Abstract
The expression cassette I10 containing the new-found flocculation gene, FLONS, was transformed into an industrial strain Saccharomyces cerevisiae YSF5. Upstream activating sequences of the S. cerevisiae alcohol dehydrogenase II (ADH2) gene promoter (PU-ADH2) were used to regulate the expression of FLONS; α-acetolactate synthase gene ILV2 was chosen for homologous recombination of I10 to the YSF5 chromosome; copper binding metallothionein (encoded by CUP1) was used for selection of transformants. Ten randomly selected transformants exhibited increased flocculation ability of 1.5 to 2.3 fold more than the original strain. Based on their sensitivity to glucose, maltose and sucrose, flocculation property of the transformants was supported to be NewFlo-type. After successive subculture, the introduced CUP1 remained in the transformants. At the end of simulated fermentation test, diacetyl content of the culture media of 5I-1 was 0.45 g l−1, lower than YSF5 (0.48 g l−1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
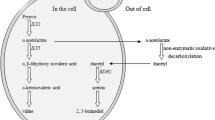
Yeast flocculation is a reversible, asexual and calcium-dependent process in which yeast cells aggregate into clumps and sediment rapidly in the medium (lager yeasts) or rise to the medium’s surface (ale yeasts) (Verstrepen et al. 2003). Mainly based on sugar sensitivity, flocculation phenotypes can be classified into two categories: the Flo1-type, which is only sensitive to mannose, and the NewFlo-type which can be inhibited by mannose, glucose, sucrose and maltose (Stratford and Assinder 1991).
Yeast flocculation involves flocculins—lectin-like cell wall proteins encoded by the FLO gene family—interacting with mannose residues on the surface of adjacent yeast cells. Flocculation allows the convenient separation of cells from the fermentation products, so yeast strains exhibiting strong flocculation toward the end of fermentation is desirable in industry. Genetic methods have been applied to improve flocculation behavior of yeasts (Cunha et al. 2006; Verstrepen et al. 2003). As NewFlo-type flocculation is sensitive to most fermentable sugars, yeast strains displaying this phenotype may possess greater potential for practical application. Lg-FLO1 encodes a flocculin which binds both mannose and glucose and is believed to be responsible for NewFlo phenotype of most lager yeasts, but only partial DNA sequences was determined due to the instability of Lg-FLO1 in E. coli (Kobayashi et al. 1998). In our previous study, FLONS was cloned from a NewFlo-type strain Saccharomyces cerevisiae YN79 (a lager yeast) and was capable of bringing NewFlo-type flocculation property to nonflocculent laboratory strain S. cerevisiae YS58. Detailed analysis of nucleotide sequence of the intact FLONS (GenBank accession number: EF182714) revealed that, with a full length of 3,843 bp containing an ORF of 3,396 bp, FLONS was a derived form of FLO1 and different from Lg-FLO1 (Liu et al. 2007).
In this study, an expression cassette I10 harboring FLONS was introduced into an industrial strain S. cerevisiae YSF5; properties of transformants were then investigated.
Materials and methods
Strains and growth conditions
Escherichia coli DH5α was used as the host for development and characterization of recombinant plasmids. E. coli cells were grown at 37°C in LB medium. Ampicillin was added when necessary at 50 μg ml−1.
Industrial strain S. cerevisiae YSF5 (Tsingtao Brewery Co., Ltd) was used as the transformation recipient of the expression cassette I10. S. cerevisiae YS59 (Flo1-type strain) and S. cerevisiae YN79 (NewFlo-type strain and donor of FLONS) were used as control in the analysis of transformants. Yeast strains were cultivated at 30°C in YPD medium. To repress or induce UAS of alcohol dehydrogenase II (ADH2)gene promoter (PU-ADH2), yeast transformants were grown in YP medium containing 30 g glucose l−1, or 10 g glucose l−1, respectively (Price 1997).
Plasmids
The E. coli-yeast shuttle vector YEp352 (Ampr, 2μ, URA3) was used for plasmid construction. pLZ-2 (Li et al. 2002) is the recombinant plasmid that α-acetolactate synthase gene ILV2 (from positions −455 to +2476) was cloned into the BamHI and SalI sites of YEp352. YFp-S (Liu et al. 2007) is the recombinant plasmid that the 3.8 kb BamHI–EcoRI FLONS fragment was cloned into the centromeric shuttle vector YCp50.
Expression cassette construction and yeast transformation
The YSF5 DNA was used as the PCR template of PU-ADH2 and CUP1. PU-ADH2 was amplified with the primers PA1 and PA2 (Table 1). Purified PCR product of PU-ADH2 was digested by XbaI and BglII and then ligated to XbaI–BglII digested pLZ-2, generating pLZ-A. The intact CUP1 was amplified with PC1 and PC2 (Table 1). PCR product of CUP1 was purified and digested by BamHI and EcoRI and then inserted into the BamHI–EcoRI digested YEp352, generating YEp-CUP. The BamHI–EcoRI FLONS fragment from YFp-S and the BamHI–EcoRI CUP1 fragment from YEp-CUP were inserted into the BglII-linearized pLZ-A, generating pIC10 (Fig. 1a).
The pIC10 was digested by BamHI and PstI. The fragment containing the expression cassette I10 (Fig. 1b) was purified and transformed into YSF5 using the lithium acetate method (Adams et al. 1997). Transformants were selected on YPD plates containing 5.5 mM copper sulfate.
Flocculation assay
Flocculation assay was performed as described (Kobayashi et al. 1998) with modifications. After derepression of ADH2 promoter for 12 h (Price 1997), yeast cells were harvested by centrifugation and washed in 0.01 M EDTA (pH 8.0) and sterile water twice and then suspended in 5 ml flocculation buffer (50 mM sodium acetate, pH 4.5) with or without 1 g CaCl2 l−1. After incubated in the water bath at 25°C for 30 min, OD600 of the upper 3 ml was measured. Flocculation ability was determined by the equation F = 1 − B/A, where F is the flocculation ability, B is the OD600 with CaCl2 and A is the OD600 without CaCl2. All the tests were repeated three times under the same conditions.
To evaluate sugar inhibition, 0.5 M sugars were added to the flocculation buffer. Yeast cells were killed by incubation for 5 min at 60°C when suspended in EDTA. This treatment did not affect the flocculating ability of yeasts (Stratford and Assinder 1991).
Glucose assay
Glucose concentration of the culture medium was determined by the dinitrosalicylic acid method (Bailey 1988).
Stability analysis
The transformants from YPD slants were cultivated in 5 ml YPD for 14 h at 30°C on a rotary shaker. Cultures were diluted and spread on YPD plates. After incubated for 48 h at 30°C, 100 colonies of each transformant were transferred to 0.5 ml sterile water and starved for 4 h at room temperature. A total of 10 μl of each starved suspension was inoculated on YPD plates containing 6 mM CuSO4 and incubated for 48 h at 30°C (He et al. 2000). Flocculation ability of these colonies (with or without glucose in flocculation buffer) was also determined. The stability of the transformants was determined by their retained copper resistance and flocculation ability.
Fermentation test and diacetyl measurement
Wort (10°Bé) was used for fermentation. Yeast strains were incubated in 5 ml wort for 12 h at 30°C and then 1 ml was inoculated into 10 ml wort and grown for 36 h. The cells were collected and inoculated into 500 ml Erlenmeyer flask containing 270 ml wort. Initial cell density was adjusted to OD600 of 0.2. The flasks were cultivated for 16 days at 12°C (Wang et al. 2007).
Diacetyl concentration of the culture media was determined by the colorimetric method (Westerfeld 1945). A total of 1 ml 5% (w/w) creatine and 1 ml α-naphthol solution (1 g α-naphthol dissolved in 20 ml 2.5 M NaOH, prepared before using) was added to 5 ml sample. The color was allowed to develop at room temperature for exactly 10 min and then A540 of the solution was measured.
Results and discussion
Construction of I10 and confirmation of transformants
The recombinant plasmid pIC10 and the expression cassette I10 are shown in Fig. 1a, b. Flocculation is a complex and strain-dependent characteristic of yeast and is influenced by genetic background, environmental factors (which can affect FLO gene expression and Flo protein activation), and factors act on the physical interactions between cells. Since FLONS possesses only 157 bp putative TATA element upstream of its ORF (Liu et al. 2007), an UAS is needed to regulate its expression to guarantee the proper flocculation behavior of the transformants. ADH2 promoter is repressed in the presence of glucose and derepressed more than 200-fold when glucose is absent (Price 1997), so it is ideal for the regulation of FLONS. As YSF5 is a wild-type yeast strain and has no nutritional marker, the CUP1 gene that encodes a metallothionein which binds copper and leads to increased resistance of yeast cells to copper ions was adopted for the selection of transformants (Karin et al. 1984). The flanking ILV2 sequences are designed for homologous recombination of I10 to ILV2 allele of the YSF5 chromosome. ILV2 encodes α-acetolactate synthase which catalyze the conversion of pyruvate to acetolactate, a precursor of diacetyl (2,3-butanedione)—an off-flavor component of beer. Removal of diacetyl is crucia1 for beer maturation, and destruction of one ILV2 gene could lower diacetyl production (Villa et al. 1995; Li et al. 2002). The deletion of ILV2 and the integration of I10 were confirmed by PCR (Fig. 1c) with the primers PA2 and PI2 (primer of ILV2; see Table 1).
Flocculation property of transformants
Flocculation level of 10 randomly selected transformants 5I-1 to 5I-10 was examined (Fig. 2a). Their flocculation ability increased by 1.5 to 2.3 fold of YSF5 and was equivalent to the positive control strains YS59 and YN79.
Flocculation property of transformants. (a) Flocculation ability of transformants 5I-1 to 5I-10 compared with YSF5 (the original strain), YS59 (Flo1-type strain) and YN79 (NewFlo-type strain and donor of FLONS). (b) Effects of sugars (▧, No sugar; ▥, mannose;
 , glucose; ▤, sucrose;
, glucose; ▤, sucrose;
 , maltose) on flocculation ability of 5I-1, YN79, YS59 and YSF5
, maltose) on flocculation ability of 5I-1, YN79, YS59 and YSF5
To infer the flocculation phenotype of the transformants, their flocculation behavior in the presence of mannose, glucose, sucrose and maltose was examined. Figure 2b shows the flocculation ability of transformant 5I-1 was inhibited by all the four kind of sugars. This was in accordance with the NewFlo-type strain YN79, but different from the Flo1-type strain YS59 which was only repressed by mannose.
In order to determine how long it would take to derepress ADH2 promoter and get flocculation ability, change of flocculation level of the transformants in the induction process of ADH2 promoter were recorded. Figure 3 shows that flocculation ability of the transformant 5I-1 displayed little increase comparing with YSF5 at the zero-hour point, but increased rapidly in the later 4 h as the glucose concentration of the media decreased. The regulation mechanism of ADH2 promoter is typical carbon catabolite repression. Besides glucose, other sugars involved in metabolism are also able to affect the catabolite repression regulation (Gancedo 1998). Maltose and sucrose are the most wildly used sugars in the fermentation industry, so their effect on ADH2 promoter was also measured. It was revealed that the effect of maltose and sucrose on the change of flocculation of 5I-1 was similar to that of glucose with peak values of 0.316 and 0.321, respectively.
Genetic stability analysis
Take 5I-1 as an example. All the transformed cells retained copper-resistant ability after successive subculture without selective pressure, indicating that CUP1 was stable on the chromosome. However, compared with the original 5I-1 strain, slight fluctuation of flocculation ability of randomly selected colonies of 5I-1 after stability analysis was detected (Fig. 4), perhaps due to the fact that the FLO genes are very unstable.
Fermentation test
A fermentation test simulating beer fermentation was carried out to test the applicability of the transformants in industry (see Fig. 5). During the fermentation process, diacetyl content of the culture media of 5I-1 was lower than that of YSF5, and the peak value of 5I-1 was 83% of that of YSF5, but no obvious difference in fermentation rate (indicated by cell density) between 5I-1 and YSF5 was observed. At the end of simulated fermentation test, diacetyl content of the culture media of 5I-1 was 0.451 g l−1, lower than YSF5 (0.483 g l−1). This could provide a reference for later fermentation test.
Conclusion
The I10 harboring FLONS, PU-ADH2, CUP1 and ILV2 were introduced into the industrial yeast strain YSF5. Since both the expression of FLONS under the control of ADH2 promoter and the function of Flonsp were repressed by the presence of fermentable sugars, the transformants displayed NewFlo flocculation property toward the end of fermentation when sugar was almost exhausted. This may be desirable in industry. By disruption of ILV2 allele, decreased diacetyl content were observed in the fermentation test of the transformants. In the stability test, the CUP1 was stable on the YSF5 chromosome, although slight change of flocculation ability was detected. In addition, other sites of the chromosome can be used instead of ILV2 to live up to different expectations. As nonyeast DNA was avoided in this study, the transformants were self-cloning strains which could bring down the risk of safety and are expected to provide an easier way to be accepted by the general public.
References
Adams A, Gottschling DE, Kaiser CA et al (1997) Methods in yeast genetics: a cold spring harbor laboratory course manual. Cold Spring Harbor Laboratory, New York
Bailey MJ (1988) A note on the use of dinitrosalicylic acid for determining the products of enzymatic reactions. Appl Microbiol Biotechnol 29:494–496
Cunha AF, Missawa SK, Gomes LH et al (2006) Control by sugar of Saccharomyces cerevisiae flocculation for industrial ethanol production. FEMS Yeast Res 6:280–287
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62:334–361
He X, Huai W, Tie C et al (2000) Breeding of high ergosterol-producing yeast strains. J Ind Microbiol Biotechnol 25:39–44
Karin M, Najarian R, Haslinger A et al (1984) Primary structure and transcription of an amplified genetic locus: the CUPI locus of yeast. Proc Natl Acad Sci USA 81:337–341
Kobayashi O, Hayashi N, Kuroki R et al (1998) Region of Flo1 proteins responsible for sugar recognition. J Bacteriol 180:6503–6510
Li Y, Tie C, Wang Z et al (2002) Construction of diacetyl-low brewer’s yeast. Liquor Making 29:77–79
Liu N, Wang D, Wang Z et al (2007) Genetic basis of flocculation phenotype conversion in Saccharomyces cerevisiae. FEMS Yeast Res 7:1362–1370
Price VL (1997) Inducible expression cassettes in yeast: ADH2. Methods Mol Biol 62:149–157
Stratford M, Assinder S (1991) Yeast flocculation: Flo1 and NewFlo phenotypes and receptor structure. Yeast 7:579–594
Verstrepen KJ, Derdelinckx G, Verachtert H (2003) Yeast flocculation: what brewers should know. Appl Microbiol Biotechnol 61:197–205
Villa KD, Lee S, Masschelein CA et al (1995) Control of vicinal diketone production by brewer’s yeast. I. Effects of ILV5 and ILV3 gene amplification on vicinal diketone production and ILV enzyme activity. J Am Soc Brew Chem 53:49–53
Wang Z, He X, Zhang B (2007) Over-expression of GSH1 gene and disruption of PEP4 gene in self-cloning industrial brewer’s yeast. Int J Food Microbiol 119:192–199
Westerfeld WW (1945) A colorimetric determination of blood acetoin. J Biol Chem 161:495–502
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, D., Wang, Z., Liu, N. et al. Genetic modification of industrial yeast strains to obtain controllable NewFlo flocculation property and lower diacetyl production. Biotechnol Lett 30, 2013–2018 (2008). https://doi.org/10.1007/s10529-008-9773-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9773-7