Abstract
The complex structure of lignocellulose requires the involvement of a suite of lignocellulolytic enzymes for bringing about an effective de-polymerization. Cellulases and hemicellulases from both fungi and bacteria have been studied extensively. This review illustrates the mechanism of action of different cellulolytic and hemi-cellulolytic enzymes and their distinctive roles during hydrolysis. It also examines how different approaches can be used to improve the synergistic interaction between fungal and bacterial glycosyl hydrolases with a focus on fungal cellulases and bacterial hemicellulases. The approach entails the role of cellulosomes and their improvement through incorporation of novel enzymes and evaluates the recent break-through in the construction of designer cellulosomes and their extension towards improving fungal and bacterial synergy. The proposed approach also advocates the incorporation and cell surface display of designer cellulosomes on non-cellulolytic solventogenic strains along with the innovative application of combined cross-linked enzyme aggregates (combi-CLEAs) as an economically feasible and versatile tool for improving the synergistic interaction through one-pot cascade reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In view of depleting petroleum reserves and a potential climate change, the need for alternative bioenergy is expected to increase sharply in coming years. In this vein, utilization of lignocellulosic biomass has emerged as the most prominent technology for efficiently producing bioethanol and other value added products.
Research efforts in the last few decades have emphasized replacing first generation biofuels with second generation biofuels as they are made from cheap and abundant lignocellulosic feedstocks. Lignocellulosic biomass obtained from agricultural residues (wheat, straw, sugarcane bagasse, corn stover), herbaceous grasses (alfalfa, clover, switch grass, miscanthus grass) and forest products (hardwoods and softwoods) provide an abundant source of carbohydrate and present substantial renewable substrates for bioethanol production (Vikari et al. 2012).
Most plant cell walls are composed of approx. 15–40 % cellulose, 10–30 % hemicellulose and pectin, and 5–20 % lignin (Prassad et al. 2007). Recalcitrance of lignocellulosic biomass is related to its complex chemical composition (lignin, hemicellulose and acetyl groups) and the physical features (cellulose crystallinity and degree of polymerization) of the plant cell wall (Van Dyk and Pletschke 2012). The principal framework of cellulose consists of anhydroglucopyranose molecules connected by β-1,4-glycosidic linkages. The complexity of strong intra- and inter-molecular hydrogen bonding found in cellulose results in the formation of microfibrillar chains which play an important role in recalcitrance of biomass and make its hydrolysis a cost intensive process (Himmel et al. 2007). Hemicellulose is a heterogeneous group of branched and linear polysaccharides, consisting mainly of d-xylose, and d-mannose, and a number of substituted sugars (Sanchez 2009). Both cellulose and hemicellulose can be hydrolyzed into simple sugars which may be then fermented to ethanol.
Enzymes involved in the degradation of lignocellulose
Many microorganisms, including fungi and bacteria, produce extracellular enzymes which are capable of degrading and utilizing cellulose and hemicellulose as a carbon source. However, a large variety of enzymes is required to degrade all the components of lignocellulose. In nature, a consortium of lignocellulolytic enzymes works synergistically to degrade lignocellulosic biomass.
Cellulases
The degradation of cellulose to glucose involves the cooperative action of at least three enzymes; exo-1,4-β-glucanases (EC 3.2.1.91) and cellobiohydrolase (EC 3.2.1.176), endo-1,4-β-glucanases (EC 3.2.1.4), β-glucosidases (EC 3.2.1.21) (also termed cellobiases). Structural characterization studies of a variety of cellulases have provided detailed information regarding the structure and function of these enzymes. In general, the structures of cellulases are composed of a catalytic domain (CD), which is responsible for the hydrolysis reaction; a cellulose-binding domain (CBD), that mediates the binding of enzyme to the substrate; and the linker (hinge) region (rich in serine, threonine and proline residues) by which the two domains are linked (Carrard et al. 2000; Rabinovich et al. 2002). New types of enzymes, originally classified as carbohydrate-binding module (CBM) 33 (now termed copper-dependent lytic polysaccharide monooxygenase AA10) and GH61 (now termed polysaccharide monooxygenase AA9), that catalyze the oxidative cleavage of polysaccharides have been identified. For an excellent review on this topic please refer to Horn et al. (2012).
Role of CBMs/CBDs
Based on the initial discovery of several modules, cellulose specific CBMs were previously classified as CBDs. Hydrolysis of cellulose occurs at catalytic modules and CBMs assist in effectively recognizing the surface of the crystalline cellulose materials. CBMs contain from 30 to about 200 amino acids and are present as single, double or triple domains in one protein. These are either linked to the N- or C-terminus of the CD (Shoseyov et al. 2006). The presence of CBMs is shown to increase the binding of the substrate and targets the enzyme towards specific substrates.
Mechanism of action
Initially, cellulose hydrolysis occurs on the surface of the solid substrates and involves endoglucanases which hydrolyze internal β-1,4-glucosidic linkages randomly in the cellulose chain. Endoglucanases appear to have cleft-shaped open active sites which produce nicks in the cellulose polymer, exposing reducing and non-reducing ends. Exoglucanases have their active sites inside a “tunnel” and progress along the reducing and non-reducing ends of cellulose, liberating cellooligosaccharides and cellobiose units. The products of cellobiohydrolases and endoglucanases are inhibitory to the activities of their enzymes, so finally β-glucosidase (BGL) is required, which acts on cellobiose to liberate glucose (Binod et al. 2011). There is a high degree of coordination between the three enzymes, such as exo/endo, exo/exo and endo/BGL synergy, which is required for the efficient hydrolysis of cellulose crystals.
Fungal and bacterial cellulases
Fungi are key micro-organisms for the degradation of cellulose and the active degraders in this group are divided into two sub-groups:
The first group includes soft rot fungi (e.g. Trichoderma reesei), and white rot fungi (e.g. Phanerochaete chrysosporium). Cellulase mixtures available commercially are mainly obtained from the enzyme cocktail produced by T. reseei (Hypocrea jecorina) and this fungus possesses a total of 200 GH genes (Martinez et al. 2008; Kubicek et al. 2009) (Table 1). Many fungi are known to produce multiple cellulases, for example T. reesei produces 2 CBHs (Cel6A and Cel7A), 8 EGs (Cel5A, Cel5B, Cel7B, Cel12A, Cel45A, Cel61A, Cel61B and Cel74A) and 7BGLs (Cel1A, Cel1B, Cel3A, Cel3B, Cel3C, Cel3D and Cel3E) (Aro et al. 2005). In fungal cellulases, the binding module seems to invariably belong to the CBMI family and targets binding to cellulose surfaces (Costaouec et al. 2013). The CD of exoglucanase Cel7A (acts on reducing end) is composed of a β sandwich structure with a long substrate tunnel lined by β-sheets, while four loops cover the tunnel. Cel7A of T. reesei degrades crystalline cellulose following the phenomenon of processivity, which is the ability of the CBHs to attach to the carbohydrate chain, and decrystallize disaccharide units from the end of the chain without dissociation (Beckham et al. 2010).The lower processivity of Cel6A (acts on non-reducing ends) is attributed to a more open and shorter active site tunnel compared to Cel7A. Endoglucanase Cel7B has an open groove type active site because it lacks four loops of β-sheets that cover the tunnel (Beckham et al. 2010).
The second group comprises of brown rot fungi (e.g. Postia placenta) that lacks both CBMs and processive cellulases, however it is found to utilize crystalline cellulose as a sole carbon source, suggesting the involvement of non-enzymatic low molecular weight oxidants through the production of reactive oxygen species (OH-, peroxide- or superoxide- radicals), also known as the Fenton reaction (Dashtban et al. 2009; Wilson 2011).
Many aerobic bacteria secrete various amounts of free cellulases that act synergistically to degrade cellulose. They contain CDs, linker peptides and CBMs which follow a non-processive manner of cellulose degradation. Various aerobic bacteria belonging Table 1 to the genera Cellulomonas, Pseudomonas, Geobacillus, Erwinia, Streptomyces, Fibrobacter, as well as Bacillus and Paenibacillus (Table 1), are known to produce different kinds of cellulases (Table 1) (Maki et al. 2009; Sethi and Scharf 2013).
Anaerobic bacteria have evolved a different type of cellulolytic system that involves complex protein structures supporting enzymes for the hydrolysis of cellulose, known as cellulosomes (Doi and Kosugi 2004). In these bacteria, for example Clostridium (Table 1), Acetivibrio, Bacteroides and Ruminococcus, different types of cellulose-degrading enzymes and hemicellulolytic enzymes are assembled on the structural scaffoldin subunits through strong non-covalent protein–protein interactions between the docking modules (dockerin) and complementary modules (cohesins) (Dashtban et al. 2009). Cellulosomes consist of a fibrillar protein scaffoldin. The scaffolding proteins are also called “scaffoldins” and consist of proteins CbpA, Cip A or Cip C (Doi et al. 2003). A few anaerobic cellulolytic thermophilic bacteria, such as Caldicellulosiruptor sp., use an intermediate strategy, i.e. they secrete many free cellulases that contain multiple CDs. The Cald. bescii CelA comprises of a GH 9 and a GH 48 CD, along with three type III cellulose-binding modules. This CelA drives cellulose hydrolysis, not only through the well-known surface ablation mechanism, but also through excavation into the surface of the substrate, resulting in the formation of extensive cavities (Brunecky et al. 2013).
A different type of cellulolytic degradation mechanism has been found in two Gram negative cellulolytic bacteria, Fibrobacter succinogenes, an anaerobic cellulolytic rumen bacterium and Cytophaga hutchinsonii, an aerobic soil bacterium. The genome sequences of these bacteria strongly suggest that they do not use free cellulases or cellulosomes, as none of their genes encode for CBMs, scaffoldins or dockerins. Thus these organisms are believed to have evolved a novel cellulolytic mechanism to degrade cellulose fibrils by a complex present on the outer membrane and transport of cellulose molecules through super channels to periplasmic spaces followed by the action of endoglucanase present in the periplasm (Wilson 2011; Sethi and Scharf 2013).
Hemicellulases
Hemicellulose, and xylan in particular, represents 20–35 % of lignocellulosic biomass. The conversion of the hemicellulosic fraction (either in the monomeric form or in the oligomeric form) is vital for increasing the overall yield of bioethanol. Hemicellulose has a more varied structure than cellulose and thus it requires a large suite of enzymes for effective hydrolysis (Van Dyk and Pletschke 2012). Enzymes that degrade hemicellulose can be divided into two groups; firstly, a group of de-polymerizing enzymes which cleave the backbone called core enzymes, and a second group called as de-branching enzymes that remove substituents which may pose steric hindrances to de-polymerizing enzymes and subsequently increase the overall yield of reducing sugars during hydrolysis. This second group of enzymes is also known as ancillary and/or auxiliary enzymes. The core enzymes required to degrade the hemicellulases include endo-β-1,4 xylanases (EC 3.2.1.8), xylan 1,4-β-xylosidases, (EC 3.2.1.37) endo-1,4-β mannanases (EC 3.2.1.78) and β-1-4 mannosidases (EC 3.2.1.25). The ancillary enzymes include α-L-arabinofuranosidase (EC 3.2.1.55), β-glucuronidase (EC 3.2.1.139), acetylxylan esterase (EC 3.1.1.72), ferulic acid esterase (EC 3.1.1.73) and p-coumaric acid esterase (EC 3.1.1-) (Lairson et al. 2008).
Role of CBMs
Hemicellulases are modular proteins and consists of CBMs and dockerins, in addition to CDs. CBMs are known to localize the soluble enzyme to its target substrate. Most of the CBMs are composed of a β-jelly roll structure of two β-sheets and one planar hydrophobic surface that helps in binding of crystalline cellulose or a deep cleft that allows the binding of a single polysaccharide molecule (Rabinovich et al. 2002).
Mechanism of enzyme action
Xylanases (EC 3.2.1.8) are the group of enzymes responsible for the hydrolysis of β-1-4 bonds in the backbone of xylan. The main enzymes involved in this group are endo 1,4- β xylanases and β-xylosidases. Most of the xylanases belong to GH family 10 and 11, and are also distributed between families 5, 8 and 43 (Sapag et al. 2002). Xylanases belonging to the GH 10 family possess a cellulose binding domain and a CD connected by a linker. Xylanases belonging to this family have a (β/α)fold(TIM barrel), whereas GH family 11 has low molecular weight enzymes which have a β jelly roll structure (Paes et al. 2012). β-xylosidases are grouped into five families—GH families 3, 39, 43, 52 and 54, while most of the fungal β-xylosidases belong to families 3 and 43 (Van den Brink and De Vries 2011). β-mannanase hydrolyzes the mannan based hemicellulose and liberates short β-1-4 manno-oligomers which can be further hydrolyzed to mannose by β-mannosidase. β-mannanase sequence comparison studies permit assignment of these enzymes to either glycoside hydrolase family 5 or 26. Most mannanases often display a modular organization and usually consist of two-domain proteins. β-mannanases in both families belong to the 3-D structure group (β/α)eightfold catalytic module characteristic of a clan A glycoside hydrolase (Stoll et al. 2000).
Several accessory enzymes are also required, such as α-D-glucuronidase, which cleaves the α-1-2-glycosidic bond of the 4-O-methyl-d-glucuronic acid side chain of xylan. α-L-Arabinofuranosidases and arabinases hydrolyze the arabinofuranosyl containing hemicellulose and are distributed within GH families 3,43,51,54 and 62. Acetylxylan esterases hydrolyze the O-acetyl groups from positions 2 and 3 on the β-d xylopyranosyl residues of acetylxylan. Feruloyl esterases hydrolyze the hydroxyl cinnamoyl ester bonds at O2 or O5 of the arabinoside chain and glucuronyl esterases hydrolyze the methyl esters bonds at the O6 position of methylated glucuronic acid (Van Dyk and Pletschke 2012).
Fungal and bacterial hemicellulases
Fungi produce a suite of hemi-cellulolytic enzymes that can degrade hemicellulose into mono- or disaccharides. The most studied fungal hemicellulases are from A. niger, which include two endo-xylanases, one β-xylosidase, one endomannanase, one β-mannosidase, two α-galactosidases, one β-galactosidases, one α-glucuronidases, one acetyl xylan esterases and two feruloyl esterases(Van den Brink and De Vries 2011). Similarly, aerobic fungi T. reesei are also known to secrete various hemicellulases (Table 1) (Shallom and Shoham 2003). Most of the fungal endo-xylanases belonging to families 10 (formerly known as F) and 11 (formerly known as G) have been reported in literature. Multiple β-xylosidases have been reported in the filamentous fungi Asp. niger and Penicillium wortmanii (Juturu and Wu, 2012). β-xylosidases from Asp. niger and Asp. awamori are included in glycosyl hydrolase families 3 and 54 and these enzymes follow a double displacement mechanism with retention of the anomeric center configuration. Similarly, β-xylosidase of the filamentous fungi Cochliobacillus carbonum follows the mechanism of inversion of the anomeric center. The most mannanolytic group among fungi belongs to genera Aspergillus, Agaricus, Trichoderma and Sclerotium (Dhawan and Kaur 2007). With the exception of a few anaerobic fungi, most of the fungal mannanases belong to the family 5 glycosyl hydrolases. Among the fungal mannanases, the T. reesei man1 gene was the first fungal mannanase gene to be characterized (Stalbrand et al. 1995). Tang et al. (2001) demonstrated that the CD of CEL4 from Agaricus bisporus displayed the highest amino acid sequence similarity with Ascomycete mannanase from Asp. aculeatus and T. reesei (43 and 42 %, respectively), which belong to glycosyl hydrolase family 5.
Bacterial hemicellulases have been reported from both Gram positive and Gram negative strains. Most of the bacterial xylanases are categorized under GH family 10 and GH family 11. Two endo-xylanases and one β-xylosidase from T. maritima have been functionally characterized (Zhengqiang et al. 2001). Multifunctional xylanases with a feruloyl esterase module and an acetyl xylan esterase module have been characterized from Clostridium thermocellum (Prates et al. 2001) and C. cellulovorans (Kosugi et al. 2002), respectively. A multi-enzyme complex with xylanase, mannanase, arabinofuranosidase and xylosidase activity was identified and characterized by Van Dyk et al. (2010). The hemicellulolytic arsenal of Thermobacillus xylanilyticus exhibiting xylanase, arabinofuranosidase, xylosidase, feruloyl esterase and acetyl esterase activity was revealed by Rakotoarivonina et al. (2012). Bacterial mannanases have been annotated to both glycosyl hydrolases families 5 and 26. Multifunctional enzymes that contain CDs belonging to different GH families, like Paenibacillus polymyxa cel44C-man26A, have been detected. The GH44 domain possesses cellulase, xylanase and lichenase activities, and the enzyme domain GH 26 exhibits mannanase activity (Han et al. 2006). Multiple β-mannanases in Cellvibrio japonicus have been classified in both families 5 and 26 (Hogg et al. 2003). Besides this, β-mannanases from different Bacillus spp are also found in both families (Hatada et al. 2005). Mannanases can be produced from various bacterial sources such as Bacillus spp., Streptomyces spp., Caldibacillus cellulovorans, Caldicellulosiruptor Rt8b, and Caldocellum saccharolyticum (Zhang et al. 2006).
Synergy between fungal and bacterial lignocellulolytic enzymes
Most of the current commercial lignocellulolytic mixtures are based on fungal cellulases and hemicellulases (Hu et al. 2011). The question arises as to what more can be done to improve the fungal enzymes or if there could there be a way to design novel cocktails based on fungal and bacterial glycosyl hydrolases. Studies have conducted a parallel comparison of fungal and bacterial enzymes but these could not provide any conclusive results (Johnson et al. 1982; Irwin et al. 1993). It is well known that fungi produce copious amounts of cellulases (Sweeney and Xu 2012); cellobiohydrolases account for nearly 70 % (w/w) of secreted proteins and enzymes in cellulolytic fungi, followed by endoglucanases (~20 % w/w), while hemicellulases account for only less than <1 % of total weight of the secreted proteins (Sweeney and Xu 2012). Many studies have documented the presence of bacterial strains (Mohanram et al. 2013) that produce cellulases with high specific activities but these have low titre values (Lynd et al. 2002). The hemicellulolytic machinery of bacterial hemicellulases has been well studied and reviewed (Maki et al. 2009). The ability of bacterial strains to inhabit extreme environmental conditions and industrial niches provides us with a unique gene pool of cellulases and hemicellulases which (in combination with modern molecular tools for engineering which impart improved properties) can be added to existing commercial cellulase and hemicellulase mixtures dominated by fungal enzymes. Only a few studies have reported on exo/exo and exo/endo synergy between fungal and bacterial enzymes (Baker et al. 1998, 1995). Gao et al. (2010) reported the use of a hybrid mixture of fungal cellulases and bacterial hemicellulases, which effectively maximized the saccharification of AFEX-treated corn stover, resulting in 95 % glucan and 65 % xylan conversion at pH 4.5 and 50 °C. The synergistic action of a diverse set of accessory hemicellulases from different bacterial sources and core fungal cellulases resulted in high glucose (80 %) and xylose (70 %) yields with moderate enzyme loadings (~20 mg protein/g glucan) compared to commercial enzymes (Gao et al. 2011).
Models for improving synergy between fungal and bacterial glycosyl hydrolases
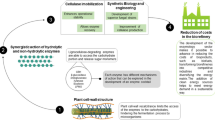
The quest for optimizing new enzyme cocktails for enhanced saccharification and fermentation of lignocellulosic biomass has to continuously evolve and, due to the structural complexity of raw substrates, different types of pre-treatment strategies and the economic constraints associated with commercialization, novel strategies have to be implemented to supplement and improve the existing commercial cellulase-hemicellulase cocktails. In this review we briefly focus on some models that could be used to exploit and implement synergistic interactions between fungal and bacterial enzymes (Fig. 1).
Designer cellulosomes
Cellulosomes are high molecular weight extracellular complexes secreted by anaerobic microorganisms consisting of scaffoldins and cellulosomal enzymes that are capable of degrading plant cell walls (Doi and Kosugi 2004). We have discussed cellulosomes in our previous section and will therefore focus on designer cellulosomes. The concept of designer cellulosomes involves the preparation of chimeric scaffoldins with specific dockerin-binding capacities by using cohesins from various scaffoldins (Doi and Kosugi 2004). The construction of divergent cohesins and matching dockerin bearing enzymes enable specific recognition and binding of cohesins and dockerins, allowing for greater control over the spatial distribution and rearrangement of the desired enzymes in designer cellulosomes (Bayer et al. 2007). Fierobe et al. (2002, 2005) developed a comprehensive set of bi-functional and tri-functional chimera cellulosomes using divergent cohesin and dockerin tagged cellulases from C. cellulolyticum and C. thermocellum. Arfia et al. (2014) demonstrated the promotion of cellulose degradation through the integration of bacterial lytic polysaccharide monooxygenases into designer cellulosomes bearing Thermomonospora fusca cellulases. The cohesion and dockerin moieties were derived from A. cellulolyticus, C. thermocellum and B. cellulovens.
In the first study of its kind, Mingardon et al. (2007) demonstrated the successful engineering of a non-cellulosomal Neocallimastix patriciarum (fungi) cellulase (Cel6A) using a bacterial dockerin and its subsequent incorporation into a mini-cellulosome with bacterial family 9 endoglucanases from C. cellulolyticum exhibiting enhanced activity on crystalline cellulose. Based on this study, elaborate designer cellulosomes containing family 6 and family 7 cellobiohydrolases from T. reseei, family 9 endoglucanase (CelF) from C. thermocellum, xylanase/acetyl xylan esterase (Xyn A) from T. fusca and Man A from C. thermocellum/C. cellulolyticum can be engineered using chimeric scaffoldin bearing specific cohesion-dockerin modules. Cellulolytic enzymes from the thermophilic fungi, Thermoascus aurantiacus and Penicillium citrinum, cellulases and hemicellulases from bacterial and archeal species across different genera, such as Bacillus, Thermobacillus, Clostridium, Fervidobacterium, Rhodothermus, Thermoplasma, Thermotoga, Pyrococcus, Sulfolobus, Thermococcus and Desulfurococcus, provide a vast pool of extremophilic cellulases and hemicellulases that can be engineered into designer cellulosomes.
Improving the saccharolytic machinery of cellulosomal bacteria
The cellulosomes of cellulolytic bacteria can be re-designed to improve their efficiency for consolidated bioprocessing (CBP). C. thermocellum with its anaerobic nature and tolerance to high temperature has been proposed as a suitable candidate for CBP. The synergistic degradation achieved by the cellulosome of C. thermocellum (due to the presence of the different enzymes) results in the formation of large concentrations of the major soluble disaccharide end product cellobiose.
Like other bacterial and fungal cellulase systems, the multienzyme cellulosome system of Clostridium thermocellum is strongly inhibited by the major end product cellobiose. The inhibition of C. thermocellum cellulosomal cellulases in the presence of cellobiose, and the inhibition of cell growth and metabolism by toxic end products such as lactic acid and acetic acid could be solved by heterogeneous expression or re-design of the cellulosome with β-glucosidases from Aspergillus niger and gene knockouts of lactic and acetic acid encoding genes, respectively (Maki et al. 2009). In a recent study, a chimeric cohesin-fused β-glucosidase (BglA-CohII) was successfully merged to an unoccupied dockerin on the scaffoldin subunit of the Clostridium thermocellum cellulosome (Gefen et al. 2012). The fusion of a cellulosome and BglA-CohII resulted in an increased hydrolysis of microcrystalline cellulose and pre-treated switch grass compared to the native cellulosome alone or in combination with wild-type BglA in solution.
Similarly, chimeric cohesins fused with fungal cellobiohydrolases like GH7 CBH-I and GH6 CBH-II can be merged to an unoccupied dockerin on the scaffoldin subunit. The opposing specificities render GH7 and 6 with a high degree of synergistic action on crystalline cellulose that could enhance the hydrolytic efficiency of the C. thermocellum cellulosome. The degradation of lignin is imperative for the commercial success of biomass conversion, because it not only reduces the cellulase/hemicellulase adsorption on lignin, but also increases the accessibility of cellulolytic enzymes for cellulose. White rot and brown rot fungi secrete significant levels of oxidoreductase enzymes, whose co-presence with hydrolytic enzymes could move the bioconversion of lignocellulose biomass to a new level. The incorporation of peroxidases and laccases on the unoccupied dockerin modules could result in a highly versatile C. thermocellum with the ability to produce ethanol from different lignocellulosic substrates (Fig. 1).
Expression of cellulolytic ability in fermentative microbes
The current cost of biomass conversion technologies can be significantly reduced by the use of organisms that hydrolyze the cellulose and hemicellulose in biomass and produce a valuable product such as ethanol at a high rate and titer. The engineering of non-cellulolytic organisms with high product yields so that they express a heterologous cellulase system able to utilize cellulose is identified as a recombinant cellulolytic strategy. A number of bacterial (E. coli, Zymomonas abilis, Klebsiella oxytoca) and yeast (Sacch. cerevisiae, Pichia pastoris, P. stipis) strains have been employed for this purpose and have been well reviewed (Elkins et al. 2010; La Grange et al. 2010). Our focus is on fungal and bacterial synergy models. The cel5 E endoglucanase gene and bgl3A β-glucosidase gene from C. thermocellum and Saccharomycopsis fibuligera, respectively, were expressed in a Sacch. cerevisiae strain, resulting in higher endoglucanase activity with improved conversion of PASC to ethanol (Jeon et al. 2009). Wen et al. (2010) used the Sacch. cerevisiae α-agglutinin anchor to tether the chimeric scaffoldin containing three C. thermocellum cohesins, as well as the C. thermocellum CBD to the yeast cell surface. C. thermocellum dockerins were added to T. reesei Cel5A (EGII) and Cel6A (CBHII) and a β-glucosidase from Asp. aculeatus. Proximity as well as enzyme–enzyme synergy was observed between the fungal and bacterial enzymes. The genomic sequence of P. stipitis shows numerous lignocellulolytic genes, including xylanase, endo-1,4-β-glucanase, exo-1, 3-β-glucosidase, β-mannosidase, and α-glucosidase. The ability of P. stipitis was enhanced by the addition of C. thermocellum endoglucanase-encoding gene celD (Piotek et al. 1998) and co-expression of fungal xylanase and xylosidase-encoding genes (Den Haan and Van Zyl 2003).
There are a number of strains such as E. coli, K. oxytoca C. acetobutylicum, K. marxianus, H. polymorpha and many others with fermentative ability. C. acetobutylicum has generated special interest as strains belonging to C. acetobutylicum have been used for the large scale production of acetone and butanol; it expresses multi-complex structures on its surface but lacks hydrolytic activity towards crystalline cellulose. Glycoside hydrolases (GHs, Cel8A, Cel9B, Cel48S and Cel9K) and a range of synthetic genes encoding C. thermocellum cellulosomal scaffoldin proteins (CipA variants), as well as synthetic cellulosomal operons that direct the synthesis of Cel8A, Cel9B and a truncated form of CipA, were integrated into the C. acetobutylicum genome using recently developed Allele-Coupled Exchange (ACE technology) (Kovacs et al. 2013). The successful expression, secretion and self-assembly of the heterologous cellulosome by C. acetobutylicum provides a fantastic opportunity to introduce CBH-I, CBH-II and β-glucosidase from T. reesei and A. niger, thereby enhancing the cellulolytic efficiency of this strain (Fig. 1).
Cross-linked enzyme aggregates
Cellulosomes have a distinct advantage over free enzymes due to their close proximity, high processivity and enhanced synergy resulting from efficient substrate channeling. However, they suffer from certain limitations that arise because of the technical challenges that accompany the successful engineering of chimeric scaffoldins and/or fusion of new enzymes into existing cellulosomes. The large size of cellulosomes may limit their movement on un-treated raw substrates as they may not be able to access the cellulose microfibrils due to the presence of lignin and non-specific adsorption on lignin. Such inactivation may have a greater impact on cellulosomes compared to free enzymes, as their high molecular weight implies that for equal loadings on mass basis, cellulosomes would be present in much lower molar concentrations, resulting in substantial loss of activity.
Considering the fact that economic feasibility of the process is imperative for commercialization of biological conversion of biomass, a model approach that includes the advantages of cellulosomes but reduces their inadvertent disadvantages can be achieved using free enzymes through the application of cross-linked enzyme aggregates (CLEAs) (Fig. 1). The preparation of combi-CLEAs involves the physical aggregation of different combinations of enzymes using precipitants followed by chemical cross-linking (cross-linking agents) (Dalal et al. 2007; Bhattacharya et al. 2013). Considering a model combi-CLEAs preparation containing a thermo-stable and processive GH 6 and 48 cellobiohydrolase from fungi and bacteria, respectively—a thermostable endoglucanase and β-glucosidase from fungi along with highly stable hemicellulase (xylanase–xylosidase, mannanase–mannosidase and de-branching enzymes) cocktail from thermophilic bacterial strains, could include all the desirable traits and allow the preparation of defined substrate based cocktails with high economic viability. The process control and the operation stability can be improved even further by the use of magnetic-CLEAs (Talekar et al. 2013; Bhattacharya and Pletschke 2014). Combi-CLEAs therefore provide an excellent option of harnessing the synergistic advantages of multi-enzyme complexes and circumventing the technical and steric hindrances associated with cellulosomes.
Future prospects
The effective de-polymerization of lignocellulosic biomass holds the key towards a greener future. In order to overcome the recalcitrance of the biomass, novel approaches have to be pursued. Due to the complexity of the substrates and the prevalence of different pre-treatment strategies, commercialization of lignocellulolytic enzymes is possible if effective sugar release is achieved under low protein loadings and with very high fermentative yields. Therefore, equal importance has to be given towards the strategic improvement of both saccharification and fermentation. Enzyme cocktails prepared based on the synergistic interaction of fungal and bacterial glycosyl hydrolases could benefit from the huge gene pool of cellulases and hemicellulases—not only on the basis of the enormous diversity that this would offer but also in bringing together the cutting edge research and development strategies in each of these separate fields. Advances in molecular techniques like directed evolution, site-directed mutagenesis, rational designing, gene fusion, DNA shuffling and other techniques have to be extended for improving the properties of the free enzymes. The application of multiple enzyme complexes and designer cellulosomes in conjunction with free enzymes has to be encouraged to create novel substrate-based defined enzyme cocktails. Improving the fermentative efficiency of cellulosomal strains and/or expression, assembly and secretion of cellulosomes in non-cellulolytic solventogenic strains will hold the key towards the validation of a successful CBP approach (Fig. 1). However, Nature may still hold the key for delivering novel enzymes. Exploring new habitats, genome analysis, data mining and proteomic analysis of secretomes (fungal and bacterial) could open the gates for novel enzymes and unravel the mysteries of enzyme interaction and synergism. It is important for us to understand that the best hydrolysis of lignocellulose occurs under natural conditions and that our aim should be to replicate those conditions which will maximize our goal towards the economically feasible production of biofuels.
References
Adav SS, Chao LT, Sze SK (2012) Quantitative secretomic analysis of Trichoderma reesei strains reveals enzymatic composition for lignocellulosic biomass degradation. Mol Proteomics 11(7):M111.012419. doi:10.1074/mcp.M111,012419
Arfia Y, Shamshouma M, Rogachevb I et al (2014) Integration of bacterial lytic polysaccharide monooxygenases into designer cellulosomes promotes enhanced cellulose degradation. Proc Natl Acad Sci USA 111:9109–9114
Aro N, Pakula T, Penttila M (2005) Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol Rev 29:719–739
Baker JO, Adney WS, Thomas SR et al (1995) Synergism between purified bacterial and fungal cellulases. Enzym Degrad Ins Carb 618:113–141
Baker JO, Ehrman CI, Adney WS et al (1998) Hydrolysis of cellulose using ternary mixtures of purified celluloses. Appl Biochem Biotech 70–72:395–403
Bayer EA, Lamed R, Himmel ME (2007) The potential of cellulases and cellulosomes for cellulosic waste management. Curr Opin Biotechnol 18:237–245
Beckham GT, Matthews JF, Bomble YJ et al (2010) Identification of amino acids responsible for processivity in a family 1 carbohydrate-binding module from a fungal cellulase. J Phys Chem B 114:1447–1453
Bhattacharya A, Pletschke BI (2014) Magnetic cross-linked enzyme aggregates (CLEAs): a novel concept towards carrier free immobilization of lignocellulolytic enzymes. Enz Microb Technol 61–62:17–27
Bhattacharya A, Shrivastava A, Sharma A (2013) Evaluation of enhanced thermostability and operational stability of carbonic anhydrase from Micrococcus species. Appl Biochem Biotechnol 170:756–763
Binod P, Janu KU, Sindhu R et al (2011) Hydrolysis of lignocellulosic biomass for bioethanol production. In: Pandey A, Larroche C, Ricke SC (eds) Biofuels: alternative feedstocks and conversion processes. Elsevier Inc., Amsterdam, pp 229–250
Boraston AB, Bolam DN, Gilbert HJ et al (2004) Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J 382:769–781
Brunecky R, Alahuta M, Xu Q (2013) Revealing nature’s cellulase diversity: the digestion mechanism of Caldicellulosiruptor bescii CelA. Science 342:1513
Carrard G, Koivula A, Soderlund H et al (2000) Cellulose-binding domains promote hydrolysis of different sites on crystalline cellulose. Proc Natl Acad Sci USA 97:10342–10347
Costaouec TL, Pakarinen A, Varnai A et al (2013) The role of carbohydrate binding module (CBM) at high substrate consistency: Comparison of Trichoderma reesei and Thermoascus aurantiacus Cel7A (CBHI) and Cel5A (EGII). Bioresour Technol 143:196–203
Dalal S, Sharma A, Gupta MN (2007) A multipurpose immobilized biocatalyst with pectinase, xylanase and cellulase activities. Chem Cent J 1:1–5
Dashtban M, Schraft H, Qin W (2009) Fungal bioconversion of lignocellulosic residues; opportunities and perspectives. Int J Biol Sci 5:578–595
Den Haan R, Van Zyl WH (2003) Enhanced xylan degradation and utilisation by Pichia stipitis overproducing fungal xylanolytic enzymes. Enz Microb Technol 33:620–662
Dhawan S, Kaur J (2007) Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol 27:197–216
Do TT, Quyen DT, Nguyen TN et al (2013) Molecular characterization of a glycosyl hydrolase family 10 xylanase from Aspergillus niger. Prot Expr Purif 92:196–202
Doi RH, Kosugi A (2004) Cellulosomes: plant-cell-wall-degrading enzyme complexes. Nat Rev Microbiol 202:541–551
Doi RH, Kosugi A, Murashima K et al (2003) Cellulosomes from mesophilic bacteria. J Bacteriol 185:5907–5914
Elkins JG, Raman B, Keller M (2010) Engineered microbial systems for enhanced conversion of lignocellulosic biomass. Curr Opin Biotechnol 21:657–662
Fierobe H-P, Bayer EA, Tardif C et al (2002) Degradation of cellulose substrates by cellulosome chimeras. Substrate targeting versus proximity of enzyme components. J Biol Chem 277:49621–49630
Fierobe H-P, Mingardon F, Mechaly A et al (2005) Action of designer cellulosomes on homogeneous versus complex substrates: controlled incorporation of three distinct enzymes into a defined trifunctional scaffoldin. J Biol Chem 280:16325–16334
Gao D, Chundawat SPS, Krishnan C et al (2010) Mixture optimization of six core glycosyl hydrolases for maximizing saccharification of ammonia fiber expansion (AFEX) pretreated corn stover. Bioresour Technol 101:2770–2781
Gao D, Uppugundla N, Chundawat SPS et al (2011) Hemicellulases and auxiliary enzymes for improved conversion of lignocellulosic biomass to monosaccharides. Biotechnol Biofuel 4:5
Gefen G, Anbar M, Morag E et al (2012) Enhanced cellulose degradation by targeted integration of a cohesin-fused β-glucosidase into the Clostridium thermocellum cellulosome. Proc Natl Acad Sci USA 109:10298–10303
Grun CH, Dekker N, Nieuwland AA et al (2006) Mechanism of action of the endo-(1-3)-α-glucanase MutAp from the mycoparasitic fungus Trichoderma harzianum. FEBS Lett 580:3780–3786
Han Y, Kye C, Hong S (2006) A cel44C-man26A gene of endophytic Paenibacillus polymyxa GS01 has multi-glycosyl hydrolases in two catalytic domains. Appl Microbiol Biotechnol 73:618–630
Hasper AA, Dekkers E, Mil M et al (2002) EglC a new endoglucanase from Aspergillus niger with major activity towards xyloglucan. Appl Environ Microbiol 68:1556–1560
Hatada Y, Takeda N, Hirasawa K et al (2005) Sequence of the gene for a high-alkaline mannanase from an alkaliphilic Bacillus sp. strain JAMB-750, its expression in B. subtilis and characterization of the recombinant enzyme. Extremophiles 9:497–500
Himmel ME, Ding SY, Johnson DK et al (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315:804–809
Hogg D, Pell G, Dupree et al (2003) The modular architecture of Cellvibrio japonicus mannanases in glycoside hydrolase families 5 and 26 points to differences in their role in mannan degradation. Biochem J 371:1027–1043
Horn SJ, Vaaje-Kolstad G, Westereng B et al (2012) Novel enzymes for the degradation of cellulose. Biotechnol Biofuel 5:45
Hu J, Arantes V, Saddler JN (2011) The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol Biofuel 4:36
Irwin DC, Spezio M, Walker LP, Wilson DB (1993) Activity studies of eight purified cellulases: specificity, synergism and binding domain effects. Biotechnol Bioeng 42:1002–1013
Jeon Eugene, Hyeon Jeong-eun, Suh Dong Jin et al (2009) Production of cellulosic ethanol in Saccharomyces cerevisiae heterologous expressing Clostridium thermocellum endoglucanase and Saccharomycopsis fibuligera β-glucosidase genes. Mol Cell 28:369–373
Johnson EA, Sakajoh M, Halliwell G, Madia A, Demain AL (1982) Saccharification of complex cellulosic substrates by the cellulase system from Clostridium thermocellum. Appl Env Microbiol 43:1125–1132
Juturu V, Wu JC (2012) Microbial xylanases. Engineering, production and applications. Biotechnol Adv 30:1219–1227
Kosugi A, Murashima K (2002) Doi RH Xylanase and acetyl xylan esterase activities of XynA, a key subunit of the Clostridium cellulovorans cellulosome for xylan degradation. Appl Environ Microbiol 68:6399–6402
Kovacs K, Willson BJ, Schwarz K (2013) Secretion and assembly of functional mini cellulosomes from synthetic chromosomal operons in Clostridium acetobutylicum ATCC 824. Biotechnol Biofuel 6:117
Kubicek CP, Mikus M, Schuster A et al (2009) Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecornia. Biotechnol Biofuels 2:19
La Grange DC, den Haan R, Willem H et al (2010) Engineering cellulolytic ability into bioprocessing organisms. Appl Microbiol Biotechnol 87:1195–1208
Lairson LL, Henrissat B, Davies GJ et al (2008) Glycosyltransferases: structures functions and mechanisms. Ann Rev Biochem 77:521–555
Lee HL, Chang CK, Jeng WY et al (2012) Mutations in the substrate entrance region of β-glucosidase from Trichoderma reesei improve enzyme activity and thermostability. Protein Eng Des Sel. doi:10.1093/protein/gzs073
Li DC, Li AN, Papageorgiou AC (2011) Cellulases from thermophilic fungi recent insights and biotechnological potential. Enzym Res. doi:10.4061/2011/308730
Liu T, Yun H, Zhang AC et al (2012) Aspergillus niger DLFCC-90 rhamnoside hydrolase, a new type of flavonoid glycoside hydrolase. Appl Environ Microbiol 78:4752–4754
Ljungdahl LG (2008) The cellulase/hemicellulase system of the anaerobic fungus Orpinomyces PC-2 and aspects of its applied use. Ann NY Acad Sci 1125:308–321
Lombard V, Golaconda Ramalu H, Drula E et al (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acid Res 42:D490–D495
Lynd LR, Weimer PJ, van Zyl WH et al (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Maki M, Leung KT, Qin W (2009) The prospects of cellulase producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci 5:500–516
Martınez D, Berka RM, Henrissat B et al (2008) Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat Biotechnol 26:513–560
Master ER, Zheng Y, Storms R et al (2008) A xyloglucan-specific family 12 glycosyl hydrolase from Aspergillus niger recombinant expression, purification and characterization. Biochem J 411:161–170
Mingardon F, Chanal A, Lopez-Contreras AM et al (2007) Incorporation of fungal cellulases in bacterial minicellulosomes yields viable, synergistically acting cellulolytic complexes. Appl Environ Microbiol 73:3822–3832
Mohanram S, Amat D, Choudhary J et al (2013) Novel perspectives for evolving enzyme cocktails for lignocellulose hydrolysis in biorefineries. Sustain Chem Proc 1:15
Nascimento AS, Muniz JRC, Aparıcio R et al (2014) Insights into the structure and function of fungal β-mannosidases from glycoside hydrolase family 2 based on multiple crystal structures of the Trichoderma harzianum enzyme. FEBS J 281:4165–4178
Olson DG, Tripathi SA, Richard J et al (2010) Deletion of the Cel48S cellulase from Clostridium thermocellum. Proc Natl Acad Sci USA 107:17727–17732
Paes G, Berrin J-G, Beaugrand J (2012) GH11 xylanases: Structure/function/properties relationships and applications. Biotechnol Adv 30:564–592
Pham Hoa PT, Quyen DT et al (2011) Cloning, expression, purification, and properties of an endoglucanase gene (glycosyl hydrolase family 12) from Aspergillus niger VTCC-F021 in Pichia pastoris. J Microbiol Biotechnol 21:1012–1020
Piotek M, Hagedorn J, Hollenberg CP et al (1998) Two novel gene expression systems based on the yeasts Schwanniomyces occidentalis and Pichia stipitis. Appl Microbiol Biotechnol 50:331–338
Prassad S, Singh A, Joshi HC (2007) Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour Conserv Recycl 50:1–39
Prates JA, Tarbouriech N, Charnock SJ et al (2001) The structure of the feruloyl esterase module of xylanase 10B from Clostridium thermocellum provides insights into substrate recognition. Structure 9:1183–1190
Rabinovich ML, Melnick MS, Bolobova AV (2002) The structure and mechanism of action of cellulolytic enzymes. Biochemistry 67:1026–1050
Rakotoarivonina H, Hermant B, Monthe N et al (2012) The hemicellulolytic enzyme arsenal of Thermobacillus xylanilyticus depends on the composition of biomass used for growth. Microb Cell Fact 11:159
Rojas AL, Fischer H, Elena V et al (2005) Structural insights into the α-xylosidase from Trichoderma reesei obtained by synchrotron small-angle X-ray scattering and circular dichroism spectroscopy. Biochemistry 44:15578–15584
Sanchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27:185–194
Sapag A, Wouters J, Lambert C et al (2002) The endoxylanases from family II: computer analysis of protein sequences reveals important structural and phylogenetic relationships. J Biotechnol 95:109–131
Sethi A, Scharf ME (2013) Biofuels: fungal, bacterial and insect degraders of lignocellulose. eLS. doi:10.1002/9780470015902.a0020374. John Wiley and Sons
Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6:219–228
Shoseyov O, Shani Z, Levy I (2006) Carbohydrate binding modules: biochemical properties and novel applications. Microbiol Mol Biol Rev 70:283–295
Shrivastava S, Shukla P, Poddar (2007) In silico studies for evaluating conservation homology among family 11 xylanases from Thermomyces lanuginosus. J Appl Sci Environ Sanit 2:70–76
Stalbrand H, Saloheimo A, Vehmaanpera J et al (1995) Cloning and expression in Saccharomyces cerevisiae of a Trichoderma reesei β-mannanase gene containing a cellulose binding domain. Appl Environ Microbiol 61:1090–1097
Stoll D, Boraston A, Stalbrand H et al (2000) Mannanase Man26A from Cellulomonas fimi has a mannan-binding module. FEMS Lett 183:265–269
Subramaniyan S, Prema P (2002) Biotechnology of microbial xylanases: enzymology, molecular biology and application. Crit Rev Biotechnol 22:33–46
Sweeney MD, Xu F (2012) Biomass converting enzymes as industrial biocatalysts for fuels and chemicals: recent developments. Catalysts 2:244–263
Talekar S, Joshi G, Joshi A et al (2013) Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv 3:12485
Tang CM, Waterman LD, Smith MH et al (2001) The cel4 gene of Agaricus bisporus encodes a β-mannanase. Appl Environ Microbiol 67:2298–2303
Van den Brink J, De Vries RP (2011) Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol 91:1477–1492
Van Dyk JS, Pletschke BI (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-factors affecting enzymes, conversion and synergy. Biotechnol Adv 30:1458–1480
Van Dyk JS, Sakkab M, Sakkab K et al (2010) Identification of endoglucanases, xylanases, pectinases and mannanases in the multi-enzyme complex of Bacillus licheniformis SVD1. Enz Microb Technol 47:112–118
Vikari L, Vehmaanpera J, Koivula A (2012) Lignocellulosic ethanol: from science to industry. Biomass Bioenergy 46:13–24
Wen F, Sun J, Zhoa H (2010) Yeast surface display of trifunctional minicellulosomes for simultaneous saccharification and fermentation of cellulose to ethanol. Appl Environ Microbiol 76:1251–1260
Wilson DB (2011) Microbial diversity of cellulose hydrolysis. Curr Opin Microbiol 14:1–5
Zhang Q, Yan X, Zhang L et al (2006) Cloning, sequence analysis, and heterologous expression of a β-mannanase gene from Bacillus subtilis Z-2. Mol Biol 40:368–374
Zhengqiang J, Kobayashi A, Ahsan MM et al (2001) Characterization of a thermostable family 10 endoxylanase (XynB) from Thermotoga maritima that cleaves p-nitrophenylbeta-D-xyloside. J Biosci Bioeng 92:423–428
Zverlov VV, Schantz N, Schmitt-Kopplin P et al (2005) Two new major subunits in the cellulosome of Clostridium thermocellum: xyloglucanase Xgh74A and endoxylanase Xyn10D. Microbiology 151:3395–3401
Acknowledgments
Ankita Shrivastava Bhattacharya and Abhishek Bhattacharya are grateful to the National Research Foundation (NRF) of South Africa for their NRF Free Standing Post Doctoral Fellowship awards. Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s) and therefore the NRF does not accept any liability in regard thereto.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharya, A.S., Bhattacharya, A. & Pletschke, B.I. Synergism of fungal and bacterial cellulases and hemicellulases: a novel perspective for enhanced bio-ethanol production. Biotechnol Lett 37, 1117–1129 (2015). https://doi.org/10.1007/s10529-015-1779-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1779-3