Abstract
Carbonic anhydrase (CA) was purified from Micrococcus lylae and Micrococcus luteus with 49.90 and 53.8 % yield, respectively, isolated from calcium carbonate kilns. CA from M. lylae retained 80 % stability in the pH and temperature range of 6.0–8.0 and 35–45 °C, respectively. However, CA from M. luteus was stable in the pH and temperature range of 7.5–10.0 and 35–55 °C, respectively. Cross-linked enzyme aggregates (CLEAs) raised the transition temperature of M. lylae and M. luteus CA up to 67.5 and 74.0 °C, while the operational stability (T 1/2) of CA at 55 °C was calculated to be 7.7 and 12.0 h, respectively. CA from both the strains was found to be monomeric in nature with subunit molecular weight and molecular mass of 29 kDa. Ethoxozolamide was identified as the most potent inhibitor based on both IC50 values and inhibitory constant measurement (K i). The K m and V max for M. lylae CA (2.31 mM; 769.23 μmol/mg/min) and M. luteus CA (2.0 mM; 1,000 μmol/mg/min) were calculated from Lineweaver–Burk plots in terms of esterase activity. Enhanced thermostability of CLEAs alleviates its role in operational stability for application at an on-site scrubber. The characteristic profile of purified CA from Micrococcus spp. advocates its effective application in biomimetic CO2 sequestration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbonic anhydrase (EC 4.2.1.1) is a Zn2+ metalloenzyme that catalyzes the interconversion of carbon dioxide and bicarbonate and is one of the fastest enzyme known [1]. In prokaryotes, carbonic anhydrases are involved in diverse biochemical and physiological processes, including photosynthesis, respiration, transport of CO2 or HCO3 −, maintaining internal pH and CO2/ HCO3 − balance required for biosynthetic reactions [2]. It also plays a crucial role in many biosynthetic and detoxification pathways where bicarbonate acts as a co-factor or as co-substrate [3]. The inhibition studies of carbonic anhydrase with different sulphonamide and anionic inhibitors have been reported from both prokaryotic and eukaryotic microbial domains. Subsequent kinetic analyses in conjugation with inhibitor studies have postulated a novel criterion for discussion regarding how active site interactions are defined by differences in their amino-acid sequences [4–6].
Recent studies with carbonic anhydrase have advocated a novel application of this ancient enzyme in the field of biomimetic sequestration [7, 8]. The biomimetic process presents an onsite solution and provides an environmentally sustainable and economically amenable approach. Success to this approach necessitates the purification and characterization of carbonic anhydrase from diverse microbial populations inhabiting unique ecological niche [9, 10]. Thermostability is one of the key factors that govern the success of biomimetic approach [11]. Use of additives provides a cost-effective method for improving the thermostability and operational stability of enzymes [12]. Current research has indicated that cross-linked enzyme aggregates (CLEAs) have many economic and environmental benefits in context to industrial biocatalysis [13].
The present study deals with the purification, molecular, biochemical and kinetic characterization of carbonic anhydrase in Micrococcus spp. isolated from calcium carbonate kilns to evaluate the effectiveness of carbonic anhydrase-CLEAs for their enhanced thermostability and operational stability at elevated temperatures with perspective for application at an on-site scrubber.
Materials and Methods
Source of Microorganisms
A total of 25 soil samples enriched with calcium carbonate were collected in sterile plastic bags from CaCO3 kilns located near Satna district of Madhya Pradesh, India. Soil (1 g) from each sample was weighed and added to 200 ml of sterilized peptone broth enriched with calcium carbonate (6 %) and incubated at 37 °C for 48 h at 150 rpm. The bacterial colonies were isolated using peptone agar plates enriched with 6 % calcium carbonate. The plates were incubated at 37 °C for 48 h, and the visible colonies were collected and purified using the conventional streaking method [14].
Determination of Carbonic Anhydrase (CO2 Hydration) Activity
Cells (A600nm = 1.2) grown in peptone broth (pH 8.0) were harvested by centrifugation at 10,000 rpm for 10 min at 4 °C. Cell pellets were suspended in Lysis buffer (sodium phosphate (50 mM; pH 7.6) containing 1 μM ZnSO4, 1 mM PMSF, 0.1 mM EDTA, 0.5 mM DTT, 25 mM glucose, 1 μg RNase A ml−1, 1 μg DNase I ml−1 and l μg lysozyme ml−1), pH 7.6 to a final concentration of 1.0 g (wet weight) of cells ml−1 and placed on ice for 10 min at 4 °C. Following incubation, the cell extract was obtained by sonication (five short bursts of 10 s at 23 kHz followed by 30 s of cooling) of cell suspension in lysis buffer. Cell lysates were centrifuged at 8,000 rpm for 30 min, and supernatant was used as crude enzyme extract to determine carbonic anhydrase activity [7].
Identification of Bacterial Isolates Producing Carbonic Anhydrase
Bacterial isolates with significant CA activity were identified on the basis of morphological, cultural and biochemical characterization [15]. The two isolates (BGCC#1078 and BGCC#1079) with highest CA activity were further confirmed on the basis of 16S rDNA sequence analysis [16]. Nucleotide sequences were di-deoxy sequenced with fluorescent terminators (Big Dye, BDT v3.1, Applied Biosystems) and run in 3130xl Applied Biosystems ABI Prism automated DNA Sequencer [16]. The identity of 16S rDNA sequence was established by performing a similarity search against the GenBank database (website: http://www.ncbi.nih.gov/BLAST).
Purification
The crude enzyme extracted from BGCC# 1078 and BGCC# 1079 was subjected to ammonium sulphate fractionation (35, 50 and 75 %) [10]. The ammonium sulphate fraction(s) exhibiting CA activity were subjected to dialysis by a 15-kDa cutoff dialysis membrane. The fraction(s) with CA activity was extensively dialyzed against 2 L of 50 mM sodium phosphate buffer (pH 7.6) for 24 h at 4 °C. Periodic changes in buffer were carried out at regular intervals of 8 h. The dialyzed sample was assayed for specific activity of carbonic anhydrase.
The soluble protein mixture was combined with an equal volume of agarose bound p-aminomethylbenzene sulphonamide agarose (p-AMBS-agarose, Sigma) pre-equilibrated with 50 mM sodium phosphate buffer, pH 7.6, containing 1 μM ZnSO4. The slurry was mixed overnight at 4 °C and loaded onto a chromatography column the following day [10]. For the purification of BGCC#1078 CA, the column was washed with 0.1 M Tris-SO4/0.3 M K2SO4/0.5 mM EDTA, pH 8.5, followed by 0.1 M Tris-SO4/0.3 M K2SO4/0.5 mM EDTA, pH 7.5. The enzyme was eluted with 0.1 M sodium acetate/0.3 M KCl and 1 μM ZnSO4 (pH 6.8), and proteins eluted were detected at 280 nm [17]. For the purification of BGCC#1079 CA, the column was washed with 0.1 M Tris-SO4/0.3 M K2SO4/0.5 mM EDTA, pH 9.0, followed by 0.1 M Tris-SO4/0.3 M K2SO4/0.5 mM EDTA, pH 7.0. The enzyme was eluted with 0.1 M sodium acetate/0.3 M KF and 1 μM ZnSO4 (pH 6.8), and proteins eluted were detected at 280 nm [17]. The active fraction of CA from both the isolates was dialyzed against Milli-Q grade water containing 1 μM ZnSO4 for 12 h to remove any salt or ion in elute. The homogenous enzyme suspension was vacuum dried using YORCO Freeze Dryer (YORCO, India). The lyophilized enzyme was used for further experiments.
Effect of pH and Temperature on CA Stability
The modified method of Sharma et al. [10] was followed. The effect of pH on stability of CA from both the isolates was determined by incubating 0.1 ml of enzyme solution (1 mg/ml stock) in 0.4 ml of citrate-phosphate buffer (pH 6.0–6.5), Tris–HCl buffer (pH 7.0–8.5) and Glycine–NaOH buffer (pH 9.0–10.0) at buffer strength of 50 mM. Following incubation for 1 h, the residual enzyme activity was determined [7]. The temperature stability of CA from both the isolates was determined by incubating 0.1 ml of enzyme solution for 1 h (1 mg/ml stock) in 50 mM Tris–HCl buffer pH 8.0, at 35, 40, 45, 50 and 55 °C. The residual carbonic anhydrase activity was measured [7].
Effect of Additives on CA Stability at Different Temperatures
The appropriate concentration of different additives such as ethylene glycol, glycerol, sorbitol and mannitol pertaining to maximum retention of enzyme activity necessary with increasing concentration of additives (0.1–2.0 M) was studied by incubating each additive at respective concentration separately with CA from Micrococcus lylae and Micrococcu luteus for 1 h in a total reaction volume of 0.1 ml at temperature 35 °C and pH 8.0 maintained using 50 mM Tris–HCl buffer.
The effect of different additives at suitable concentration on thermostability of CA (1 mg/ml) from both the isolates was studied individually at different temperatures ranging from 35 to 70 °C. The reaction mixture (0.1 ml) was incubated for 1 h at pH 8.0 (pH 8.0, 50 mM Tris–HCl buffer) at respective temperature. CA activity at 35 °C was considered maximum, and residual enzyme activity was calculated as percentage residual activity. Transition temperature was calculated from a plot of percentage activity versus temperature as the temperature at which activity falls to 50 % of the initial value [12].
Preparation of M. lylae CA cross-linked enzyme aggregates (MLC-CLEAs) and M. luteus CA cross-linked enzyme aggregates (MTC-CLEAs)
The purified CA from M. lylae and M. luteus was concentrated using 80 % w/v ammonium sulphate at 4 °C and pH 8.0 (100 mM Tris–HCl). The cross-linking optimization reaction at protein concentration of 2 mg/ml was carried out with glutaraldehyde within the concentration range of 0.1–2.0 % in a total volume of 0.1 ml containing 1 M sorbitol. The reaction mixture was incubated for 1 h at 4 °C, and the suspension was quenched with 0.9 ml Tris–HCl buffer (100 mM, pH 8.0). The hydration activity of the suspension containing the CLEAs and residual free enzyme was assayed. A parallel set of sample was centrifuged to separate the CLEAs. The CA activity of the residual free enzyme was determined. The difference in activities corresponds to the activity of CLEAs [18, 19].
Enzyme Immobilization
The immobilization of M. luteus and M. lylae was carried out on chitosan-KOH beads as described by Sharma et al. [8].
Comparative Evaluation of Enzyme Thermostability
The thermostability of native CA, CA with additive (sorbitol), immobilized CA and CLEAs was comparatively evaluated within a temperature range of 35–70 °C for M. lylae and 35–75 °C for M. luteus by incubating the reaction mixture for 1 h at pH (8.0, 100 mM Tris-buffer). The residual activity and transition temperature were calculated as described earlier. The operational stability for all the variables under study was measured at 55 °C by incubating the reaction mixture for varying time periods 0–9 h for M. lylae and 0–15 h for M. luteus. Maximum enzyme activity was considered at 0 h, residual activity was determined at corresponding temperature. The half life (T 1/2) was calculated from the plot of residual activity versus incubation time.
Effect of Metal Ions and Inhibitors on CA Stability
The modified method of Sharma et al. [10] was followed. Enzyme solution (0.1 ml) in 0.4 ml of Tris–HCl buffer was incubated with 5 mM concentration of Ni2+, Mn2+, Cu2+, Hg2+,Co2+, Se2+, Pb2+, Zn2+, Cd2+, Fe2+ and Ar3+ individually for 1 h at room temperature. The residual carbonic anhydrase activity was measured [7]. The IC50 concentration of sulphonamides (acetazolamide, ethoxozolamide and sulphanilamide) and anionic inhibitors (CN−, N3 −, I−, Br−, Cl−, F−, ClO4 −, SO4 2−, NO3 −, SCN−) for CA from both the isolates was determined by incubating different concentration of inhibitors individually with the reaction mixture (0.1 ml of enzyme in 50 mM Tris–HCl, pH 8.0) for 1 h at room temperature [10]. The residual carbonic anhydrase activity was determined [7].
Esterase Activity of CA
Activity for p-Nitro phenyl acetate hydrolysis was determined at 25 °C, following the method of Smith and Ferry [20]. The enzyme activity is expressed in International Units (IU).
Polyacrylamide Gel Electrophoresis
Electrophoresis using 12.0 % polyacrylamide gels (0.8 % bisacrylamide) was performed by following the method of Laemmli [21]. The gel was allowed to run at 10 mA through stacking phase followed by an increase in current up to 20 mA for separating phase. The protein bands were visualized by staining with Coomassie brilliant blue R- 250 (0.1 %) followed by overnight destaining. An 8 % polyacrylamide concentration was optimized to perform non-denaturing PAGE. Following electrophoresis at 10 mA, the gel was cut into two halves. The first half was stained with Coomassie brilliant blue R-250, and the second half was used for zymography [10].
Molecular Mass Determination
The molecular mass of the purified protein was determined using Sephadex G-75 (Sigma) column (2.6 × 6.5 cm). The column was balanced for 24 h with the buffer (50 mM Na3PO4, 1 mM DTT and 1 μM ZnSO4, pH 7.6) until no absorbance at 280 nm was obtained. The concentration of the protein solution used was 0.2 mg/ml. The flow rate through the column was adjusted to 18 ml/h [10].
Determination of Kinetic Parameters
Determination of K m and V max values of M. lylae CA and M. luteus CA was carried out by measuring the esterase activity of CA at five different substrate concentrations (2.0–6.0 mM) using p-Nitrophenyl acetate as substrate. The enzymatic reaction was carried at pH 7.0 (100 mM phosphate buffer), 25 °C, and the change in absorbance was measured at 348 nm. Both ethoxozolamide and acetazolamide at 5 and 10 mM concentration were used to determine the type of inhibition, and an average K i value was calculated for each inhibitor. The K m, V max and K i values were calculated from Lineweaver–Burk double-reciprocal plot [22].
Results and Discussion
Bacterial Isolates Involved in the Study
Overall, 115 bacterial isolates were obtained from calcium carbonate-enriched soil samples. Six potential (BGCC#1078, BGCC#1079, BGCC#1080, BGCC#1081, BGCC#1083 and BGCC#1089) isolates with considerable CO2-hydration activity were identified on the basis of morphological, cultural and biochemical parameters. Significant enzyme activity was observed in two isolates, BGCC#1078 (3.07 U/mg protein) and BGCC#1079 (3.64 U/mg protein), and were confirmed by 16S rRNA gene sequencing as M. lylae (JN 411681) and M. luteus (JN411682), respectively, and were selected for further studies. The enzyme CA is critical to many energy yielding reactions and is conduit for maintaining the ionic balance and distribution of CO2 and bicarbonate within the cell. CaCO3 enriched soil offers a unique niche represented by ancient lithified cyanobacterial communities that participated in the deposition of atmospheric CO2 and formation of stromatolites at early stages of Earth’s history [23].
Purification of Intracellular Carbonic Anhydrase
The purification studies for both the species indicated similar purification profile (Table 1); maximum specific activity was recorded for M. luteus CA (66.22 U/mg protein), followed by M. lylae CA (61.11 U/mg protein). Under similar conditions, the specific activity of purified CA from the indigenous isolates was comparable to that of commercial BCA [7]. More than 46 % enzyme yield was recorded for CAs from both the species following affinity purification. Rawat and Moroney [24] reported 20 % yield of CIA-5 from Chlamydomonas reinhardtii, while 20 and 40 % recovery of purified CA from Methanosarcina thermophila and Acetobacterium woodii were reported by Alber and Ferry [25] and Braus-Stromeyer et al. [26], respectively. Ramanan et al. [27] also reported the purification of CA from Bacillus subtilis with 15 % yield. However, the extracellular CA from Pseudomonas fragi [10] was recovered with 86 % yield but with comparatively low specific activity (11.03 U/mg protein).
Effect of pH and Temperature on CA Stability
Carbonic anhydrase from M. lylae and M. luteus retained 100 % stability at pH 7.0 and 8.5, respectively (Fig. 1). One hundred percent stability corresponds to retention of maximum CA activity, i.e. 61.11 and 66.22 U/mg by M. lylae and M. luteus, respectively following 1 h of incubation. Eighty percent enzyme activity was retained between pH 6.0 and 8.0 for M. lylae CA and between pH 7.5 and 10.0 for M. luteus CA. The results showed distinct variability in the stability range of intracellular CA from the two indigenous isolates. Similarly, difference in the temperature stability of enzyme from two isolates was demarcated during the study (Fig. 2); 80 % stability was retained within the range of 35–45 °C for M. lylae CA and between 35 and 55 °C for M. luteus CA. However, the CA from Enterobacter taylorae and Aeromonas caviae were found to be stable in the pH range of 7.5–8.5 and temperature range of 35–45 °C [9]. Contrastingly, CA from M. thermophila was found to be active at 75 °C [28]. The extracellular CA from Microcoleus cthonoplastes was found to have optimal activity at pH 7.5 and pH 10.0 [29]. In contrast, CA from Helicobacter pylori showed high acid tolerance, functioning optimally in the acidic environment of human stomach [30]. Comparatively, the optimal activity for B. subtilis CA was recorded at pH 8.3. The enzyme was also found to be stable at temperature range below 60 °C [27]. All these facts indicate the remarkable functional diversity of CA and the ability of this enzyme to perform different roles for the organisms surviving in extreme microenvironments and diverse ecological niche. It will be interesting to decipher the significant differences in the pH optima of M. lylae and M. luteus. Both the strains belong to the same genus and were isolated from calcium carbonate-enriched soils, thus could be envisioned to share similar physiological and metabolic roles. However, stark differences in the pH optimum for two strains were evident in the present work. A study pertaining to the elucidation of physiological role of CA in these strains could designate certain novel characters to this enzyme.
Effect of Additives on CA Stability at Different Temperatures
The effect of different concentration of additives on CA from both M. lylae and M. luteus indicated appreciable loss of enzyme activity over 1 M concentration for all the additives (Table 2). At 1 M, more than 94 % activity was retained for CA from both the isolates. Further study with any of the additives was carried out 1 M respective concentration. In the presence of all the four additives, the thermostability of enzyme improved over the entire temperature range (I and II of Fig. 3) Maximum thermo-stabilizing effect was observed in the presence of sorbitol with increase in transition temperature up to 64.0 °C followed by mannitol (T m, 54 °C), glycerol (T m, 55.6 °C) and ethylene glycol (T m, 55.6 °C) compared to native M. lylae CA (T m, 52.5 °C). A similar profile for M. luteus CA (Fig. 3.2) was observed in presence of sorbitol (T m, 69.5 °C), mannitol (T m 64.5 °C), glycerol (T m, 63.5 °C) and ethylene glycol (63.0 °C) compared to native M. luteus CA (57.0 °C). The stabilizing effect of the polyols against thermal denaturation of the enzyme might be explained with the preservation of the water shell around the protein molecule [31]. Sorbitol and mannitol are long chain polyols (6 carbon), and thus, their effect could be more pronounced; however, the higher effectiveness of sorbitol can only be explained by a better orientation of the compound with the enzyme which also varies with the nature of the enzyme [32].
Comparative Evaluation of Enzyme Thermostability
The maximum CA activity retained by MLC-CLEAs and MTC-CLEAs was 53.0 and 49.0 U/mg protein, respectively, when 0.5 % glutaraldehyde was used as a cross-linking agent. Correspondingly, the aggregate percentage was calculated as 79.6 and 80.3 % for M. lylae and M. luteus CA (Table 3). It is imperative to determine the appropriate concentration of the cross-linking agent as a lower concentration results in low degree of cross-linking with greater protein loss. Contrastingly, a higher degree of cross-linking could mask the active site of the enzyme and/or result in the conformational change at active site. The immobilized M. lylae CA (52.2 U/mg protein) and M. luteus CA (51.1 U/mg protein) were used in comparative thermostability analysis.
Both MLC-CLEAs (I of Fig 4) and MTC-CLEAs (II of Fig 4) provided greater thermostability compared to immobilized CA and sorbitol (additive). This was evident as the transition temperature for MLC-CLEAs and MTC-CLEAs was calculated as 67.5 and 74.0 °C, respectively. However, the T m for immobilized MLCA and MTCA was estimated as 63.5 and 67.0 °C, respectively. The T m for native MLCA and CA with sorbitol as additive was recorded as 52.5 and 64.0 °C, respectively. Similarly, T m for native MTCA and with sorbitol as additive was recorded as 57.0 and 69.5 °C, respectively. Decrease in thermostability with increasing incubation period was minimum for both MLC-CLEAs (III of Fig 4) and MTC-CLEAs (IV of Fig. 4), while it was maximum for CA from both the isolates. At 55 °C, the T 1/2 for native MLCA and MTCA was estimated to be 3.5 and 7.8 h, respectively. The T 1/2 for native MLCA and MTCA in the presence of sorbitol was calculated as 5.7 and 9.8 h, respectively. The T 1/2 for immobilized CA from M. lylae (5 h) and M. luteus (9.9 h) was also calculated. Similarly, the T m for MLC-CLEAs (7.7 h) and MTC-CLEAs (12 h) was also calculated from the plot.
I Comparative evaluation of thermostability at different temperatures on native M. lylae CA, CA in the presence of sorbitol, immobilized CA on chitosan-KOH beads and cross-linked enzyme aggregates. II Comparative evaluation of thermostability at different temperatures on native M. luteus CA, in the presence of sorbitol, immobilized CA on chitosan-KOH beads and cross-linked enzyme aggregates. III Comparative evaluation of operational stability at 55 °C with respect to native M. lylae CA, in the presence of sorbitol, immobilized CA on chitosan-KOH beads and cross-linked enzyme aggregates. IV Comparative evaluation of operational stability at 55 °C with respect to native M. luteus CA, CA in the presence of sorbitol, immobilized CA on chitosan-KOH beads and cross-linked enzyme aggregates
The high efficiency of CLEAs could be explained accordingly; the activation of enzymes by additives, such as surfactants and crown ethers, is well documented and is generally attributed to the enzyme being induced to adopt a more active conformation. Co-precipitation of such additives with the enzyme followed by cross-linking of the enzyme aggregates can lock the enzyme in a more favourable conformation [13]. The orientation of enzyme in a favourable conformation is associated with increase in free energy of the enzyme and correspondingly greater thermostability.
This piece of work allows the conceptual designing of a membrane-bound bioreactor with carbonic anhydrase CLEAs for biomimetic CO2 sequestration. It is clearly evident that CLEAs have resulted in an increase in the thermal and operational stability of the enzyme at high temperature; this is of extreme significance with respect to bioreactor application at onsite scrubber where thermostability of the enzyme holds the prime significance. However, such application will require a comprehensive knowledge regarding kinetics of CLEAs under operational parameters.
Effect of Metal Ions and Inhibitors on CA Stability
During the present study, Co2+, Zn2+ and Cd2+ were found to stimulate enzyme activity of M. lylae and M. luteus CA (Table 4). The extracellular P. fragi CA was found to be stimulated by addition of Fe2+, Zn2+ and Cd2+ [7]. The fact that all these ions are part of the active metal centre in different classes of CA [33] means they could have a stabilization effect on the enzyme thus enhancing the activity. In the present study, Zn2+ had maximum stimulatory effect on CA activity; this holds true for α-CA as Zn (II) forms the part of the metal coordination sphere [14]. In contrast, the CA activity was strongly inhibited by Se2+ , Hg2+, Pb2+ and As3+. Sharma et al. [9] reported that inhibition due to Hg2+ suggests the presence of thiol groups in the active site of the enzyme. In a similar study, Ramanan et al. [27] reported enhancement of Citrobacter freundii CA activity in the presence of Co2+, Zn2+, Cd2+ and Fe2+ . They also reported considerable inhibition of CA activity in the presence of lead and mercury, while slight inhibition was observed in the presence of calcium and manganese ions. Likewise, the same group reported enhancement of CA activity from B. subtilis in the presence of Co2+, Zn2+, Cu2+, Fe2+ and Mg2+ and also enzyme inhibition in the presence of Hg2+, Pb2+ and EDTA [34].
The inhibition profile (Table 2) of M. lylae CA (ethoxozolamide > CN− > acetazolamide > sulphanilamide > SCN− > F− > NO3 − = HCO3 − = Cl− > I− = Br− > SO4 2−), and M. luteus CA (ethoxozolamide > acetazolamide > sulphanilamide > CN− > SCN− > I− > Cl− = Br− = NO3 − = HCO3 − > SO4 2− = F−) demarcated a clear picture upholding the characteristic phenomena of alpha class carbonic anhydrase [33]. Both acetazolamide (IC50 = 2 × 10−5 mM) and sulfanilamide (IC50 = 7.0 × 10−3 mM) were found to strongly inhibit extracellular CA from P. fragi [7]. The inhibition profile resembled HCA II, HCA I and other α-CAs [33]. The esterase activity of purified carbonic anhydrase from M. lylae and M. luteus was recorded as 4,019.34 and 5,321.65 IU, respectively. Interestingly, CA from both the species showed remarkable esterase activity. Smith and Ferry [28] concluded that esterase activity is associated only with alpha class carbonic anhydrase and has not been documented with other known classes of CA. The experimental support was duly justified by the work of Armstrong et al. [35] who determined esterase activity in α–class HCA and by Ekinci et al. [22] who established the simultaneous in vitro inhibition of hydratase and esterase activities of human carbonic anhydrase–I and II in the presence of certain drugs. Kaur et al. [36, 37] established the absence of esterase activity in ß-CA from Azospirillum brasilense; the same group also reported non-existence of esterase activity in γ-CA from A. brasilense.
Two main classes of CA inhibitors are known; the metal complexing anions that may bind either in tetrahedral geometry of the metal ion or as trigonal bipyramidal adducts. Sulfonamides bind in a tetrahedral geometry of the Zn (II) ion, in deprotonated state, with the nitrogen atom of the sulphonamide moiety [38]. Interestingly, M. luteus CA was found to be more sensitive towards sulphonamide inhibitors compared to M. lylae CA. However, apart from CN− and SCN−, all other anionic inhibitors involved in the study showed elevated inhibition against M. lylae CA compared to M. luteus CA. The present study also highlighted notable resemblances of both these bacterial CAs towards the α-CA superfamily based on their interactions with inhibitors and metal ions. The IC50 values of ethoxozolamide and acetazolamide against intracellular CA from C. reinhardtii (2 × 10−5 and 3 × 10−4 M), periplasmic CA from C. reinhardtii (5 × 10−9 and 8 × 10−9 M) and purified spinach CA (5 × 10−7 and 3 × 10−5 M) were in accord with CA from M. lylae (1.0 × 10−6 and 3.0 × 10−5 M) and M. luteus (4.0 × 10−7 and 9.0 × 10−7 M). Similarly, the inhibitory effect (IC50 values) of acetazolamide against alpha CA from Rhodopseudomonas palustris (3.1 × 10−8 M) and Oryza sativa expressed in Escherichia coli (2 × 10−6) indicated a strong resemblance of CA from the two indigenous Micrococcus species with the α–CA superfamily.
Molecular Characterization of Intracellular Carbonic Anhydrase
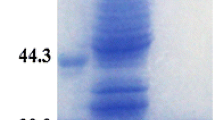
During the present course of the study, the subunit molecular weight (29 kDa) of M. lylae CA (I of Fig. 5) and M. luteus CA along with estimated molecular mass (29 kDa) determined by gel filtration chromatography indicated that the enzyme is a monomer. Native-PAGE along with zymography (II of Fig. 5, II of Fig. 6) indicated the presence of a single isoform in intracellular CA from both the indigenous Micrococcus species. There are very few reports of monomeric CA in bacterial domain including; Neisseria sicca (29 kDa; [28], Neisseria gonorrhoea (25 kDa [28]), H. pylori (29 kDa), B. subtilis (37 kDa, [34]), C. freundii (24 kDa; [27]) and recombinant Gca1 protein (21 kDa) from A. brasilense [36]. In an interesting study, our group [7] reported the purification of a 31-kDa extracellular CA from P. fragi. The enzyme was characterized as a trimer with a molecular mass of 91 kDa with two isoforms. The presence of dimeric [17, 20] and tetrameric CA [37] has also been reported in bacterial domain.
Determination of Kinetic Parameters
The K m and V max values of M. lylae CA (2.31 mM; 769.23 μmol/mg/min; Fig. 7) and M. luteus CA (2.0 mM; 1,000 μmol/mg/min; Fig. 8) were estimated from the intercepts on X and Y axes of the LB plot (Figs. 7 and 8). The turnover number (K cat) and catalytic efficiency (K cat/K m) of CA from M. lylae (37.27 s−1; 1.61 × 104) and M. luteus (46.51 s−1; 2.32 × 104) were calculated. The Lineweaver–Burk plots indicated competitive inhibition of carbonic anhydrase in the presence of both acetazolamide and ethoxozolamide. The inhibitory constant (K i) of acetazolamide and ethoxozolamide was found to be 2.78 and 0.918 mM for M. lylae CA, respectively (Fig. 7); similarly, K i of acetazolamide and ethoxozolamide was found to be 1.623 and 1.235 mM for M. luteus CA, respectively (Fig 8).
The carbonic anhydrase enzyme also catalyzes some non-physiological reactions apart from catalyzing the reversible hydration of carbon dioxide for maintaining homeostasis [29]. Therefore, esterase activity has been used as a tool to study the kinetic parameters [22, 27, 33]. In the present study, V max of M. lylae CA (769.23 μmol/mg/min) and M. luteus CA (1,000 μmol/mg/min) was found to be higher compared to B. subtilis CA (714.28 μmol/mg/min). The K m values of M. lylae CA (2.307 mM) and M. luteus CA (2.00 mM) were found to be lower in contrast to B. subtilis CA (9.09 mM), HCA I (3.025 mM) and HCA II (30.53 mM), thus indicating the high affinity of the enzyme for the substrate. The catalytic efficiency of CA from both the indigenous strains was found to be higher compared to HCA I, HCA II and mCA XIII in terms of esterase activity. The high turnover number (K cat) and catalytic efficiency (K cat/K m) of CA from both the Micrococcus species indicated that the CA plays an important role in the physiology of these organisms. In the present study, acetazolamide and ethoxozolamide were found to competitively inhibit CA from both the strains. The inhibitory constant (K i) values indicated that ethoxozolamide acted as better potency inhibitor compared to acetazolamide for CA from both the strains involved in the study. The characteristic inhibitory profile of M. lylae and M. luteus CA resembled closely to human isoforms HCA I and HCA II but was in stark contrast with both alpha and beta CA from H. pylori (hpCA) and gamma CA from M. thermophila which were more sensitive towards acetazolamide than ethoxozolamide [38]. The studies with inhibitors indicated the divergence within carbonic anhydrase superfamily that arises due to subtle differences in the amino acid sequence at the active site of the enzyme especially the hydrophilic and hydrophobic pockets, where complex interaction occurs between amino acid residues and the different functional groups present in the inhibitors [38].
The study for the first time reports the purification and characterization of alpha type CA from two indigenous strains belonging to genus Micrococcus. Although CA from both the strains have similar molecular mass and subunit molecular weight, substantial differences exist in their biochemical and kinetic properties, reflecting the specific and significant role this enzyme could play in regulating the physiology and metabolism of the organism pertaining to their bioenergetic requirements and also the possible influence of ecological niche and its microcosm on the enzymology of carbonic anhydrase.
The recent studies have indicated the role of carbonic anhydrase in the pathogenecity of established pathogens and subsequent designing of isozyme specific inhibitors as possible drug targets have opened up a whole new dimension to the role of CA in prokaryotes [6, 38]. The present study reflects how CA from closely related species exhibit quite distinct inhibitory profile thus necessitating the importance of understanding the intricate interaction between the enzyme and inhibitors based on their structural and thermodynamic relationship. The current scenario linked with elevated CO2 concentration has inclined the development of novel strategies to mitigate the problem of global warming. The biomimetic approach involves the use of an enzyme carbonic anhydrase for sequestering CO2 into CaCO3 [11]. Carbonic anhydrase from P. fragi, M. lylae and M. luteus has been evaluated with the perspective of biomimetic sequestration for application at an on-site scrubber [7, 8]. This study holds a key prospective with respect to the application of CLEAs at on-site scrubbers and opens up the door for efficient designing of membrane-bound and sol–gel systems for bioreactor application. Our lab is currently working on the recyclability efficiency of the immobilized CLEAs system compared to the biopolymeric [8] and SEN system [39] being used for CA immobilization. Such unique application of this enzyme entails further identification, purification and characterization of carbonic anhydrase from diversified genus of bacteria spread across composite habitats.
Conclusion
The study for the first time reports the purification and characterization of an alpha-type carbonic anhydrase from the genus Micrococcus. CA from both the strains is monomeric in nature exhibiting high esterase activity. The present study holds a pioneering ground reflecting the convoluted differences in the kinetic and biochemical properties along with reasonable similarities in molecular characteristics of carbonic anhydrase from M. lylae and M. luteus isolated from similar niche, thus substantiating the phenomena of convergent evolution in CA cutting across different phylogenetic realms. Low K m value and high catalytic efficiency of both the CAs validated their importance in maintaining homeostasis and regulating inorganic carbon transport. The study also emphasizes that the CA from the two strains although resembled α–CA superfamily but exemplified the attributable differences in their inhibitory profile. This pioneering study establishes the efficiency of CLEAs as an effective system for enhancing the thermostability and operational stability for application at an on-site scrubber pertaining to CO2 sequestration.
References
Lindskog, S. (1997). Pharmacology and Therapeutics, 74, 1–20.
Zimmerman, S. A., & Ferry, J. G. (2008). Current Pharmacologica Design, 14, 716–721.
Liu, N., Bond, G. M., Abel, A., McPherson, B., & Stringer, J. (2005). Fuel Processing Technology, 86, 1615–1625.
Klengel, T., Liang, W. J., Chaloupka, J., Ruoff, C., Schroppel, K., Naglik, J. R., et al. (2005). Current Biology, 15, 2021–2026.
Bahn, Y. S., Cox, G. M., Perfect, J. R., & Heitman, J. (2005). Current Biology, 15, 2013–2020.
Elleuche, S., & Poggler, S. (2010). Microbiology, 156, 23–29.
Sharma, A., & Bhattacharya, A. (2010). Journal of Molecular Catalysis B: Enzymatic, 67, 122–128.
Sharma, A., Bhattacharya, A., & Shrivastava, A. (2011). Enzyme and Microbial Technology, 48, 416–426.
Sharma, A., Bhattacharya, A., Pujari, R., & Shrivastava, A. (2008). Indian Journal Microbiology, 48, 365–371.
Sharma, A., Bhattacharya, A., & Singh, S. (2009). Process Biochemistry, 44, 1293–1297.
Bond, G. M., Stringer, J., Brandvold, D. K., Simsek, F. A., Medina, N. G., & Egeland, G. (2001). Energy & Fuels, 15, 309–331.
Gulla, K. C., Gouda, M. D., Thakur, M. S., & Karnath, N. G. (2004). Biosensors and Bioelectronics, 19, 621–625.
Sheldon, R. A. (2007). Advanced Synthesis and Catalysis, 349, 1289–1307.
American Public Health Association (APHA) (1985). Standard methods for examination of water and wastewater, 16th ed (pp. 199–200). Washington, DC: American Public health Association.
Kreig, R. N., & Holt, J. G. (1984). Bergey’s manual of systematic bacteriology (Vol. 1). Baltimore: William and Wilkins Co.
Edwards, U., & Rogall, T. H. (1989). Nuclear Acid Research, 17, 7843–7853.
Pushkas, L. G., Inui, M., Zahan, K., & Yukawa, H. (2000). Microbiology, 46, 2957–2966.
Oritz-Soto, M. E., Rudino-Pinera, E., Rodriguez-Algeria, M. E., & Munguia, A. L. (2009). BMC Biotechnology, 9(68), 1–8.
Schoevaart, R., Wolbers, M. W., Golubovic, M. W., Ottens, M., & Kieboom, A. P. G. (2004). Biotechnology and Bioengineering, 87(6), 754–762.
Smith, K. S., & Ferry, J. G. (1999). Journal of Bacteriology, 181, 6247–6253.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Ekinci, D., Beydemir, S., & Kufreivioglu, O. I. (2007). Journal Enzymology Inhibition and Medicinal Chemistry, 22(6), 745–750.
Sergeev, V. N., Gerasimenko, L. M., & Zavarzin, G. A. (2002). Mikrobiologiia, 71, 623–637.
Rawat, M., & Moroney, J. V. (1995). Plant Physiology, 109, 937–944.
Alber, B. E., & Ferry, J. G. (1994). Proceedings National Academy Science USA, 91, 6909–6913.
Braus Stromeyer, S. A., Schnappauf, G., Braus, G. H., Gossner, A. S., & Drake, H. L. (1997). Journal of Bacteriology, 179, 7197–7200.
Ramanan, R., Kannan, K., Vinayagamoorthy, N., Ramkumar, K. M., Sivanesan, S. D., & Chakrabarti, T. (2009). Biotechnology and Bioprocess Engineering, 14, 32–37.
Smith, K. S., & Ferry, J. G. (2000). FEMS Microbiology Reviews, 24, 335–366.
Kupriyanova, E., Villarejo, A., Markelova, A., Gerasimenko, L., Zavarzin, G., Samuelsson, G., et al. (2007). Microbiology, 153, 1149–1156.
Stahler, M. F., Ganter, L., Katherin, L., Manfred, K., & Stephen, B. (2005). FEMS Immunology and Medical Microbiology, 44, 183–189.
Costa, S. A., Tzanov, T., & Carneiro, A. F. (2002). Enzyme Microbial Technology, 30, 387–391.
Gouda, M. D., Singh, S. A., Appu Rao, A. G., Thakur, M. S., & Karnath, N. G. (2003). Journal of Biological Chemistry, 278(27), 24324–24333.
Innocenti, A., Muhlschegel, F., Hall, R. A., Steegborn, C., Scozzafava, A., & Supuran, C. T. (2008). Bioorganic & Medicinal Chemistry Letters, 18, 5066–5070.
Ramanan, R., Kannan, K., Sivanesan, S. D., Mudliar, S., Kaur, S., Tripathi, A. K., et al. (2009). World Journal of Microbiology and Biotechnology, 25, 981–987.
Armstrong, J. M., Myers, D. A., Verpoorte, J. A., & Edsall, J. T. (1966). Journal of Biological Chemistry, 241(2), 5137–5149.
Kaur, S., Mishra, M. N., & Tripathi, A. K. (2010). BMC Microbiology, 10, 184–189.
Kaur, S., Mishra, M. M., & Triphathi, A. K. (2009). FEMS Microbiology Letters, 299, 149–158.
Supuran, C. T. (2008). Current Pharmaceutical Design, 14, 603–614.
Yadav, R., Satyanaryana, T., Kotwal, S., & Rayalu, S. (2011). Current Science, 100(3), 520–524.
Acknowledgments
The authors are thankful to DBT, New Delhi for providing the financial assistance, and A. Bhattacharya is thankful to CSIR New Delhi for granting the CSIR-SRF fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharya, A., Shrivastava, A. & Sharma, A. Evaluation of Enhanced Thermostability and Operational Stability of Carbonic Anhydrase from Micrococcus Species. Appl Biochem Biotechnol 170, 756–773 (2013). https://doi.org/10.1007/s12010-013-0226-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0226-y