Abstract
Aerial conidia of Beauveria bassiana in an emulsifiable formulation germinated by >95% after 24 h exposure to the regimes of 20, 25 and 30°C with 51%, 74% and 95% RH. Ovicidal activities of the formulation towards two-spotted spider mite, Tetranychus urticae, were assayed at the concentrations of 0, 18, 160 and 693 conidia mm−2 sprayed separately onto fava bean leaves including 39 (25–76) eggs per capita. All the sprayed eggs on the leaves were directly exposed to the different regimes for hatch after 24 h maintenance in covered Petri dishes. Generally, hatched proportions increased over post-spray days and decreased with the elevated fungal concentrations; no more eggs hatched from day 9 or 10 onwards. Based on the counts of the hatched/non-hatched eggs in the different regimes, the final egg mortalities were 15.0–40.4%, 48.9–66.6% and 62.9–87.5% at the low, medium and high concentrations, respectively, but only 5.6–11.3% in blank controls. The RH effect on the fungal action was significant at 20 and 25°C but not at 30°C whereas the effect of temperature was significant at 51% and 74% RH but not at 95% RH. Probit analysis of the egg mortalities versus the fungal sprays generated median lethal concentrations (LC50) of 65–320 conidia mm−2 at all the regimes, and of only 65–78 conidia mm−2 at 25–30°C with 74–95% RH. The results highlight ovicidal activities of the emulsifiable formulation against the mite species at the tested regimes and its potential use in spider mite control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The two-spotted spider mite, Tetranychus urticae Koch [including synonymous T. cinnabarinus (Boisduval); Ros and Breeuwer 2007], infests a large variety of economic plants worldwide (Hazan et al. 1974; Ho et al. 1997). Spider mite control in the past few decades has relied upon a number of acaricides, such as organochlorides and organophosphates (Gerson and Cohen 1989). This reliance on chemicals has generally caused mite resistance and public concerns on their high residues in products (Guo et al. 1998; Dagli and Tunc 2001). Some acaricides, such as dicofol, cyhexatin and fenbutatin oxide, have thus been prohibited from mite control on vegetables, melons, fruits and tea in China, making it necessary to search for alternative control measures. Fungal pathogens of mites are considered to be potential for the purpose (Poinar 1998; Chandler et al. 2000; Van der Geest et al. 2000).
Entomopathogenic hyphomycetes, such as Beauveria bassiana (Balsamo) Vuellemin, Metarhizium anisopliae (Metschnikoff) Sorokin and Paecilomyces fumosoroseus (Wize) Brown & Smith, are well-known fungal biocontrol agents (Feng et al. 1994; Faria and Wraight 2001; Roberts and Leger 2004) and have been formulated for wide application to insect control (Langewald et al. 1997; Wraight et al. 2000; Wraight and Ramos 2002; Feng et al. 2004a, b; Pu et al. 2005). They are also potential mite pathogens despite rare prevalence in the field (Chandler et al. 2000). Recently, some fungal isolates derived from host insects have proven to kill spider mite eggs under laboratory conditions and unformulated conidia of a B. bassiana isolate have an ovicidal LC50 of 548 conidia mm−2 (Shi and Feng 2004), which can be reduced greatly by low application rates of pyridaben included in fungal sprays (Shi et al. 2005). In other studies, the fungal insect pathogens are also found capable of infecting active stages of spider mites (Alves et al. 2002; Wekesa et al. 2005, 2006; Maniania et al. 2008; Shi et al. 2008a) and ectoparasitic mites (Shaw et al. 2002; Lekimme et al. 2006, 2008; Meikle et al. 2008).
Environmental temperature and relative humidity (RH) are known to affect conidial germination, colony growth, and host infection of the fungal pathogens (Feng et al. 1994; Roberts and Leger 2004). Appropriate temperature and high RH are usually crucial to successful infection of the fungal agents (Milner et al. 1997; Luz and Fargues 1999). Although common fungal agents in unformulated form have proven to infect various stages of mite pests under controlled conditions (Shi and Feng 2004; Lekimme et al. 2006; Wekesa et al. 2005, 2006), the possible effects of selected formulations and variable environments on their acaricidal activities have not yet been understood. This has hindered a sound evaluation of their potential in mite control. In the present study, aerial conidia of the ovicidal B. bassiana isolate found previously (Shi and Feng 2004) were formulated into an oil-based, emulsifiable carrier and then sprayed onto leaves where T. urticae eggs were laid in advance. Our goals were to evaluate ovicidal activities of the formulation at gradient application rates and to determine the effects of different temperature and humidity regimes on the hatch rates and mortalities of the mite eggs. The data presented in this paper would help to value the potential of the fungal formulation for incorporation into mite pest management systems.
Materials and methods
Preparation of aerial conidia and emulsifiable formulation
The ovicidal isolate, B. bassiana SG8702, was derived from a naturally mycosed aphid (Feng et al. 1990) with accession number ARSEF 2860 (USDA-ARS Collection of Entomopathogenic Fungal Cultures, Ithaca, NY, USA). This isolate has been formulated for control of greenhouse whiteflies (Feng et al. 2004a) and tea leafhoppers (Feng et al. 2004b; Pu et al. 2005). It was preserved as a mixture of dried conidia with sterile sands at −72°C. To produce conidia in this study, the preserved conidia were used to inoculate the plates of Sabouraud dextrose agar plus 1% yeast extract (SDAY) for 7 days incubation at 25°C. The resultant conidia were suspended in Sabouraud dextrose broth (SDB) and incubated for 2 days at 25°C by shaking at 110 rpm. The resultant liquid culture was mixed with steamed rice at the rate of 10% (v/w) and the mixture was then poured into 15-cm-diameter Petri dishes (100 g per dish). After 7 days growth and conidiation at 25°C, the rice cultures were dried overnight in a ventilation chamber at 33°C and then passed through an electrically vibrating sieve (10 threads mm−1) for harvest of conidia, followed by vacuum drying to ca. 5% water content at ambient temperature (Ye et al. 2006).
The dried conidial powder was uniformly suspended in a mixture of 95% (v/v) industrial paraffin as oil carrier and 5% (v/v) fatty alcohol polyethylene glycol ether ‘AEO-3’ as emulsifier (Xiaoshan Chemical Additives, Hangzhou, Zhejiang, China). The emulsifiable formulation was standardized to 1 × 1010 conidia ml−1 and used immediately or stored at 6°C in dark for bioassays below.
Viability assays at different regimes
Aqueous dilution (1 × 106 conidia ml−1) of the emulsifiable formulation was prepared and 100 μl aliquots were smeared evenly onto the 90-mm-diameter plates of SDAY supplemented with 0.1% chloramphenicol to prevent possible bacterial contamination. Not covered with lids, the smeared SDAY plates were maintained in incubators at 20, 25 and 30 ± 1°C with 12:12 L:D, respectively. Each incubator included three Perspex chambers (135 × 135 × 185 mm), in which 51%, 74% and 95% RH were achieved by pumping continuously moisture-specific air from the last of three rubber-tube-connected jars into each chamber (Feng et al. 1999). Aqueous solutions of 59.8%, 31.4% and 18.8% (v/v) glycerin were separately half-filled into each set of the jars to generate the RHs at 20–30°C (Doberski 1981). The conidia smeared on the plates were thus exposed to nine treatments of temperature and RH combinations, each including three plates as replicates.
Germinated and non-germinated conidia at each of the regimes were counted after 12 and 24 h incubation under microscope at 400× magnification (three counts of >100 conidia per plate). Conidial viability at a given regime was determined as percentages of the germinated conidia (with visible germ tubes) in total.
Preparation of the mite eggs
The eggs of T. urticae were prepared using a detached leaf system described by Shi and Feng (2004). A laboratory population of the mite species was maintained on fava bean (Vicia faba L.) plants in a walk-in growth room at the regime of 23 ± 2°C and 12:12 L:D. Twenty vigorous adult females arbitrarily taken from the population were transferred to a detached leaf in Petri dish (6.5 cm diameter), in which root hairs grew from the petiole into an agar plate below the leaf. The females were allowed to lay eggs freely for 18 h and then removed. A certain number of eggs (usually 30–40) were left on each leaf to receive treatments as follows. The detached leaf system could support a mite colony for 15 days or so, warranting normal hatch of the mite eggs with no need for leaf change during a bioassay.
Ovicidal assays of B. bassiana at different regimes
Aqueous dilutions (1 × 108, 1 × 107 and 1 × 106 conidia ml−1) of the emulsifiable formulation were sprayed onto the eggs on the leaves for inoculation using a non-touch leaf method (Shi and Feng 2004). Briefly, each uncovered dish of the detached leaf bearing the mite eggs was placed on the center of the bottom specimen dish (11 cm diameter) of an Automatic Potter Spray Tower (Burkard Scientific, Uxbridge, Middx, UK) to receive a 2-ml spray of each conidial dilution from its top nozzle at the working pressure of 0.7 kg cm−2 (the manufacturer’s guide). Separate equal-volume sprays of the three aqueous dilutions resulted in different concentrations of the conidia deposited onto the mite eggs and leaves. Each concentration was determined as no. conidia mm−2 using microscopic counts of the conidia deposited onto a glass slip (20 × 20 mm; five 0.2165-mm2 view fields per slip), which was placed beside the dish under each spray. The same-volume spray of 100-fold aqueous dilution of the liquid carrier alone (i.e., the mixture of 95% paraffin and 5% emulsifier) was included as blank control of the three fungal sprays in each bioassay.
After exposure to the fungal sprays, all the eggs on detached leaves in Petri dishes were covered with lids and maintained overnight at 25°C and 12:12 L:D to favor conidial germination. Subsequently, all the dishes with the sprayed leaves and mite eggs were uncovered and arranged randomly into the regimes of 20–30°C and 51–95% RH (humidity chambers) as described above with each regime including the three fungal concentrations as treatments. Egg hatches were daily examined until no more eggs hatched for three consecutive days at any of the regimes. All non-hatched eggs, together with the detached leaves, were examined under a dissection microscope for verification of fungal infection. Final egg mortalities in different treatments were computed based on the last-day counts of the hatched and non-hatched eggs. All the bioassays were repeated three times during a period of 75 days.
Data analysis
The 12- and 24-h germination rates of the formulated conidia exposed to the temperature and RH regimes were analyzed using two-way ANOVA. Hatched proportions of the mite eggs observed at the concentrations of 0–693 conidia mm−2 from the regimes of 20–30°C and 51–95% RH were plotted over post-spray days. Variation in egg mortalities at a given RH or temperature was differentiated among the fungal concentrations by two-way ANOVA. The egg mortalities caused by the fungal sprays at each of the combined regimes were corrected using background mortality in the corresponding blank control and then subjected to probit analysis. A linear concentration- mortality relationship from each analysis was used to estimate median lethal concentration (LC50) and associated 95% confidence limits (CL) as an index for ovicidal activity of the fungal formulation at each regime. An updated version of DPS software (Tang and Feng 2007) was used in all the analyses.
Results
Effects of temperature and RH on the viability of oil-formulated conidia
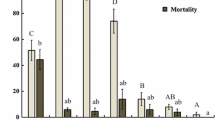
The viabilities of B. bassiana conidia formulated were significantly affected by temperature (12 h: F 2,16 = 171.9, P < 0.01; 24 h: F 2,16 = 57.0, P < 0.01) but not by RH (12 h: F 2,16 = 2.6, P = 0.11; 24 h: F 2,16 = 1.9, P = 0.18) among the concerned regimes. The interaction of both variables also had significant effect on the viabilities (12 h: F 4,16 = 3.3, P = 0.04; 24 h: F 4,16 = 21.7, P < 0.01). Germination ranged from 5.1% at the regime of 20°C and 51% RH to 22.4% at 25°C and 95% RH after 12 h incubation but reached 95.6–98.3% in all the regimes by 24 h (Fig. 1). Thus, the oil-formulated conidia had high viabilities despite some variation perhaps due to the temperature and RH interaction.
Comparison of the viabilities of the formulated Beauveria bassiana conidia incubated for 12 (shading bars) and 24 h (white bars) on SDAY plates at the regimes of 20, 25 and 30°C with 51%, 74% and 95% RH, respectively. Bars with different letters differed significantly in height (Tukey’s HSD, P < 0.05). Error bars: SD
After12 h incubation, the overall mean germination rates (± SD) at 51–95% RH were significantly higher at 25°C (18.1 ± 3.6%) than at 20 (8.3 ± 3.5%) or 30°C (6.4 ± 1.5%) (Tukey’s HSD, P < 0.05). By the end of 24 h incubation, however, mean germination rates in the three RH treatments were very close among the temperatures, i.e., 98.0 ± 0.5% at 20°C, 98.3 ± 0.2% at 25°C, and 96.4 ± 0.7% at 30°C. Moreover, overall mean germination rates at 95% RH (12 h: 10.9 ± 4.3%; 24 h: 97.7 ± 1.0%) did not differ significantly from those at 51% RH (12 h: 11.8 ± 8.1%; 24 h: 97.3 ± 1.4%) or at 74% RH (12 h: 10.2 ± 5.7%; 24 h: 97.7 ± 0.6%) when the three-temperature observations were pooled (Tukey’s HSD, P > 0.05).
Hatch trends of sprayed mite eggs at different regimes
Sprays of the three conidial dilutions generated mean concentrations of 17.9 (±3.0), 160.4 (±17.1) and 693.1 (±183.6) conidia mm−2 deposited on the leaves with 39 (25–76) eggs per capita in the repeated bioassays. Thus, a total number of 4,218 mite eggs sprayed at 0–693 conidia mm−2 were exposed to the regimes of 20, 25 and 30°C with 51%, 74% and 95% RH.
The trends of hatched proportions of the mite eggs at all the regimes are illustrated over days after each fungal spray (Fig. 2). Generally, observations within each of the regimes were dependent on both fungal concentrations and post-spray days. Most of the mite eggs in blank controls or sprayed at the low fungal concentration hatched within 7–9 days but no egg hatch was observed in the first 2 days. Differences in hatch rates were small among the fungal treatments during the first 3–4 days but became larger thereafter. Very few eggs were observed hatching from day 9 or 10 onwards. As a result, different numbers of the mite eggs were not hatched in the fungal treatments irrespective of the regimes.
Egg mortalities caused by fungal sprays at different regimes
Variations in the final mortalities of the mite eggs at the different fungal concentrations were differentiated by two-way ANOVA (Table 1). The fungal concentration was consistently most influential on the egg mortalities (maximal F with minimal P) at a given temperature or RH. This indicates that the egg mortalities were attributed to infection by B. bassiana. The RH effects on the mortalities were significant only at 20 and 25°C (P < 0.01) but insignificant at 30°C (P = 0.79). The RH and concentration interaction was not significant at a given temperature (P > 0.05). The effect of temperature on the mortalities was significant only at 51% or 74% RH (P < 0.01) but not at 95% RH (P = 0.23). However, a significant effect was found in the interaction of temperature with the fungal concentration at 95% RH (P < 0.01).
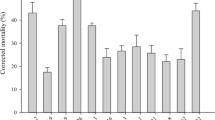
The egg mortalities caused by the fungal formulation fell in the range of 62.5–87.9% at the high concentration and of 48.9–66.6% at the medium, varying with the temperature/RH regimes (Fig. 3). These were significantly higher than the background mortalities of 5.6–11.3% in the blank controls (Tukey’s HSD, P < 0.05). The low fungal concentration resulted in the mortalities of 15.0–40.4% but only those at the regimes of 25°C or 74–95% RH were significantly higher than the mortalities in the controls.
Comparison of the final mortalities of Tetranychus urticae eggs at the different concentrations of Beauveria bassiana formulation (no. conidia mm−2; 0 = blank control) at the regimes of 20, 25 and 30°C with 51%, 74% and 95% RH, respectively. Bars with different lowercase letters in each graph differed significantly in height (Tukey’s HSD, P < 0.05). Error bars: SD
LC50s as indices of ovicidal activities at different regimes
The linear concentration-mortality relationships determined by probit analysis generated the LC50 values and associated 95% CL for the tested formulation against the mite eggs at all the regimes (Table 2). The fungal formulation was highly ovicidal with the LC50 declining with increased RH at a given temperature. The maximal LC50 at the regime of 20°C and 51% RH was 320 conidia mm−2. Those at the regimes of 25–30°C with 74–95% RH were only 65–78 conidia mm−2.
Discussion
In summary, the formulated B. bassiana conidia were highly viable at the regimes of 20–30°C with 51–95% RH. The hatched proportions of T. urticae eggs after exposure to fungal sprays of 18–693 conidia mm−2 were generally lower than those in blank controls despite some variations. The final egg mortalities in the fungal treatments were always higher than those in blank controls irrespective of the temperature/RH regimes. The RH effect on the fungal action was significant at 20 and 25°C but not at 30°C whereas the effect of temperature was significant at 51% and 74% RH but not at 95% RH. The ovicidal LC50 s of the formulation spanned from 65 to 320 conidia mm−2 at all the regimes but fell in a very narrow range of 65–78 conidia mm−2 at 25–30°C with 74–95% RH. The results indicate a conspicuous ovicidal activity of the fungal formulation towards the spider mite species at the concerned regimes.
A high viability of fungal conidia in a formulation sprayed onto target pests is a prerequisite for their germination and infection. Germination in vitro is related to expression of fungal virulence (Jackson et al. 1989; Altre et al. 1999). Since conidial germination is known to largely depend on RH and temperature (Feng et al. 1994; Roberts and St. Leger 2004) and spider mite eggs are usually laid on the surfaces of leaves or shoots with some moisture (e.g., metabolic water, dew), the formulated conidia in this study were allowed to germinate for 24 h on uncovered SDAY plates entirely exposed to the regimes of 51–95% RH and 20–30°C. These are normal conditions for heavy infestation of spider mite pests in the field. The observed high viabilities help to interpret the high egg mortalities caused by B. bassiana at the same regimes. This indicates that the emulsifiable formulation would be able to act on spider mites under field conditions. Other reports have also shown substantial infections of B. bassiana and/or M. anisopliae to southern pine beetle adults at 55–94% RH (Moore 1973), elm bark beetle larvae at 51–100% RH (Doberski 1981), grasshoppers at 12–100% RH (Marcandier and Khachatourians 1987), and the Chagas’ disease vector Rhodnius prolixus Stål at 43–97% RH (Fargues and Luz 2000), despite higher mortalities associated with higher RH.
The LC50 of the tested B. bassiana formulation against T. urticae eggs ranged from 65 conidia mm−2 at 30°C and 95% RH to 320 at 20°C and 51% RH. We think that the emulsifiable formulation has greatly enhanced ovicidal activities of the fungal agent in comparison with an LC50 of 548 (393–857) or 546 (406–818) conidia mm−2 (plain conidia suspended in 0.02% Tween-80) toward the eggs of the same mite species at 25°C under moist conditions (Shi and Feng 2004; Shi et al. 2005). This supports previous reports on oil-increased efficacy of M. anisopliae against whiteflies (Malsam et al. 2002) and of B. bassiana against aphids (Ye et al. 2005), and on improved adaptation of oil formulations to low-humidity environments for insect control (Bateman et al. 1993; Kooyman and Godonou 1997). Although possible mechanisms involved in the enhancement of fungal activities by the oil-based formulation are not clear at present, we postulate that the enhancement may result from better attachment of the formulated conidia to target pests and from improved protection of the conidia from desiccation after spray. This warrants more studies.
The ovicidal activities of the emulsifiable formulation tested at different temperature/RH regimes highlight its potential for practical incorporation into mite pest management due to its adaptation to the low-RH environments. In field trials, this formulation sprayed twice at the rate of ca. 1.5 × 1013 conidia ha−1 has provided significant control of citrus red mites, Panonychus citri (McGregor), in the orchards of east China (Shi and Feng 2006) and of cotton spider mites, mainly Tetranychus truncates Ehara and T. turkestani (Ugarov & Nikolskii), in the Tarim Basin of northwest China (Shi et al. 2008b). However, spider mites in southern China and other subtropical areas often infest crops heavily during hot summer, which is a challenge for the tolerance of the fungal formulation to outdoor thermal stress often around 40°C. If fungal candidates with greater thermotolerance and other improved traits (Ying and Feng 2004; Zou et al. 2006) are formulated into the oil-based carrier, application of fungal formulations to more stressed seasons or environments for spider mite control would be more promising. This also warrants future studies.
References
Altre JA, Vandenberg JD, Cantone FA (1999) Pathogenicity of Paecilomyces fumosoroseus isolates to diamondback moth, Plutella xylostella: correlation with spore size, germination speed, and attachment to cuticle. J Invertebr Pathol 73:332–338. doi:10.1006/jipa.1999.4844
Alves SB, Rossi LS, Lopes RB, Tamai MA, Pereira RM (2002) Beauveria bassiana yeast phase on agar medium and its pathogenicity against Diatraea saccharalis (Lepidoptera: Crambidae) and Tetranychus urticae (Acari: Tetranychidae). J Invertebr Pathol 81:70–77. doi:10.1016/S0022-2011(02)00147-7
Bateman RP, Carey M, Moore D, Prior C (1993) The enhanced infectivity of Metarhizium flavoviride in oil formulations to desert locusts at low humidities. Ann Appl Biol 122:145–152. doi:10.1111/j.1744-7348.1993.tb04022.x
Chandler D, Davidson G, Pell JK, Ball BV, Shaw K, Sunderland KD (2000) Fungal biocontrol of Acari. Biocontrol Sci Technol 10:357–384. doi:10.1080/09583150050114972
Dagli F, Tunc I (2001) Dicofol resistance in Tetranychus cinnabarinus: resistance and stability of resistance in populations from Antalya, Turkey. Pest Manag Sci 57:609–614. doi:10.1002/ps.334
Doberski JW (1981) Comparative laboratory studies on three fungal pathogens of the elm bark beetle, Scolytus scolytus: effect of temperature and humidity on infection by Beauveria bassiana, Metarhizium anisopliae and Paecilomyces farinosus. J Invertebr Pathol 37:195–200. doi:10.1016/0022-2011(81)90075-6
Faria M, Wraight SP (2001) Biological control of Bemisia tabaci with fungi. Crop Prot 20:767–778. doi:10.1016/S0261-2194(01)00110-7
Fargues J, Luz C (2000) Effects of fluctuating moisture and temperature regimes on the infection potential of Beauveria bassiana for Rhodnius prolixus. J Invertebr Pathol 75:202–211. doi:10.1006/jipa.1999.4923
Feng MG, Johnson JB, Kish LP (1990) Survey of entomopathogenic fungi naturally infecting cereal aphids (Homoptera: Aphididae) of irrigated grain crops in southwestern Idaho. Environ Entomol 19:1534–1542
Feng MG, Poprawski TJ, Khachatourians GG (1994) Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Technol 4:3–34
Feng MG, Xu Q, Xu JH (1999) Humidity control in biological experiments: modified device and methodology. Chin J Appl Ecol 10:357–361 in Chinese
Feng MG, Chen B, Ying SH (2004a) Trials of Beauveria bassiana, Paecilomyces fumosoroseus and imidacloprid for management of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on greenhouse grown lettuce. Biocontrol Sci Technol 14:531–544. doi:10.1080/09583150410001682269
Feng MG, Pu XY, Ying SH, Wang YG (2004b) Field trials of an oil-based emulsifiable formulation of Beauveria bassiana conidia and low application rates of imidacloprid for control of false-eye leafhopper Empoasca vitis in southern China. Crop Prot 23:489–496. doi:10.1016/j.cropro.2003.10.004
Gerson U, Cohen E (1989) Resurgences of spider mites (Acari: Tetranychidae) induced by synthetic pyrethroids. Exp Appl Acarol 6:29–46. doi:10.1007/BF01193231
Guo FY, Zhang ZQ, Zhao ZM (1998) Pesticide resistance of Tetranychus cinnabarinus (Acari: Tetranychidae) in China: a review. Syst Appl Acarol 3:3–7
Hazan A, Gerson U, Tahori AS (1974) Spider mite webbing I The production of webbing under various environmental conditions. Acarologia 16:68–84
Ho CC, Lo CC, Chen WH (1997) Spider mite (Acari: Tetranychidae) on various crops in Taiwan. J Agric Res China 46:333–346
Jackson CW, Heale JB, Llewellyn M (1989) Characteristics relating to the pathogenicity of Metarhizium anisopliae toward Nilaparvata lugens. J Invertebr Pathol 53:25–31. doi:10.1016/0022-2011(89)90070-0
Kooyman C, Godonou I (1997) Infection of Schistocerca gregaria (Orthoptera: Acrididae) hoppers by Metarhizium flavoviride (Deuteromycotina: Hyphomycetes) conidia in an oil formulation applied under desert conditions. Bull Entomol Res 87:105–107
Langewald J, Kooyman C, Douro-Kpindou O, Lomer CJ, Dahmoud AO, Mohamed HO (1997) Field treatment of desert locust (Schistocerca gregaria Forskal) hoppers in Mauritania using an oil formulation of the entomopathogenic fungus Metarhizium flavoviride. Biocontrol Sci Technol 7:603–611. doi:10.1080/09583159730659
Lekimme M, Mignon B, Tombeux S, Focant C, Marechal F, Losson B (2006) In vitro entomopathogenic activity of Beauveria bassiana against Psoroptes spp. (Acari: Psoroptidae). Vet Parasitol 139:196–202. doi:10.1016/j.vetpar.2006.02.041
Lekimme M, Focant C, Farnir F, Mignon B, Losson B (2008) Pathogenicity and thermotolerance of entomopathogenic fungi for the control of the scab mite, Psoroptes ovis. Exp Appl Acarol 45(this issue)
Luz C, Fargues J (1999) Dependence of the entomopathogenic fungus, Beauveria bassiana, on high humidity for infection of Rhodnius prolixus. Mycopathology 146:33–41. doi:10.1023/A:1007019402490
Malsam O, Kilian M, Oerke EC, Dehne HW (2002) Oils for increased efficacy of Metarhizium anisopliae to control whiteflies. Biocontrol Sci Technol 12:337–348. doi:10.1080/09583150220128121
Maniania NK, Bugeme DM, Wekesa VW, Delalibera I Jr, Knapp M (2008) Role of entomopathogenic fungi in the control of Tetranychus evansi and Tetranychus urticae (Acari: Tetranychidae) pests of horticultural crops. Exp Appl Acarol 45(this issue)
Marcandier S, Khachatourians GG (1987) Susceptibility of the migratory grasshopper, Melanoplus sanguinipes (Fab.) (Orthoptera: Acrididae), to Beauveria bassiana (Bals.) Vuillemin (Hyphomycete): influence of relative humidity. Can Entomol 119:901–907
Meikle WG, Mercadier G, Holst N, Girod V (2008) Impact of two treatments of a formulation of Beauveria bassiana (Deuteromycota: Hyphomycetes) conidia on Varroa mites (Acari: Varroidae) and on honeybee (Hymenoptera: Apidae) colony health. Exp Appl Acarol 45(this issue)
Milner RJ, Staples JA, Lutton GG (1997) The effect of humidity on germination and infection of termites by the hyphomycete, Metarhizium anisopliae. J Invertebr Pathol 69:64–69. doi:10.1006/jipa.1996.4636
Moore GE (1973) Pathogenicity of three entomogenous fungi to the southern pine beetle at various temperatures and humidities. Environ Entomol 2:54–57
Poinar GO Jr (1998) Parasites and pathogens of mites. Annu Rev Entomol 43:449–469. doi:10.1146/annurev.ento.43.1.449
Pu XY, Feng MG, Shi CH (2005) Impact of three application methods on the field efficacy of a Beauveria bassiana-based mycoinsecticide against the false-eye leafhopper, Empoasca vitis (Homoptera: Cicadellidae) in tea canopy. Crop Prot 24:167–175. doi:10.1016/j.cropro.2004.07.006
Roberts DW, St. Leger RJ (2004) Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects. Adv Appl Microbiol 54:1–70. doi:10.1016/S0065–2164(04)54001-7
Ros VID, Breeuwer JAJ (2007) Spider mite (Acari: Tetranychidae) mitochondrial COI phylogeny reviewed: host plant relationships, phylogeography, reproductive parasites and barcoding. Exp Appl Acarol 42:239–262. doi:10.1007/s10493-007-9092-z
Shaw KE, Davidson G, Clark SJ, Ball BV, Pell JK, Chandler D et al (2002) Laboratory bioassays to assess the pathogenicity of mitosporic fungi to Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of the honeybee, Apis mellifera. Biol Control 24:266–276. doi:10.1016/S1049-9644(02)00029-4
Shi WB, Feng MG (2004) Lethal effect of Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces fumosoroseus on the eggs of Tetranychus cinnabarinus (Acari: Tetranychidae) with a description of a mite egg bioassay system. Biol Control 30:165–173. doi:10.1016/j.biocontrol.2004.01.017
Shi WB, Feng MG (2006) Field efficacy of application of Beauveria bassiana formulation and low rate pyridaben for sustainable control of citrus red mite Panonychus citri (Acari: Tetranychidae) in orchards. Biol Control 39:210–217. doi:10.1016/j.biocontrol.2006.06.016
Shi WB, Jiang Y, Feng MG (2005) Compatibility of ten acaricides with Beauveria bassiana and enhancement of fungal infection to Tetranychus cinnabarinus (Acari: Tetranychidae) eggs by sublethal application rates of pyridaben. Appl Entomol Zool (Jpn) 40:659–666. doi:10.1303/aez.2005.659
Shi WB, Zhang L, Feng MG (2008a) Time-concentration-mortality responses of carmine spider mite (Acari: Tetranychidae) females to biocontrol agents of three hypocrealean fungi in a standardized bioassay system. Biol Control 46. doi:10.1016/j.biocontrol.2008.04.006
Shi WB, Zhang LL, Feng MG (2008b) Field trials of four formulations of Beauveria bassiana and Metarhizium anisopliae for control of cotton spider mites (Acari: Tetranychidae) in the Tarim Basin of China. Biol Control 45:48–55. doi:10.1016/j.biocontrol.2007.11.006
Tang QY, Feng MG (2007) DPS data processing system: experimental design, statistical analysis and data mining. Science Press, Beijing
Van der Geest LPS, Elliot SL, Breeuwer JAJ, Beerling EAM (2000) Diseases of mites. Exp Appl Acarol 24:497–560. doi:10.1023/A:1026518418163
Wekesa VW, Maniania NK, Knapp M, Boga HI (2005) Pathogenicity of Beauveria bassiana and Metarhizium anisopliae to the tobacco spider mite Tetranychus evansi. Exp Appl Acarol 36:41–50. doi:10.1007/s10493-005-0508-3
Wekesa VW, Knapp M, Maniania NK, Boga HI (2006) Effects of Beauveria bassiana and Metarhizium anisopliae on mortality, fecundity and egg fertility of Tetranychus evansi. J Appl Entomol 130:155–159. doi:10.1111/j.1439-0418.2006.01043.x
Wraight SP, Ramos ME (2002) Application parameters affecting field efficacy of Beauveria bassiana foliar treatments against Colorado potato beetle Leptinotarsa decemlineata. Biol Control 23:164–178. doi:10.1006/bcon.2001.1004
Wraight SP, Carruthers RI, Jaronski ST, Bradley CA, Garza CJ, Galaini-Wraight S (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol Control 17:203–217. doi:10.1006/bcon.1999.0799
Ye SD, Dun YH, Feng MG (2005) Time and concentration dependent interactions of Beauveria bassiana with sublethal rates of imidacloprid against the aphid pests Macrosiphoniella sanborni and Myzus persicae. Ann Appl Biol 146:459–468. doi:10.1111/j.1744-7348.2005.040147.x
Ye SD, Ying SH, Chen C, Feng MG (2006) New solid-state fermentation chamber for bulk production of aerial conidia of fungal biocontrol agents on rice. Biotechnol Lett 28:799–804. doi:10.1007/s10529-006-9004-z
Ying SH, Feng MG (2004) Relationship between thermotolerance and hydrophobin-like proteins in aerial conidia of Beauveria bassiana and Paecilomyces fumosoroseus as fungal biocontrol agents. J Appl Microbiol 97:323–331. doi:10.1111/j.1365-2672.2004.02311.x
Zou G, Ying SH, Shen ZC, Feng MG (2006) Multi-sited mutations of beta-tubulin are involved in benzimidazole resistance and thermotolerance of fungal biocontrol agent Beauveria bassiana. Environ Microbiol 8:2096–2105. doi:10.1111/j.1462-2920.2006.01086.x
Acknowledgements
Funding of this study was provided jointly by the grants from the Natural Science Foundation of China (30571250), the Ministry of Science and Technology of China (2006BAD08A02 and 2007DFA3100), and the Zhejiang Provincial R&D Program (2007C12035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, WB., Feng, MG. & Liu, SS. Sprays of emulsifiable Beauveria bassiana formulation are ovicidal towards Tetranychus urticae (Acari: Tetranychidae) at various regimes of temperature and humidity. Exp Appl Acarol 46, 247–257 (2008). https://doi.org/10.1007/s10493-008-9172-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-008-9172-8