Abstract
The entomopathogenic fungus, Beauveria bassiana, is capable of infecting pest mites, while the effect on different mite stages has rarely been reported. The present study evaluates the effect of several B. bassiana isolates (GZGY-1-3, LNSZ-26, SDDZ-9, XJWLMQ-32, SCWJ-2 and JXJGS-1) on Tetranychus urticae by a series of assays and observations. A potted bean plant assay indicated the fungal sprays resulted in greater reduction of adult T. urticae populations, but poor suppression in eggs and immature stages. During the one-month experiment, the different fungal isolates reduced the numbers of T. urticae eggs, immatures and adults by 38.7–55.2, 3.7–18.7 and 61.0–72.1%, respectively. Laboratory bioassays showed their corrected mortalities were 2.7–3.8, 17.5–25.8 and 63.2–71.2%, respectively, at seven days days post-fungal treatment. Fecundity of female mites was significantly reduced after fungal spray due to the lethal effect on females, while egg hatchability was not affected. Light microscopy observations indicated fungal outgrowths were evident in mite cadavers, but were not visible on eggs. Scanning electronic microscopy observations demonstrated fungal mycelia grew prolifically from the adult mite 60 h following fungal spray, although no symptoms of fungal infection were exhibited in most immature cadavers. Despite the fact that fungal conidia were able to adhere to and germinate on eggshells, and the germ tubes elongated on the shell surface, they were never observed to penetrate the eggs. Our results demonstrating that the failure to control T. urticae on bean plants using B. bassiana, which we attributed to its poor infectivity to mite eggs and immature stages, may provide useful information in future attempts to develop effective mite control strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The two spotted spider mite or red spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), is an extremely polyphagous pest that has been reported on more than 200 economically important crops, including ornamentals, vegetables, and fruits (Mondel and Ara 2006; Grbić et al. 2011; Wu et al. 2019). Because of its rapid developmental rate and high reproductive potential, this mite can colonize crops shortly with damaging outbreaks which usually occur during the peak of the growing season (Wilson and Morton 1993; Farazmand and Amir-Maafi 2018; Ullah et al. 2012). Spider mite control has mainly relied on the use of chemical insecticides, particularly synthetic pyrethroids (Kakoki et al. 2019; Prischmann et al. 2005). However, the overuse of pesticides has generally caused resistance among mite populations, as well as contributing to widespread concerns relative to environmental contamination and the presence of high residues in agricultural products (Wang et al. 2018; Guo et al. 1998; Dagli and Tunc 2001; Herron et al. 2004). As a result, several chemical acaricides, including dicofol, cyhexatin and fenbutatin oxide have been prohibited in many crops for mite control (Shi et al. 2008a, b), creating an urgent need to develop alternative measures that are sustainable and environmentally friendly.
The use of fungal pathogens as biological control measures in integrated pest management (IPM) programs is being explored and expected to reduce the dependence on acaricides (Alves et al. 2002; Maniania et al. 2008; Shin et al. 2017; Zhang et al. 2016, 2018). Among these fungi, Beauveria bassiana (Balsamo) Vuillemin is a well-known fungal biocontrol agent on a wide range of arthropod pest species (Feng et al. 1994) that has shown potential for mite control (Ullah and Lim 2017; Irigaray et al. 2003; Gatarayiha et al. 2011). Successful use of B. bassiana depends on environmental conditions, host population levels and physical properties of the pathogen, such as its efficacy against target hosts and having a low virulence in natural enemies of the target host. Among the host factors, the developmental stage of the intended target host has been found to affect its susceptibility to fungi (Ferron 1985). Recently, new isolates of B. bassiana derived from host insects have proven to be highly pathogenic to T. urticae adults while showing no detrimental effects in predatory mites under laboratory conditions (Wu et al. 2016). These findings have demonstrated that these fungal strains are promising biological control agents that may be employed as alternatives to harmful acaricides for mite management.
The aim of this study was to assess the effect of a B. bassiana suspension on T. urticae after direct application to all stages of the mite populations via potted plants and laboratory assays. Based on our previous results, one isolate of B. bassiana, shown to be highly virulent against T. urticae adults, was chosen to evaluate its effects on the oviposition of female mites and the hatch rate of eggs after inoculation. In order to confirm the effect of B. bassiana on T. urticae, light microscopy and scanning electron microscopy (SEM) were used to verify fungal infection. This information will be helpful in evaluating the potential for incorporating B. bassiana into future mite pest management strategies.
Materials and methods
Fungal isolate and preparation of conidial suspension
The origin and source of six isolates of B. bassiana are shown in Table 1. All isolates were maintained, and conidia produced on Sabouraud Dextrose Agar (SDA) kept at 26 ± 1 °C and incubated for seven days. Conidia powder was harvested using an inoculation loop. Fungal conidia were suspended in sterile 0.05% Tween-80 and diluted to 1 × 107 ml−1 conidia (the concentration recommended for bioassays by Shi et al. 2008a, b), according to the method described by Goettel and Inglis (1997). Conidial viability was confirmed on SDA medium using a hemocytometer, with the germination rate of the conidia determined to be > 90%. The five isolates (GZGY-1-3, LNSZ-26, SDDZ-9, XJWLMQ-32, SCWJ-2), which was derived from Ostrinia furnacalis Guenée larvae (Lepidoptera: Pyralidae), were chosen for this study, because they have previously been screened from 12 fungal isolates and shown to be virulent against T. urticae adults (Wu et al. 2016). The isolate JXJGS-1 was derived from T. urticae adults.
Preparation of different T. urticae stages
Two spotted spider mites, T. urticae, were obtained from the Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China, and reared on kidney beans (Phaseolus vulgaris) in a walk-in growth room at a regime of 20–30 °C, 60–70% RH and L:D 12:12. To obtain eggs, immatures (larvae, protonymphs and deutonymphs) and adults for bioassays, vigorous adult females were removed from the plants and transferred onto fresh leaves situated on moist cotton wool in a Petri dish (7 cm diameter) and allowed to freely oviposit for 20–24 h. The females were then removed, leaving the eggs allowed to hatch and develop until reaching the desired life stage. Bean leaves were changed every five days. The rearing was timed in such a way that different life stages could be obtained at any given time.
Potted bean plant assay
To evaluate the actual effect of all fungal isolates on a T. urticae population, this study was conducted on potted kidney bean plants in an experimental greenhouse. Twenty adult, gravid, T. urticae females were infested onto each seven-day-old plant. Four plants were enclosed in a cage consisting of a fine transparent mesh screen (0.1 mm mesh opening). Each cage of four plants was an experimental unit and served as one replicate. The experiment was replicated six times. Seven days after introduction, an initial count of T. urticae was made using a hand magnifier. Twelve leaves per cage were sampled non-destructively. These leaves were selected by visually dividing the plants in the cage equally into three vertical strata (lower, middle and upper) and from each stratum randomly sampling four leaves. The average number of eggs and individuals of mobile stages of T. urticae, including immatures (larvae, protonymphs and deutonymphs) and adults per leaf was determined. B. bassiana suspension was then sprayed on the plants with mite stages, using a hand-held pressure sprayer (15 ml). Care was taken that all parts of the plants were coated with spray droplets. Each potted bean plant was sprayed until run off, using approximately 8 ml of conidial suspension. Subsequent counts of mite densities were conducted every five days after inoculation using the above method. The second spray was conducted two weeks later. In the control treatment, the plants were sprayed with a sterile 0.05% Tween-80 solution. The same procedure for each fungal isolate as described above was carried out. The potted experiment lasted one month. Temperatures and RH were recorded throughout the test period.
Assessment of mite mortality in bioassays
Laboratory bioassays with B. bassiana on T. urticae were performed in Petri dishes (7 cm diameter), lined with a freshly excised bean leaf upside down on moisten filter paper. The leaf stalk was wrapped with moist cotton to slow leaf desiccation. The different stages of T. urticae, i.e., eggs, immatures (larvae, protonymphs and deutonymphs) and adults were separately transferred to a Petri dish. The effect of B. bassiana on the mortality in each mite stage was evaluated by spraying with fungal suspensions on the leaves using a hand-held pressure sprayer (2 ml). Each fungal treatment was replicated eight times with 20 individuals of the corresponding stage being tested in each replication. When immatures were bioassayed, the number of individuals used was five larvae and 15 nymphs (protonymphs and deutonymphs) per replicate. The concentration of fungal conidia deposited on the leaves was 735 ± 102 conidia mm−2. This estimate was based on the methods described by Wu et al. (2016).
After exposure to fungal spray, detached leaves were allowed to air dry for approximately 5 min in a Petri dish, which was then covered with a polyvinyl chloride (PVC) film, containing adequate fine holes for ventilation (Wu et al. 2016). The Petri dishes were incubated at 25 °C and L:D 12:12 in a climate chamber. The humidity inside the Petri dishes exceeded 80% (83–96%) RH, which was measured by using digital data loggers (Apresys®) prior to the formal assays. The number of dead individuals was recorded daily for seven days using an optical microscope (Nikon, SMZ1500, Japan) after the PVC cover had temporarily been removed to facilitate observation. Cumulative mortalities of untreated control individuals that had been sprayed with a sterile 0.05% Tween-80 solution never exceeded 8.5% on day 7. Immobile mites that failed to respond by obvious movement of their appendages when prodded with a fine paintbrush were considered to be deceased. Unhatched eggs and cadavers of mites were transferred onto moist filter paper in a Petri dish and incubated for 5–6 days. Individual mites and unhatched eggs that developed fungal mycelia were considered to have been killed as a result of fungal infection. The cumulative corrected mortality rates of different mite stages were determined over a seven-day period.
Effects of fungal sprays on fecundity of females
Mated 2–3 days old T. urticae females were transferred to a fresh bean leaf (five females per leaf) in Petri dishes, treated with fungal spray as above, and placed in a climate chamber and allowed to oviposit. Untreated control individuals were sprayed with a sterile 0.05% Tween-80 solution. Eggs laid in the fungal treatments were counted daily under a stereomicroscope until all treated mites had died (usually by day 10). The observed dead mites were removed from the dishes. Observations in the control set were continued until mites ceased to oviposit (usually by day 25). Each fungal treatment was replicated eight times.
Effects of fungal sprays on egg hatch
Twenty adult females were transferred onto a bean leaf in a Petri dish and allowed to oviposit for 20–24 h. The females were subsequently removed, leaving ~ 25–50 eggs per leaf for bioassay. The system used to retain moisture in the detached leaves usually maintained adequate moisture in the leaves for over ten days and allowed normal hatching of the mite eggs. Observations on the viability of eggs were continued for ten days following direct fungal spray on the eggs. Untreated control eggs were handled in a similar manner, except sterile 0.05% Tween-80 was used in place of fungal suspension. Each fungal treatment was replicated eight times.
Observations using light microscopy and scanning electronic microscopy (SEM)
According to the results from the tests above that the six fungal strains showed similar effects on mite population, mortality, fecundity of females and egg hatch, one of B. bassiana strains GZGY-1-3 was chosen in this test. In the potted bean plant assay, a mixed population of eggs and individuals of various mobile stages were first sprayed with B. bassiana as described above. The infection status of the mite mixture was examined ten days after spraying using an optical microscope. In the laboratory bioassays, mite cadavers were also viewed under a light microscope after six days. To verify the effect of B. bassiana on different T. urticae stages, the SEM was used to observe the micromorphology that occurred due to fungal conidial inoculation. After 60 h, the unhatched eggs and mite cadavers were randomly sampled and then fixed overnight in 5% (v/v) glutaraldehyde in phosphate buffer. The fixed samples were dehydrated in an ascending series of ethyl alcohol (60, 70, 80, 90, 95 and 100%, 5 min each), followed by air-drying for a few seconds. Dried samples were then coated with gold and viewed with the SEM.
Statistical analysis
In the potted assay, a two-way repeated-measures ANOVA (‘proc mixed’ procedure in SAS) (Littell et al. 2000) was used to compare the density of T. urticae between treatments (fungal spray and untreated control) throughout the sampling period. In the mixed model, treatments and sampling time were fixed factors, and the replicate was a random factor. The sampling time had six levels due to no significant differences being found in the initial densities in all T. urticae stages tested (all P’s > 0.05, one-way ANOVA, Tukey’s test). When a significant interaction was observed, the “slice” option was chosen to test the significance level of interaction between the treatment and sampling time. The levels of different stages of T. urticae control resulting from the fungal spray were evaluated by comparing their density decline at the end of each sampling time. Density decline (D) was calculated as D = (dT0 − dTi)/dT0 × 100%, where dT0 and dTi were the mean densities of corresponding mite stages in untreated controls and fungal treatments. Differences between means were compared by one-way ANOVA followed by Tukey’s test at P < 0.05. Comparison of the fecundity of females and hatching rate of eggs between fungal spray and untreated control treatments were conducted using the repeated-measures ANOVA. The corrected mite mortality attributable to B. bassiana under laboratory conditions was calculated using Abbott’s formula (Abbott 1925). A comparison of corrected mortalities between different mite stages was conducted using a one-way ANOVA. Data were normalized using square-root transformation prior to statistical analyses.
Results
Potted bean plant assay
The mean temperature and RH in the greenhouse during the experiment period were 24.6 °C (19–27 °C) and 79.8% (73–82%), respectively. The mean densities of different stages of T. urticae seven days after inoculation with the fungal sprays and the control treatments were 7.8–9.3 and 7.7–10.3 (no. eggs per leaf), 2.0–2.5 and 1.7–3.8 (no. immatures per leaf), 2.5–3.5 and 2.2–4.1 (no. adults per leaf). The initial densities of the T. urticae showed no significant differences between each fungal treatment and the corresponding untreated control (all P’s > 0.05). Overall, the population trends of T. urticae adults showed a rapid decrease after fungal sprays, followed by a consistent lower level at almost every subsequent sampling time. The number of eggs showed a decline beginning with the initial fungal sprays. However, the population trend of the immature stages following each fungal treatment was similar to the control group (Fig. 1 and Supplementary Fig. S1). The repeated-measures ANOVA showed that these fungal treatments had significant effects on the egg and adult stages of T. urticae (all P’s < 0.05 for both effects), while having no significant effect on the immature stages (all P’s > 0.05) (Table 2 and Supplementary Table S1–S5). The mean density declines of T. urticae eggs, immatures and adults at the conclusion of the sampling time, compared to the control, were 38.7–55.2, 3.7–18.7 and 61.0–72.1%, respectively.
Population fluctuations of T. urticae on potted bean plants following fungal spray (B. bassiana strain: GZGY-1-3). Initial densities of T. urticae were determined immediately prior to treatment on day 0. B. bassiana, GZGY-1-3 suspension, was sprayed at 1 × 107 fungal conidia ml−1 . a Eggs; b immatures (larvae, protonymphs and deutonymphs) of T. urticae; c adult T. urticae. Each data point and bar represent the mean and SE, respectively. Black arrows signify fungal spray
Assessment of mite mortality in bioassays
The fungal sprays caused corrected mortalities of 2.7–3.8, 17.5–25.8 and 63.2–71.2% in mite eggs, immatures and adults, respectively, seven days post-treatment. The mortality of adults was significantly higher than that of eggs and immatures (Fig. 2 and Supplementary Fig. S2) (all P’s < 0.05). Of those cadavers, approximate 15% of the immatures and 70% of the adults had visible mycelial growth from their bodies 5–6 days after incubation. However, unhatched eggs in the fungal treatments as well as the untreated control, although being noticeably shriveled, had no visible fungal hyphae. The mortalities of mite eggs, immatures and adults in the untreated control treatments were 1.7 ± 0.6% (mean ± SE), 5.2 ± 1.1% and 8.4 ± 2.6%, respectively.
Effects of fungal sprays on fecundity of females and egg hatch
Tetranychus urticae females gradually died after exposure to the fungal spray, and after ten days had oviposited, on average, only 4.0–6.3 eggs per mite. Untreated mites, however, averaged 34.6–37.4 eggs per mites after surviving for 25 days (Fig. 3a and Supplementary Fig. S3a). The repeated-measures ANOVA showed that the fungal treatment had a significant effect on female fecundity (all P’s< 0.0001). The cumulative hatch rate of fungus-treated and control eggs showed very similar trends after ten days, at 79.0–86.3% and 78.4–88.6%, respectively (Fig. 3b and Supplementary Fig. S3b), indicating that the fungal treatment had no significant effect on egg hatchability (all P’s > 0.05).
Light microscopy and scanning electronic microscopy (SEM) observations
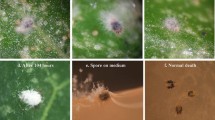
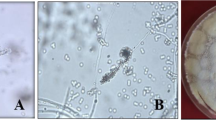
Ten days after spraying with B. bassiana, approximate 40% of the mobile stages of T. urticae (larva, immatures and adults) on the bean plants had succumbed, with the cadavers exhibiting symptoms of fungal infection (Fig. 4a). Because eggs were not infected and consequently gradually hatched, the eggs shown in the figure were actually newly oviposited. In the excised bean leaves in Petri dishes, fungal outgrowths were noticeable on most adult cadavers by six days post-treatment (Fig. 4b). The SEM shows the fungal mycelium had colonized the entire body of the adult mite (Fig. 4c) and immature (Fig. 4d) 60 h after fungal treatment. However, approximate 85% of the immature cadavers, produced no fungal hyphae. SEM images also demonstrated that fungal spray did result in conidial germination on the egg surface with hyphae extending from the germ tubes, although, none of the germ tubes were observed penetrating the eggs (Fig. 4e).
Observations on the status of T. urticae eggs and cadavers after treatment with B. bassiana (strain: GZGY-1-3) under light microscopy and scanning electronic microscopy. a Symptoms of fungal infection were observed from mite cadavers at ten days post-treatment on the bean plants; b fungal hyphae were noticeable on cadaver of adult mites at six days post-treatment in Petri dishes; c fungal mycelium colonizing the entire body of an adult mite cadaver at 60 h post-treatment; d fungal mycelium colonizing the entire body of an immature mite cadaver at 60 h post-treatment. e germinated conidia attached to egg shell at 60 h post-treatment
Discussion
Evaluation of potential fungal biocontrol candidates of an insect pest is not only based on their virulence in laboratory bioassays, but also depends on their practical suppression of the pest population on their host plant. Our previous study reported that several isolates of B. bassiana from non-acarine hosts were highly pathogenic to T. urticae adults (Wu et al. 2016). However, this study using potted bean plants resulted in a failure to control T. urticae on their host plants which were sprayed with B. bassiana including an isolate from acarine hosts, due to poor suppression of the eggs and immature stages. Although B. bassiana was sprayed twice at a 15-day interval, eggs in the original treated population hatched and developed into adults. Moreover, a majority of the immatures was found to be free of fungal infection. These viable eggs and immatures would subsequently be able to re-establish populations on their hosts. Results of laboratory bioassays of B. bassiana on eggs and immature individuals differ from those dealing with adult mites, further explaining the results obtained on potted plants.
Different developmental stages of T. urticae have been shown to vary in their susceptibility to fungal infection (Samish et al. 2001; Gindin et al. 2002. Wekesa et al. 2006). Our results demonstrated that eggs and immature stages were less susceptible to infection by B. bassiana. Similar results have been reported in other arthropods, including the spider mite T. evansi (Baker & Pritchard), with both B. bassiana and Metarhizium anisopliae (Metschn.) (Wekesa et al. 2006), the cassava green mite, Mononychellus tanajoa (Bondar), with Neozygites floridana (Weiser & Muma) (Oduor 1995), tick species (Samish et al. 2001; Gindin et al. 2002), and the western flower thrips, Frankliniella occidentalis (Pergande) (Vestergaard et al. 1995; Wu et al. 2014) with entomopathogenic fungi. The higher infection rate found in bean plant-collected adults of T. urticae compared to immature stages in this study, verifies the results of our laboratory bioassays. The different susceptibility could be attributed to interactions between the cuticle of arthropods being penetrated by the fungus and ecdysis. Moulting has been regarded as an important factor in arthropods’ defense to fungal infection, especially in those species with short intervals between molts (Wekesa et al. 2006). The larval, deutonymphal and protonymphal stages of T. urticae last 1–2 days at 26 °C (Hoque et al. 2008). Our previous study demonstrated that B. bassiana conidia attaches to the cuticle of T. urticae adults 2–12 h after fungal spray, and germinates within 24–36 h. The germinated conidia were able to successfully penetrate the mite cuticle at 48 h (Wu et al. 2016). The poor infectivity to immatures probably because of many conidia shedding from the mite’s cuticle during its ecdysis.
Egg quantity and viability are crucial for the development of pest mite populations (Bostanian et al. 2007), and, thus, a key target for population control. Because the mite eggs are too small and delicate to be transplanted by brush without inflicting damage, all eggs used in this study were naturally oviposited on detached kidney beans in Petri dishes and then inoculated with fungal spray (see Shi and Feng 2004). Unlike the mobile stages of insects/mites, spider mite eggs lack a true hemocoel where fungal cells would be able to propagate by budding following penetration. They are also well protected by their “solid” shells, which serve as a barrier to external hazards threatening the developing embryos (Witalinski 1993). However, some fungal strains have been found to have ovicidal activities against spider mites (Wekesa et al. 2006; Zhang et al. 2014). Based on our results, B. bassiana can be used to control T. urticae adults but has little, if any, effects on egg viability. The ovicidal activities of entomopathogenic fungi towards mite eggs rely upon the extending hyphae of the fungus for uptake of egg nutrition, resulting in embryo disruption (Zhang et al. 2014). Therefore, the difference in the susceptibility to spider mite eggs may be due to different features between fungal strains. Although poor infectivity to mite eggs and immature stages was found in our study, these fungal strains showed relatively high mortalities to female adults, probably resulting to the significant reduction in subsequent oviposition and egg populations. This hypothesis is also supported by our laboratory and potted plant assays.
Crops are normally infested with all stages of a spider mite pest in the field or greenhouse. An understanding of which stages are susceptible to fungal infection is critical in developing successful spider mite management tactics (Butt et al. 2001). Although this study does include some “negative” results, it does serve as an important assessment on the use of B. bassiana in controlling spider mites and provides information that will be of use in the development of biological control programs for T. urticae.
References
Abbott WS (1925) A method for computing the effectiveness of insecticides. J Econ Entomol 18:265–267
Alves SB, Rossi LS, Lopes RB, Tamai MA, Pereira RM (2002) Beauveria bassiana yeast phase on agar medium and its pathogenicity against Diatraea saccharalis (Lepidoptera: Crambidae) and Tetranychus urticae (Acari: Tetranychidae). J Invertebr Pathol 81:70–77
Bostanian NJ, Hardman JM, Racette G, Franklin JL (2007) The relationship between winter egg counts of the European red mite Panonychus ulmi (Acari: Tetranychidae) and its summer abundance in a reduced spray orchard. Exp Appl Acarol 42:185–195
Butt TM, Jackson CW, Magan N (eds) (2001) Fungi as biocontrol agents: progress, problems and potential. CABI Publishing, Wallingford
Dagli F, Tunc I (2001) Dicofol resistance in Tetranychus cinnabarinus: resistance and stability of resistance in populations from Antalya, Turkey. Pest Manag Sci 57:609–614
Farazmand A, Amir-Maafi M (2018) A population growth model of Tetranychus urticae Koch (Acari: Tetranychidae). Persian J Acarol 7:193–201
Feng M, Poprawski T, Khachatourians GG (1994) Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Technol 4:3–34
Ferron P (1985) Fungal control. In: Kerkut GA, Gilbert LI (eds) Comparative insect physiology, biochemistry and pharmacology, vol 12. Pergamon Press, Oxford, pp 313–346
Gatarayiha MC, Laing MD, Miller RM (2011) Field evaluation of Beauveria bassiana efficacy for the control of Tetranychus urticae Koch (Acari: Tetranychidae). J Appl Entomol 135:582–592
Gindin G, Samish M, Zangi G, Mishoutchenko A, Glazer I (2002) The susceptibility of different species and stages of ticks to entomopathogenic fungi. Exp Appl Acarol 28:283–288
Goettel MS, Inglis DG (1997) Fungi: Hyphomycetes. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, London, pp 213–249
Grbić M, van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, Osborne EJ, Dermauw W, Ngoc PCT, Ortego F, Hernández-Crespo P, Diaz I, Martinez M, Navajas M, Sucena É, Magalhães S, Nagy L, Pace RM, Djuranović S, Smagghe G, Iga M, Christiaens O, Veenstra JA, Ewer J, Villalobos RM, Hutter JL, Hudson SD, Velez M, Yi SV, Zeng J, Pires-daSilva A, Roch F, Cazaux M, Navarro M, Zhurov V, Acevedo G, Bjelica A, Fawcett JA, Bonnet E, Martens C, Baele G, Wissler L, Sanchez-Rodriguez A, Tirry L, Blais C, Demeestere K, Henz SR, Gregory TR, Mathieu J, Verdon L, Farinelli L, Schmutz J, Lindquist E, Feyereisen R, van de Peer Y (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492
Guo FY, Zhang ZQ, Zhao ZM (1998) Pesticide resistance of Tetranychus cinnabarinus (Acari: Tetranychidae) in China: a review. Syst Appl Acarol 3:3–7
Herron GA, Rophail J, Wilson LJ (2004) Chlorfenapyr resistance in two-spotted spider mite (Acari: Tetranychidae) from Australian cotton. Exp Appl Acarol 34:315–321
Hoque MF, Islam W, Khalequzzaman M (2008) Life tables of two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae) and its predator Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). J Biosci 16:1–10
Irigaray FJS, Marco-Mancebón V, Pérez-Moreno I (2003) The entomopathogenic fungus Beauveria bassiana and its compatibility with triflumuron: effect on the two spotted spider mite, Tetranychus urticae. Biol Control 26:168–173
Kakoki S, Kamimuro T, Ikenoue Y, Inokuchi M, Tsuda K, Sakamaki Y (2019) The response of three species of phytoseiid mite (Acari: Phytoseiidae) to synthetic pyrethroid pesticides in the laboratory and the field. Exp Appl Acarol 77:27–41
Littell RC, Pendergast J, Natarajan R (2000) Tutorial in biostatistics: modelling covariance structure in the analysis of repeated measures data. Stat Med 19:1793–1819
Maniania NK, Bugeme DM, Wekesa VW, Delalibera I Jr, Knapp M (2008) Role of entomopathogenic fungi in the control of Tetranychus evansi and Tetranychus urticae (Acari: Tetranychidae), pests of horticultural crops. Exp Appl Acarol 46:259–274
Mondel M, Ara N (2006) Biology and fecundity of the two-spotted spider mite, Tetranychus urticae Koch. (Acari: Tetranychidae) under laboratory conditions. J Life Earth Sci 1:43–47
Oduor GI (1995) Abiotic factors and the epizootiology of Neozygites cf. floridana, a fungus pathogenic to the cassava green mite. PhD Thesis, University of Amsterdam, The Netherlands
Prischmann DA, James DG, Wright LC, Teneyck RD, Snyder WE (2005) Effects of chlorpyrifos and sulfur on spider mites (Acari: Tetranychidae) and their natural enemies. Biol Control 33:324–334
Samish M, Gindin G, Alekseev E, Glazer I (2001) Pathogenicity of entomopathogenic fungi to different developmental stages of Rhipicephalus sanguineus (Acari: Ixodidae). J Parasitol 87:1355–1359
SAS Institute (2009) Base SAS® 9.2 Procedures Guide. SAS Institute, Cary
Shi WB, Feng MG (2004) Lethal effect of Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces fumosoroseus on the eggs of Tetranychus cinnabarinus (Acari: Tetranychidae) with a description of a mite egg bioassay system. Biol Control 30:165–173
Shi WB, Feng MG, Liu SS (2008a) Sprays of emulsifiable Beauveria bassiana formulation are ovicidal towards Tetranychus urticae (Acari: Tetranychidae) at various regimes of temperature and humidity. Exp Appl Acarol 46:247–257
Shi WB, Zhang L, Feng MG (2008b) Time-concentration-mortality responses of carmine spider mite (Acari: Tetranychidae) females to three hypocrealean fungi as biocontrol agents. Biol Control 46:495–501
Shin TY, Bae SM, Kim DJ, Yun HG, Woo SD (2017) Evaluation of virulence, tolerance to environmental factors and antimicrobial activities of entomopathogenic fungi against two-spotted spider mite, Tetranychus urticae. Mycoscience 58:204–212
Ullah MS, Lim UT (2017) Synergism of Beauveria bassiana and Phytoseiulus persimilis in control of Tetranychus urticae on bean plants. Syst Appl Acarol 22:1924–1935
Ullah MS, Haque MA, Nachman G, Gotoh T (2012) Temperature-dependent development and reproductive traits of Tetranychus macfarlanei (Acari: Tetranychidae). Exp Appl Acarol 56:327–344
Vestergaard S, Gillespie AT, Butt TM, Schreiter G, Eilenberg J (1995) Pathogenicity of the hyphomycete fungi Verticillium lecanii and Metarhizium anisopliae to the western flower thrips, Frankliniella occidentalis. Biocontrol Sci Technol 5:185–192
Wang Z, Cang T, Wu S, Wang X, Qi P, Wang X, Zhao X (2018) Screening for suitable chemical acaricides against two-spotted spider mites, Tetranychus urticae, on greenhouse strawberries in China. Ecotoxicol Environ Saf 163:63–68
Wekesa VW, Knapp M, Maniania NK, Boga HI (2006) Effects of Beauveria bassiana and Metarhizium anisopliae on mortality, fecundity and egg fertility of Tetranychus evansi. J Appl Entomol 130:155–159
Wilson LJ, Morton R (1993) Seasonal abundance and distribution of Tetranychus urticae (Acari: Tetranychidae), the two spotted spider mite, on cotton in Australia and implications for management. Bull Entomol Res 83:291–303
Witalinski W (1993) Egg-shells in mites—vitelline envelope and chorion in acaridida (Acari). Exp Appl Acarol 17:321–344
Wu SY, Gao YL, Zhang YP, Wang ED, Xu XN, Lei ZR (2014) An entomopathogenic strain of Beauveria bassiana against Frankliniella occidentalis with no detrimental effect on the predatory mite Neoseiulus barkeri: evidence from laboratory bioassay and scanning electron microscopic observation. PLoS ONE 9(1):e84732
Wu SY, Xie HC, Li MY, Xu XN, Lei ZR (2016) Highly virulent Beauveria bassiana strains against the two-spotted spider mite, Tetranychus urticae, show no pathogenicity against five phytoseiid mite species. Exp Appl Acarol 70:421–435
Wu M, Adesanya AW, Morales MA, Walsh DB, Lavine LC, Lavine MD, Zhu F (2019) Multiple acaricide resistance and underlying mechanisms in Tetranychus urticae on hops. J Pest Sci 92:543–555
Zhang L, Shi WB, Feng MG (2014) Histopathological and molecular insights into the ovicidal activities of two entomopathogenic fungi against two-spotted spider mite. J Invertebr Pathol 117:73–78
Zhang XN, Jin DC, Zou X, Guo JJ (2016) Laboratory and field evaluation of an entomopathogenic fungus, Isaria cateniannulata strain 08XS-1, against Tetranychus urticae (Koch). Pest Manag Sci 72:1059–1066
Zhang XN, Guo JJ, Zou X, Jin DC (2018) Pathogenic differences of the entomopathogenic fungus Isaria cateniannulata to the spider mite Tetranychus urticae (Trombidiformes: Tetranychidae) and its predator Euseius nicholsi (Mesostigmata: Phytoseiidae). Exp Appl Acarol 75:69–84
Acknowledgements
We thank Dr. Cecil L. Smith (University of Georgia, USA) for helping with the language editing. This work was supported by Natural Science Foundation of China (Grant No. 31501704).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Helen Roy
Electronic supplementary material
Below is the link to the electronic supplementary material.
10526_2019_9970_MOESM1_ESM.tif
Supplementary Fig. S1 Population fluctuations of T. urticae on potted bean plants following fungal sprays. Initial densities of T. urticae were determined immediately prior to treatment on day 0. B. bassiana suspension, was sprayed at 1 × 107 ml−1 fungal conidia/ml. (a). Eggs; (b). immatures (larvae, protonymphs and deutonymphs) of T. urticae; (c). Adult T. urticae. Each data point and bar represent the mean and SE, respectively. Black arrows signify fungal spray (B. bassiana strains: LNSZ-26; SDDZ-9; XJWLMQ-32; SCWJ-2 and JXJGS-1) (TIFF 11344 kb)
10526_2019_9970_MOESM2_ESM.tif
Supplementary Fig. S2 Comparison of mean corrected mortality (± SE) of B. bassiana in different stages of T. urticae at 7 days post-fungal treatment in the laboratory (B. bassiana strains: LNSZ-26; SDDZ-9; XJWLMQ-32; SCWJ-2 and JXJGS-1). Bars with different letters indicate that the corrected mortalities in different stages of T. urticae are significant different (all P’s< 0.05)
10526_2019_9970_MOESM3_ESM.tif
Supplementary Fig. S3 Daily oviposition by female T. urticae and egg hatchability after being separately treated with B. bassiana (strain: LNSZ-26, SDDZ-9, XJWLMQ-32, SCWJ-2 and JXJGS-1). (a). Oviposition; (b). Egg hatch rate. Each data point and bar represent the mean and SE, respectively (B. bassiana strains: LNSZ-26; SDDZ-9; XJWLMQ-32; SCWJ-2 and JXJGS-1) (TIFF 5258 kb)
Rights and permissions
About this article
Cite this article
Wu, S., Sarkar, S.C., Lv, J. et al. Poor infectivity of Beauveria bassiana to eggs and immatures causes the failure of suppression on Tetranychus urticae population. BioControl 65, 81–90 (2020). https://doi.org/10.1007/s10526-019-09970-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09970-0