Abstract
A novel two-step fermentation process was developed to enhance arachidonic acid (ARA) production by Mortierella alpina ME-1 in a 5 l fermentor. Agitation speed and aeration rate were adjusted from 180 to 40 rpm and from 0.6 to1 vvm, respectively, after 5 days cultivation, to decrease physical damage to the mycelia and to extend the stationary phase. Moreover, 3% (w/v) and 2% (w/v) ethanol were fed after 5 and 7 days cultivation, respectively, to enhance ARA content of total lipid. Eventually, an ARA yield of 19.8 g/l was achieved, which was 1.7 times higher than that of a one-step fed-batch cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachidonic acid (ARA; 5,8,11,14-cis-eicosatetraenoic acid) is a major constituent and plays the role of maintaining membrane fluidity in some biological cells (Higashiyama et al. 2002). As a precursor of prostaglandins, thromboxane, prostacyclin, and leucotrienes, ARA has various physiological functions (Gill and Valivety 1997; Horrobin and Huang 1987), and has applications in medicine, pharmacology, cosmetics, the food industry, agriculture and other fields (Eroshin et al. 2000).
Although animal liver, fish oil and egg yolk are well known as sources of ARA (Gill et al. 1997; Ratledge 2004), Mortierella fungi seems to be the most prominent source (Sakuradani et al. 2004). During the past 20 years, the effect of cell morphology, dissolved O2 concentration and medium compositions, including carbon, nitrogen sources and mineral addition, on ARA productivity were widely investigated and known as the key factors (Higashiyama et al. 1998; Park et al. 1999; Koike et al. 2001). However, most of those works limited the fermentation period to 6–8 days as the fungus is physically fragile (Higashiyama et al. 2002) with both shaking and agitation inhibiting or even damaging the growth of mycelia during the stationary phase of fermentation which, in turn, inhibits the synthesis of fatty acids and shortens the stationary phase. On the other hand, the stationary phase itself is an excellent phase for fatty acids, especially ARA, synthesis (Jin et al. 2007). Therefore, the present investigation highlights the use of the stationary phase to enhance ARA production by developing a two-step fermentation system of M. alpina. The first step was similar to conventional fermentation but in the second step the agitation was adjusted to an extremely low level to decrease physically damage and extend the period of stationary phase.
Changes of the lipid composition in the presence of ethanol have been investigated in several microorganisms other than M. alpina, showing an increase in the ratio of unsaturated to saturated fatty acids (D’Amore and Stewart 1987; Teixeira et al. 2002; Jones 1989). Moreover, ARA is the main unsaturated fatty acid in M. alpina. Accordingly, ethanol was introduced in the second step of the two-step fermentation system to further enhance ARA content of the lipid.
Materials and methods
Microorganism
Mortierella alpina ME-1 was obtained by UV mutation of the original ATCC 16266 strain at 20 W for 15 min. It generates a lot of yellow agglomerations on mycelia when cultured on slant for 8 days.
Culture conditions
The inoculum medium contained (g/l): glucose 30; yeast extract 6; KH2PO4 3; NaNO3 3; MgSO4 · 7H2O 0.5. Inocula were prepared in 250 ml baffled flasks containing 50 ml medium. The culture was grown for 3 days at 25°C with shaking at 120 rpm. The 5 l fermentor containing 3 l production medium was inoculated at 10% (v/v), and incubated at 23°C, aeration rate 0.6 vvm, and agitation speed 180 rpm with pH uncontrolled.
Analytical methods
The dinitrosalicylic acid method was used to assay the glucose content. Dry cell weight (DCW) concentration was determined gravimetrically. Total lipids were extracted with chloroform/methanol (2:1, v/v) following the method of Bligh and Dyer (1957). Fatty acids were methylated by BF3 in methanol (Metcalfe and Schmitz 1961). Fatty acid methyl esters and ethanol were determined by GC–MS and GC (Jin et al. 2007; Hu et al. 2007).
Results and discussion
One step fed-batch cultivation
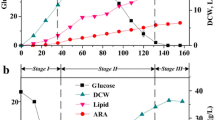
Time courses of cell growth, glucose consumption, and total lipid and ARA biosynthesis of M. alpina ME-1 during one-step fed batch cultures are shown in Fig. 1 and fatty acids profiles of lipids are presented in Table 1. The biomass reached its maximal value (33.5 g/l) after 7 days. The mycelial morphology of M. alpina ME-1 was maintained in a fluffy pellet, which is suggested to be suitable for ARA production (Park et al. 1999). Lipid reached 18.9 g/l and ARA 11.7 g/l after 8.5 days. Then, however, the biomass declined (see Fig. 1) with a certain amount of glucose still remaining. At the same time the mycelia became fragmented, possibly caused by a high shear stress. This fragility of the biomass may thus explain the decreased biomass.
Two-step cultivation with feeding glucose
A high shear stress clearly disrupted the mycelia in the stationary phase of the one-step fed-batch cultivation (see Fig. 1). Thus a two-step cultivation system was introduced, in which the agitation speed was reduced to 40 rpm and aeration rate was adjusted to 1 vvm in the second phase (see Fig. 2). Compared with the one-step fed batch cultivation, physically damage was reduced and the mycelia disruption did not begin until 10.5 days. As a result, the total lipid content of biomass increased to 65%, and the ARA yield now was 15 g/l.
Profiles of cell growth, glucose consumption, and total lipid and ARA production during two-step cultivation of M. alpina ME-1 with agitation speed and aeration rate adjusted from 180 to 40 rpm and from 0.6 to 1 vvm, respectively and feeding 2% (w/v) glucose and 0.3% (w/v) NaNO3 after 5 days. Each datum is the mean value of three identical samples
Two-step cultivation with feeding ethanol
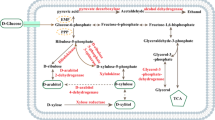
To achieve a higher ARA content, 5% (w/v) ethanol instead of glucose was fed after 5 days (see Fig. 3). The final ARA content of total lipid was increased to 72% and ARA yield was 17.9 g/l after 11 days. Ethanol in its metabolism might generate additional reducing power, NADPH, (Sijtsma et al. 2005) for the various desaturases needed to produce ARA and which may be a limiting factor for ARA production.
Profiles of cell growth, glucose consumption, and total lipid and ARA production during two-step cultivation of M. alpina ME-1 with agitation speed and aeration rate adjusted from 180 to 40 rpm and from 0.6 to 1 vvm, respectively and feeding 5% (w/v) ethanol and 0.3% (w/v) NaNO3 after 5 days. Each datum is the mean value of three identical samples
During the stationary phase with a low concentration of nitrogen source, the carbon flux through the glycolytic pathway was decreased (Jin et al. 2007), leading to a relative low glucose consumption (see Fig. 2). When ethanol was used as a carbon source, it avoided the glycolytic pathway and the consumption of carbon source was improved (see Fig. 3). In addition, ethanol could be converted directly to acetyl-CoA and provided additional reducing power NADPH, which is needed for lipogenesis (Sijtsma et al. 2005). Therefore, a higher fatty acids production rate (see Fig. 3) was achieved. Eventually, the total lipid content of biomass was enhanced to 67%.
During the initial 24 h after feeding 5% (w/v) ethanol, there was a slight inhibition on biomass synthesis (see Fig. 3). To maximize the final product concentration, the effect of substrate inhibition was removed by first feeding 3% (w/v) ethanol after 5 days fermentation and adding 2% (w/v) ethanol at the 7th day (Fig. 4). Eventually, ARA content of total lipid was enhanced to 75% and ARA accumulated up to 19.8 g/l after 11 days cultivation, which was 1.7 times higher than that of one-step fed batch cultivation and was also higher than the previously reported yields (Singh and Ward 1997; Hwang et al. 2005).
Profiles of cell growth, glucose consumption, and total lipid and ARA production during two-step cultivation of M. alpina ME-1 with agitation speed and aeration rate adjusted from 180 to 40 rpm and from 0.6 to 1 vvm, respectively and feeding 3% (w/v) ethanol with 0.3% (w/v) NaNO3 and 2% (w/v) ethanol after 5 and 7 days, respectively. Each datum is the mean value of three identical samples
Conclusions
A novel two-step fermentation process was developed to enhance arachidonic acid (ARA) production by M. alpina ME-1 in a 5 l fermentor. In the first step, the agitation speed and aeration rate were maintained to 180 rpm and 0.6 vvm, respectively. During the second step, the agitation speed was reduced to 40 rpm and aeration rate was adjusted to1 vvm to reduce the physically damage caused by agitation to the mycelia and extend the period of stationary phase, which is an excellent phase for fatty acids, especially ARA, synthesis. Moreover, ethanol was introduced to enhance ARA content of total lipid by feeding 3% (w/v) and 2% (w/v) ethanol after 5 and 7 days cultivation, respectively. Eventually, an ARA yield of 19.8 g/l, which constituted 75% of total lipid, was achieved after 11 days cultivation. It was 1.7 times higher than that of one-step fed-batch cultivation. The relatively high ARA yield displays the potential of using the two-step fermentation process in developing bioprocess for commercial production of ARA.
References
Bligh EG, Dyer WJ (1957) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
D’Amore T, Stewart GG (1987) Ethanol tolerance of yeast. Enzyme Microb Tech 9:322–330
Eroshin VK, Satroutdinov AD, Dedyukhina EG, Chistyakova TI (2000) Arachidonic acid production by Mortierella alpina with growth-coupled lipid synthesis. Process Biochem 35:1171–1175
Gill I, Valivety R (1997) Polyunsaturated fatty acid, part 1: occurrence, biological activities and applications. Trends Biotechnol 15:401–409
Higashiyama K, Fujikawa S, Park EY, Shimizu S (2002) Production of Arachidonic acid by Mortierella Fungi. Biotechnol Bioprocess Eng 7:252–262
Higashiyama K, Yaguchi T, Akimoto K, Fujikawa S, Shimizu S (1998) Effects of mineral addition on the growth morphology of and arachidonic acid production by Mortierella alpina 1S-4. J Am Oil Chem Soc 75:1815–1819
Horrobin DF, Huang YS (1987) The role of linoleic acid and its metabolites in the lowering of plasmacholesterol and the prevention of cardiovascular disease. Int J Cardiol 17:173–180
Hu YC, Huang H, Shi HF, Hu Y, Yan J, Chen L (2007) Catalytic dehydration of ethanol to ethylene using transition metal modified HZSM-5. Chem Bioeng 24:19–21
Hwang BH, Kim JW, Park CY, Park CS, Kim YS, Ryul YW (2005) High-level production of arachidonic acid by fed-batch culture of Mortierella alpina using NH4OH as a nitrogen source and pH control. Biotech Lett 27:731–735
Jin MJ, Huang H, Zhang K, Yan J, Gao Z (2007) Metabolic flux analysis on arachidonic acid fermentation. J Chem Eng Chinese Univ 21:316–321
Jones RP (1989) Biological principles for the effects of ethanol. Enzyme Microb Technol 11:130–153
KoikeY, Cai HJ, Higashiyama K, Fujikawa S, Park EY (2001) Effect of consumed carbon to nitrogen ratio on mycelial morphology and arachidonic acid production in cultures of Mortierella alpina. J Biosci Bioeng 91:382–389
Metchalfe LD, Schmitz AA (1961) The rapid preparation of fatty acid esters for gas chromatographic analysis. Anal Chem 33:363–372
Park EY, Koike Y, Higashiyama K, Fujikawa S, Okabe M (1999) Effect of nitrogen source on mycelial morphology and arachidonic acid production in cultures of Mortierella alpina. J Biosci Bioeng 88:61–67
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 86:807–815
Sakuradani E, Hirano Y, Kamada N, Nojiri M, Ogawa J, Shimizu S (2004) Improvement of arachidonic acid production by mutants with lower n-3 desaturation activity derived from Mortierella alpina 1S-4. App Microbiol Biotechnol 66:243–248
Sijtsma L, Anderson AJ, Ratledge C (2005) Alternative carbon sources for heterotrophic production of docosahexaenoic acid by the marine alga Crythecodinium cohnii. In: Cohen Z, Ratledge C (eds) Single cell oils. AOCS press, Illinois
Singh A, Ward OP (1997) Production of high yields of arachidonic acid in a fed-batch system by Mortierella alpina ATCC 32222. Appl Microbiol Biotechnol 48:1–5
Teixeira H, GonÇalves MG, Rozès N, Ramos A, San Romão MV (2002) Lactobacillic acid accumulation in the plasma membrane of Oenococcus oeni: a response to ethanol stress? Microb Ecol 43:146–153
Acknowledgements
The authors would like to thank the financial support from the National Natural Science Foundation of China (No. 20606018) and the Ministry of Science and Technology of China (National Basic Research Program of China (No. 2007CB707805)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, MJ., Huang, H., Xiao, AH. et al. A novel two-step fermentation process for improved arachidonic acid production by Mortierella alpina . Biotechnol Lett 30, 1087–1091 (2008). https://doi.org/10.1007/s10529-008-9661-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9661-1