triton X-100 for 10 min. The cells were then incubated in a blocking buffer containing (1 % (w/v) BSA, 0.05 % (v/v) Tween 20, PBS) at 37 °C for 1 h and washed again with PBS. Rabbit anti-histag antibody (1:50) (Affinity BioSciences, USA) was added to each of the cell samples and incubated at 37 °C for 1 h followed by the addition of FITC-conjugated goat anti-rabbit IgG (1:200) (Beyotime). The cells were imaged by using an inverted fluorescence microscope. Labeled cells were also analyzed using flow cytometry.
Results and discussion
Design of humanized anti-HER2 scFv
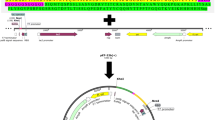
Anti-HER2 scFv can target many HER2-overexpressing cancer cells, such as bladder, pancreatic, breast, and gastric carcinoma (Ménard et al. 2001; Mitri et al. 2012; Qiu and Xu 2013). To obtain humanized anti-HER2 scFv for in vivo applications, the sequence of anti-HER2 scFv was designed according to that of the humanized monoclonal antibody trastuzumab (IMGT/mAb-DB database Chain ID INN 7637_H and 7637_L) and was constructed in the VH-(G4S)3-VL mode (Fig. 1). Codon optimization of the gene encoding the expressed protein can dramatically increase the expression level in P. pastoris (Hu et al. 2006; Sinclair and Choy 2002; Woo et al. 2002). For instance, the expression level of codon optimized human glucocerebrosidase was increased 10-fold (Sinclair and Choy 2002) when compared to that of the native enzyme. Accordingly, the gene of humanized anti-HER2 scFv (NCBI accession number: KM016462) was synthesized based on the codon preference of P. pastoris (Sinclair and Choy 2002; Zhao et al. 2000).
Design and construction of anti-HER2 scFv. The sequence of anti-HER2 scFv was designed based on the amino acid sequence of trastuzumab and was constructed in the VH-Linker-VL mode. The linker sequence is (GGGGS)3. A polyhistidine sequence (6× His) was introduced to C-terminus of scFv for antibody detection and purification. The α-factor signal sequence was designed to facilitate the anti-HER2 scFv secretion to the medium
Screening for higher expresser
After pXDC2 was transferred into P. pastoris GS115, multi-copy inserts of the target gene that promise high level expression of anti-HER2 scFv, were selected from plates containing G418 (Nordén et al. 2011). PCR showed that the gene encoding anti-HER2 scFv (744 bp) has been successfully inserted into the genome of P. pastoris GS115 in all the selected G418-resistant colonies.
The expression of anti-HER2 scFv was carried out in baffled flasks and analyzed by SDS-PAGE. A band that showed a molecular weight of approx. 29 kDa was detected in the supernatant, although the calculated molecular weight of anti-HER2 scFv was 26.6 kDa. The difference might be attributed to the post-translational modification. The expression level increased initially with time; however, the expression level at 72 h was similar to that at 96 h, suggesting 72 h-induction was sufficient for anti-HER2 scFv production (Fig. 2). No target protein was detected at 0 h. Moreover, the transformant No. 6 displayed the highest expression level of anti-HER2 scFv among the ten selected transformants (Fig. 2b).
Expression of humanized anti-HER2 scFv in P. pastoris. Transformants resistant to 4 mg/ml G418 were grown in BMMY medium. The equal amount of culture supernatants of various transformants at different times were examined by using 12 % SDS-PAGE. Afterwards, the gel was stained with Coomassie blue. Lane M: protein molecular weight marker (from the top: 97.2, 66.4, 44.3, 29.0, 20.1, 14.3 kDa). a Lanes 1–4: supernatant of transformant no. 1 at 24, 48, 72, 96 h; Lanes 5–8: supernatant of transformant no. 2 at 24, 48, 72, 96 h; Lanes 9–12: supernatant of transformant no. 3 at 24, 48, 72, 96 h. b Lanes 1–4: supernatant of transformant no. 4 at 24, 48, 72, 96 h; Lanes 5–8: supernatant of transformant no. 5 at 24, 48, 72, 96 h; Lanes 9–12: supernatant of transformant no. 6 at 24, 48, 72, 96 h. c Lanes 1–4: supernatant of transformant no. 7 at 24, 48, 72, 96 h; Lanes 5–8: supernatant of transformant no. 8 at 24, 48, 72, 96 h; d Lanes 1–4: supernatant of transformant no. 9 at 24, 48, 72, 96 h; Lanes 5–8: supernatant of transformant no. 10 at 24, 48, 72, 96 h
The relative gene copy number of the ten selected strains was then analyzed. As a result, all the strains possessed approximately the same relative copy number (Supplementary Table 1), suggesting that the copy number had no obvious effect on the expression of anti-HER2 scFv. Increased gene dosage may have positive effect on protein expression in some cases (Athmaram et al. 2012; Mansur et al. 2005; Vassileva et al. 2001; Yu et al. 2009), although negative effects have also been reported (Hohenblum et al. 2004; Inan et al. 2006; Zhu et al. 2009). Other studies, in contrast, have demonstrated that there is no apparent correlation between the two factors for cases such as murine anti-HER2 scFv, NK1-fragment of human hepatocyte growth factor, and extracellular domain of mouse tissue factor (Hu et al. 2006; Mack et al. 2009), which is consistent with the results obtained in this work.
Purification of humanized anti-HER2 scFv
The transformant No. 6 that afforded the highest expression level was used for the production of anti-HER2 scFv. The 6× his-tagged scFv could be readily purified by using Ni–NTA resin. Mixing the supernatant with the Ni–NTA resin before loading onto the column apparently increased the binding efficiency, as indicated by the small amount of scFv remained in the flow-through fraction (Fig. 3a, lane 1). After washing with 4 ml binding buffer containing 10 mM imidazole to remove non-specific binding proteins (Fig. 3a, lane 2), the anti-HER2 scFv was eluted with binding buffer containing 100 mM imidazole (Fig. 3a, lanes 3–6). One clear band with a molecular weight (29 kDa) comparable to the theoretical value of anti-HER2 scFv (26.6 kDa) was detected in the eluted fractions by both SDS-PAGE and western blot analysis (Fig. 3b). The amount, purity, and yield of scFv from each purification step are listed in Table 1. About 800 μg anti-HER2 scFv with the purity of 92 % was obtained from 0.1 L culture. The yield (8 mg/l) was comparable to murine anti-HER2 scFv (scFv800E6) produced in P. pastoris KM71H (10 mg/l) (Sommaruga et al. 2011). However, the latter appeared to suffer from impurities, as two major protein bands were reported for the SDS-PAGE analysis of the product, while the current product is of high purity and shows a single protein band. It was suspected by the authors of the previous study that different isoforms of scFv800E6 were probably formed due to inefficient cleavage by Ste13 aminopeptidase during the in vivo precursor processing. That is apparently not an issue for the current work considering the high purity observed.
Purification of humanized anti-HER2 scFv product. a The secreted scFv was purified by Ni2+-affinity chromatography, subjected to 12 % SDS-PAGE analysis, and visualized by using Coomassie staining. Lane 1, flow-through; Lane 2, washing buffer; Lane M, protein molecular weight marker (from the top: 97.2, 66.4, 44.3, 29.0, 20.1 kDa); Lanes 3–6, eluted with 100 mM imidazole. b Purified scFv was subjected to 12 % SDS-PAGE and then transferred onto PVDF membrane (Millipore) using semi-dry electroblotter (Bio-Rad)
Anti-proliferative effect of anti-HER2 scFv
To test the anti-proliferative effect of anti-HER2 scFv on the breast cancer cell line SKBR3, different concentrations of scFv were applied to incubation culture of the cells. The results showed that the application of 100 and 120 μg anti-HER2 scFv/ml could significantly inhibit cell growth (P < 0.05), while no significant effect was observed for SKBR3 cells treated with 20, 40, 60, 80 μg anti-HER2 scFv/ml (P > 0.05) (Fig. 4). Monnier et al. (2013) suggested that scFv lacking the Fc fragment had a limited therapeutic potential because it could only induce cytotoxicity by binding to the target without Fc-mediated cytotoxicity. Nejatollahi et al. (2014) also found individual anti-HER2 scFv inhibited cell proliferation to a less extent than a cocktail of anti-HER2 scFv antibodies. Our results agreed well with these previous findings. Although 100 and 120 μg anti-HER2 scFv/ml had a significant effect on cell proliferation, cell viability was kept to 76 and 71 %, respectively.
Cell viability of SKBR3 cells against anti-HER2 scFv. Cells were plated in 96-well plates and treated with 20, 40, 60, 80, 100, 120 μg/ml anti-HER2 scFv for 48 h at 37 °C. After the treatment, 10 μl MTT (5 mg/ml) was added to each well and incubated for 4 h at 37 °C. The medium was then removed and 150 μl DMSO was added following incubation for 10 min. Cell viability was calculated by comparing the absorbance value at 490 nm of treated cells to the absorbance value of untreated cells. The asterisk indicates significant difference (P < 0.05) in cell viability between treated cells and untreated cells [analysis of variance (ANOVA)]
Binding efficiency of anti-HER2 scFv to SKBR3 cells
The binding activity of the anti-HER2 scFv product was further tested with the HER2-overexpressing breast cancer cell line SKBR3. After the treatment with scFv, SKBR3 cells apparently showed green fluorescence from FITC-labeled second antibody as detected with fluorescence microscopy (Fig. 5c, 5d). As a comparison, no fluorescence was detected on the control, the PBS-treated cells (Fig. 5a, 5b). Moreover, when human normal breast cell line Hs 578Bst (lacking HER2 expression) was incubated with scFv, no significant FITC fluorescence was detected either (Fig. 5e, 5f). Binding of anti-HER2 scFv to SKBR3 cells was also quantitatively evaluated by using flow cytometry. The shift of the FITC intensity peak was indicative of the binding of anti-HER2 scFv to SKBR3 cells (Fig. 5g). The mean fluorescent intensity of SKBR3 cells obtained by flow cytometry studies was 3.2-times higher (Fig. 5g) in comparison to that of Hs 578Bst cells (Fig. 5h). These findings demonstrated anti-HER2 scFv could specifically bind to HER2-overexpressing cells, but not to the normal cells.
Binding activity of humanized anti-HER2 scFv to SKBR3 cells. SKBR3 cells were incubated with anti-HER2 scFv at 37 °C for 2 h (c, d, g). After being washed with PBS buffer, the cells were detected with rabbit anti-histag antibody and FITC-conjugated goat anti-rabbit IgG with fluorescence microscope. SKBR3 cells incubated with PBS buffer (a, b) and human normal breast cell Hs 578Bst incubated with scFv (e, f) were used as control. The shifted fluorescent signal was evident for SKBR3 cells treated with anti-HER2 scFv [solid line in (g)], while not detected for SKBR3 cells treated with PBS [gray line in (g)] and normal cells (Hs 578Bst cells) treated with anti-HER2 scFv (h)
In conclusion, we demonstrate a simple but effective design and production protocol for the production of humanized single-chain antibodies for cancer therapy. A soluble form of the humanized anti-HER2 scFv was expressed in P. pastoris. The process enabled the production of a high purity product at 8 mg protein/l. An anti-proliferative assay and binding studies with HER2-overexpressing cancerous cells confirmed that the recombinant scFv offered promising therapeutic and binding efficiencies that are desired for efficient targeted cancer therapies.
References
Afshar S, Olafsen T, Wu AM, Morrison SL (2009) Characterization of an engineered human purine nucleoside phosphorylase fused to an anti-her2/neu single chain Fv for use in ADEPT. J Exp Clin Cancer Res 28:147
Ahmad M, Hirz M, Pichler H, Schwab H (2014) Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol 98:5301–5317
Athmaram TN, Saraswat S, Singh AK et al (2012) Influence of copy number on the expression levels of pandemic influenza hemagglutinin recombinant protein in methylotrophic yeast Pichia pastoris. Virus Genes 45:440–451
Cheng LS, Liu AP, Yang JH et al (2003) Construction, expression and characterization of the engineered antibody against tumor surface antigen, p185(c-erbB-2). Cell Res 13:35–48
Colombo M, Corsi F, Foschi D et al (2010) HER2 targeting as a two-sided strategy for breast cancer diagnosis and treatment: outlook and recent implications in nanomedical approaches. Pharmacol Res 62:150–165
Galeffi P, Lombardi A, Donato MD et al (2005) Expression of single-chain antibodies in transgenic plants. Vaccine 23:1823–1827
Galeffi P, Lombardi A, Pietraforte I et al (2006) Functional expression of a single-chain antibody to ErbB-2 in plants and cell-free systems. J Transl Med 4:39
Gerber DE (2008) Targeted therapies: a new generation of cancer treatments. Am Fam Physician 77:311–319
Hohenblum H, Gasser B, Maurer M et al (2004) Effects of gene dosage, promoters, and substrates on unfolded protein stress of recombinant Pichia pastoris. Biotechnol Bioeng 85:367–375
Hu S, Li L, Qiao J et al (2006) Codon optimization, expression, and characterization of an internalizing anti-ErbB2 single-chain antibody in Pichia pastoris. Protein Expr Purif 47:249–257
Inan M, Aryasomayajula D, Sinha J, Meagher MM (2006) Enhancement of protein secretion in Pichia pastoris by overexpression of protein disulfide isomerase. Biotechnol Bioeng 93:771–778
Liu D, Wang C, Li C et al (2011) Production and characterization of a humanized single-chain antibody against human integrin αvβ3 protein. J Biol Chem 286:24500–24507
Lombardi A, Sperandei M, Cantale C et al (2005) Functional expression of a single-chain antibody specific for the HER2 human oncogene in a bacterial reducing environment. Protein Expr Purif 44:10–15
Mack M, Wannemacher M, Hobl B et al (2009) Comparison of two expression platforms in respect to protein yield and quality: Pichia pastoris versus Pichia angusta. Protein Expr Purif 66:165–171
Mansur M, Cabello C, Hernández L et al (2005) Multiple gene copy number enhances insulin precursor secretion in the yeast Pichia pastoris. Biotechnol Lett 27:339–345
Ménard S, Casalini P, Campiglio M et al (2001) HER2 overexpression in various tumor types, focussing on its relationship to the development of invasive breast cancer. Ann Oncol 12(Suppl 1):S15–S19
Mitri Z, Constantine T, O’Regan R (2012) The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract 2012:743193
Monnier PP, Vigouroux RJ, Tassew NG (2013) In vivo applications of single chain Fv (variable domain) (scFv) fragments. Antibodies 2:193–208
Muzard J, Bouabdelli M, Zahid M et al (2009) Design and humanization of a murine scFv that blocks human platelet glycoprotein VI in vitro. FEBS J 276:4207–4222
Nejatollahi F, Jaberipour M, Asgharpour M (2014) Triple blockade of HER2 by a cocktail of anti-HER2 scFv antibodies induces high antiproliferative effects in breast cancer cells. Tumour Biol 35:7887–7895
Nordén K, Agemark M, Danielson JÅH et al (2011) Increasing gene dosage greatly enhances recombinant expression of aquaporins in Pichia pastoris. BMC Biotechnol 11:47
Pavlinkova G, Colcher D, Booth BJ et al (2001) Effects of humanization and gene shuffling on immunogenicity and antigen binding of anti-TAG-72 single-chain Fvs. Int J Cancer 94:717–726
Qiu MZ, Xu RH (2013) The progress of targeted therapy in advanced gastric cancer. Biomark Res 1:32
Reynolds JG, Geretti E, Hendriks BS et al (2012) HER2-targeted liposomal doxorubicin displays enhanced anti-tumorigenic effects without associated cardiotoxicity. Toxicol Appl Pharmacol 262:1–10
Sinclair G, Choy FYM (2002) Synonymous codon usage bias and the expression of human glucocerebrosidase in the methylotrophic yeast, Pichia pastoris. Protein Expr Purif 26:96–105
Sommaruga S, Lombardi A, Salvadè A et al (2011) Highly efficient production of anti-HER2 scFv antibody variant for targeting breast cancer cells. Appl Microbiol Biotechnol 91:613–621
Spadiut O, Capone S, Krainer F et al (2014) Microbials for the production of monoclonal antibodies and antibody fragments. Trends Biotechnol 32:54–60
Vassileva A, Chugh DA, Swaminathan S, Khanna N (2001) Effect of copy number on the expression levels of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris. Protein Expr Purif 21:71–80
Woo JH, Liu YY, Mathias A et al (2002) Gene optimization is necessary to express a bivalent anti-human anti-T cell immunotoxin in Pichia pastoris. Protein Expr Purif 25:270–282
Yu H, Yan X, Shen W et al (2009) Expression of methyl parathion hydrolase in Pichia pastoris. Curr Microbiol 59:573–578
Zdobnova TA, Stremovskiy OA, Lebedenko EN, Deyev SM (2012) Self-assembling complexes of quantum dots and scFv antibodies for cancer cell targeting and imaging. PLoS ONE 7:e48248
Zhao X, Huo KK, Li YY (2000) Synonymous codon usage in Pichia pastoris. Sheng Wu Gong Cheng Xue Bao 16:308–311
Zhu T, Guo M, Tang Z et al (2009) Efficient generation of multi-copy strains for optimizing secretory expression of porcine insulin precursor in yeast Pichia pastoris. J Appl Microbiol 107:954–963
Acknowledgments
This work was supported by China Postdoctoral Science Foundation Grant (2013M540341), Key grant cultivating interdisciplinary studies of Chinese Education Ministry (WF1113014), National Natural Science Foundation of China (21303050 and 31471659), and Pujiang Talent Program of Shanghai Municipality (13PJD012).
Supporting information
Supplementary Table 1—Comparison of relative copy numbers of gene encoding anti-HER2 scFv in different strains using real-time PCR.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, X., Yu, H., Chen, C. et al. Expression and characterization of recombinant humanized anti-HER2 single-chain antibody in Pichia pastoris for targeted cancer therapy. Biotechnol Lett 37, 1347–1354 (2015). https://doi.org/10.1007/s10529-015-1804-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1804-6